

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM252182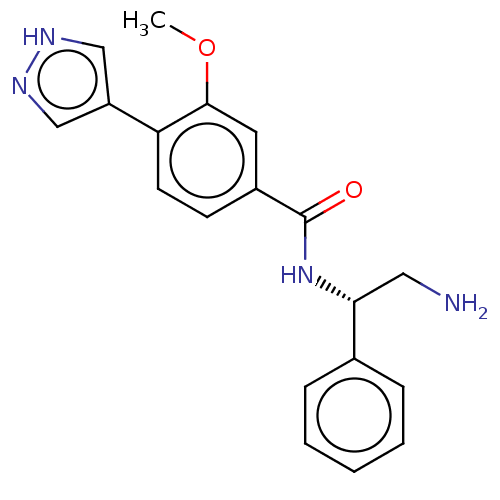 (US9458110, 254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM251935 (US9458110, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546544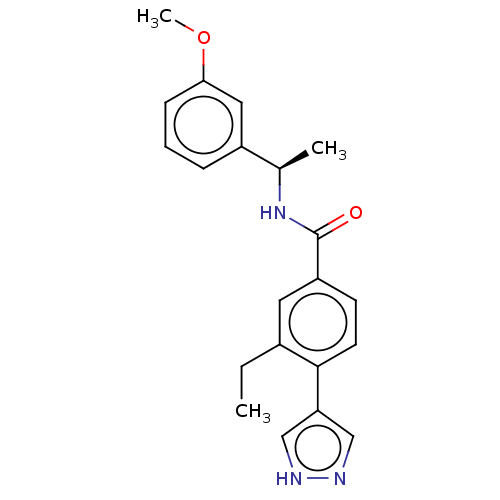 (CHEMBL4791701) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506498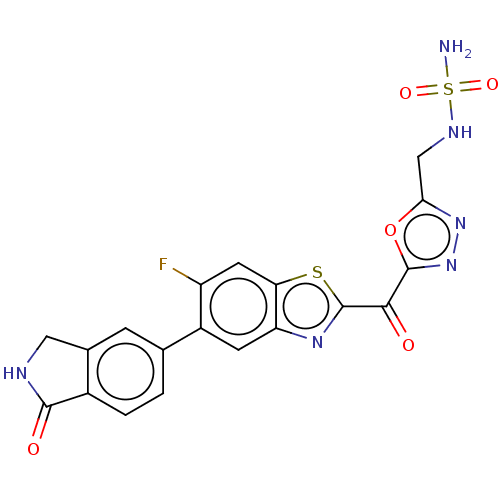 (CHEMBL4581886) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506506 (CHEMBL4465833) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506504 (CHEMBL4460663) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506505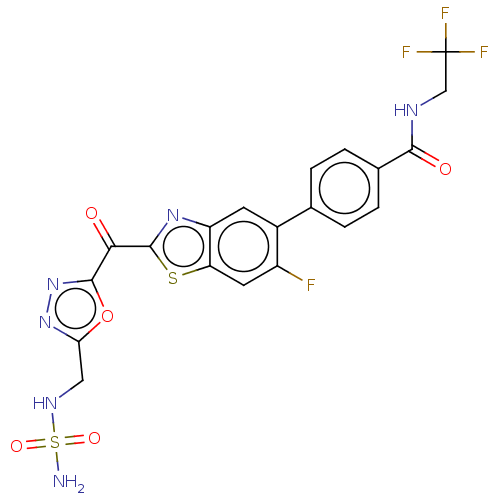 (CHEMBL4460376) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM252032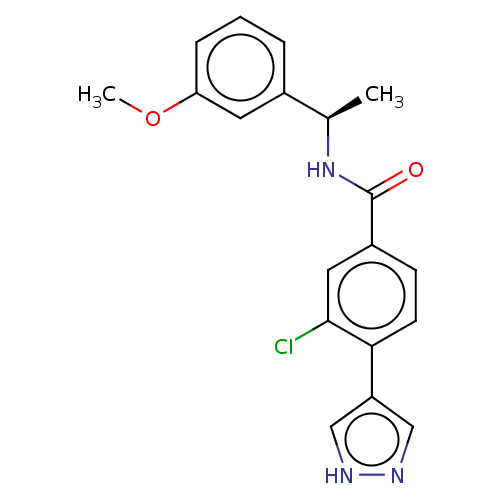 (US9458110, 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546544 (CHEMBL4791701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK1 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM252182 (US9458110, 254) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK1 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506501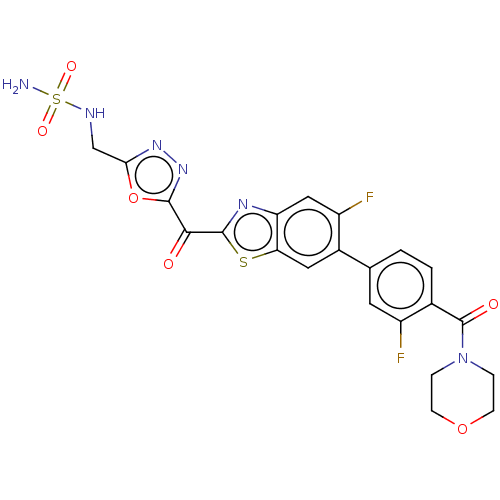 (CHEMBL4563019) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM251935 (US9458110, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK1 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516404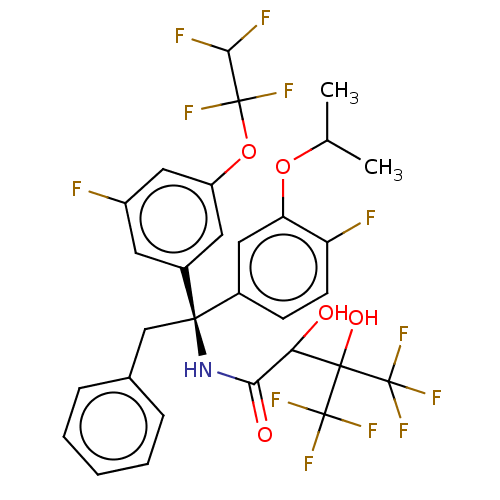 (CHEMBL4439823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506500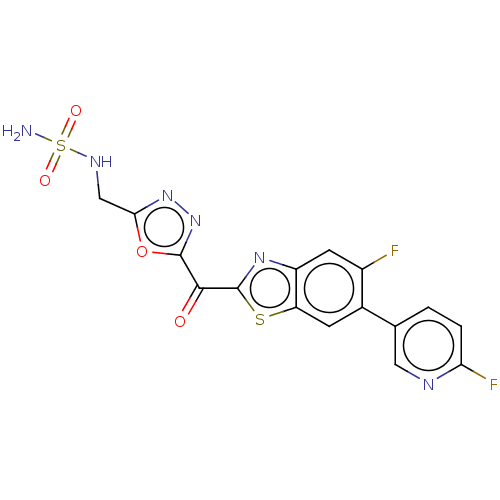 (CHEMBL4471611) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM252039 (US9458110, 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392514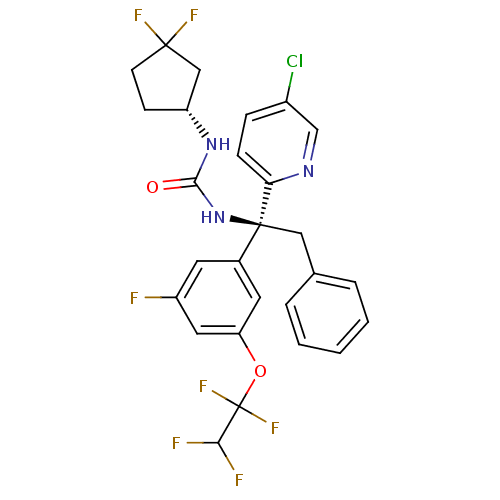 (CHEMBL2152167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM252036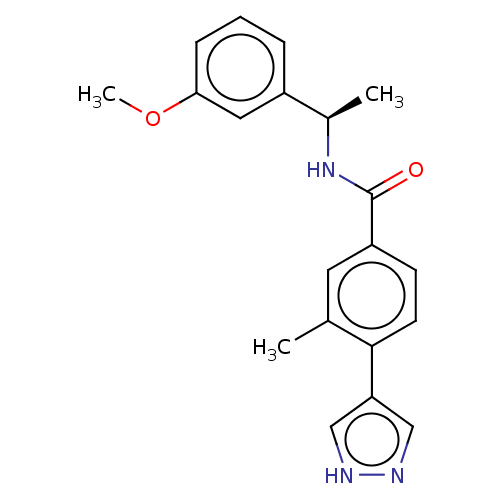 (US9458110, 107) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM251982 (US9458110, 55) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM252001 (US9458110, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546545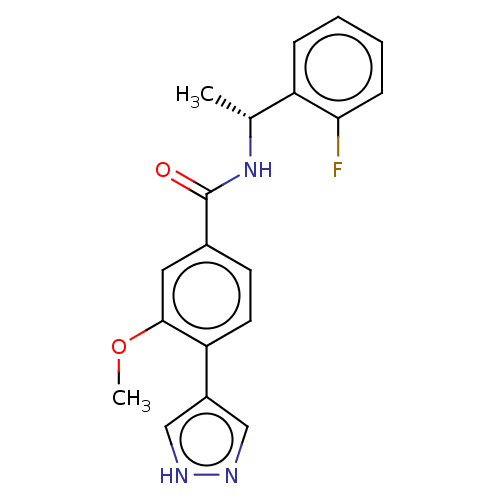 (CHEMBL4753754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178701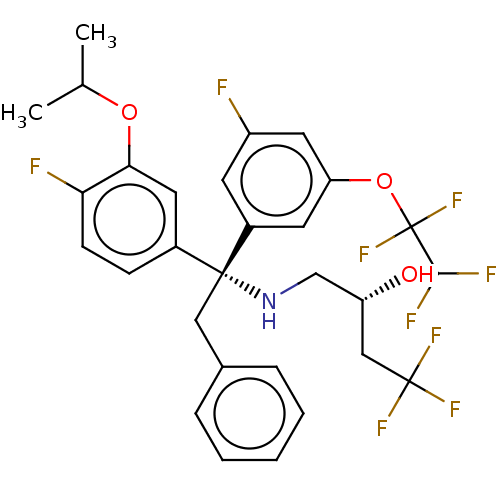 (CHEMBL3814374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM252065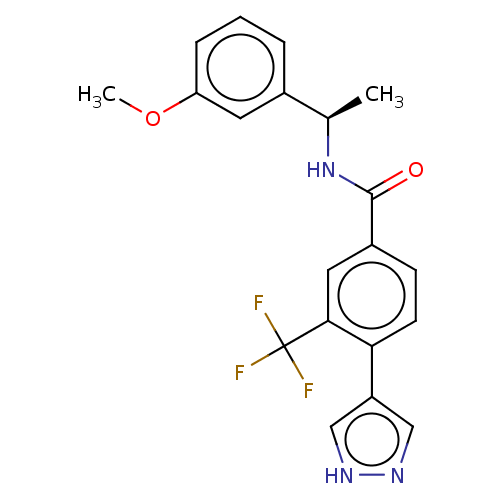 (US9458110, 136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178698 (CHEMBL3813720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546535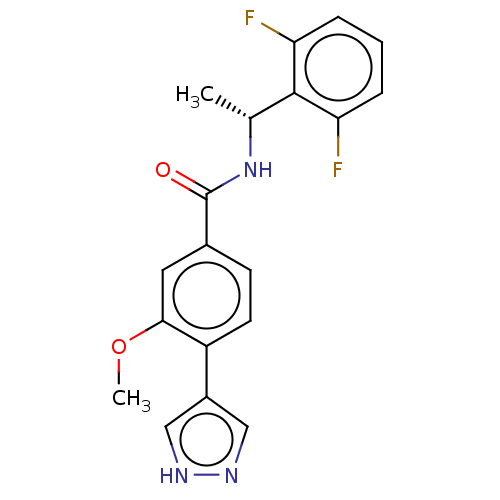 (CHEMBL4746508) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178699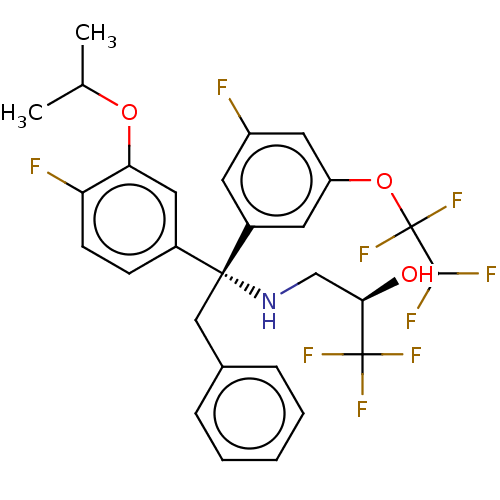 (CHEMBL3813836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50506499 (CHEMBL4463265) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516410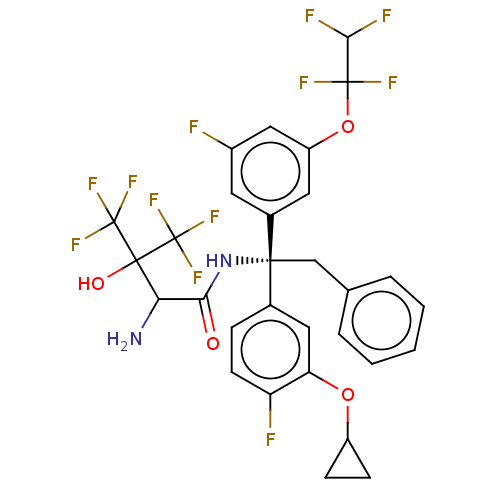 (CHEMBL4574163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392527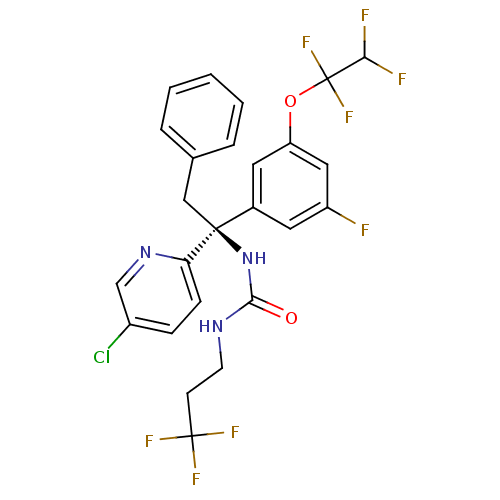 (CHEMBL2152180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546534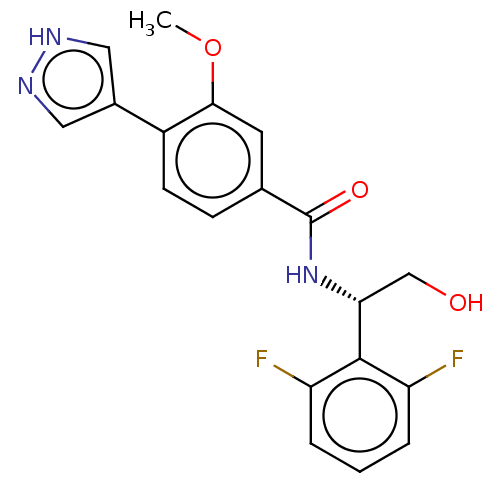 (CHEMBL4799704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546539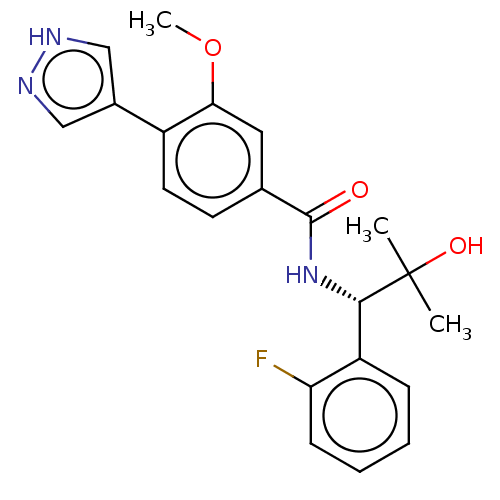 (CHEMBL4757844) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM251931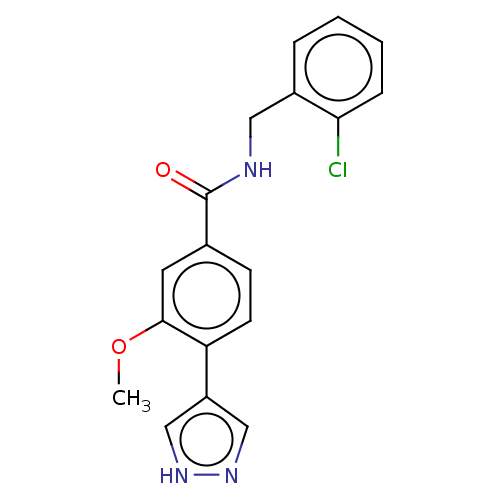 (US9458110, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM251933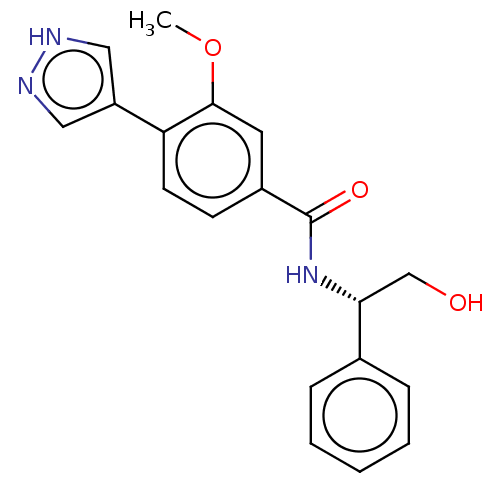 (US9458110, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178702 (CHEMBL3814963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178700 (CHEMBL3814418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human HL expressed in COS7 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by subs... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 BindingDB Entry DOI: 10.7270/Q2TF01QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM187287 (US9169240, 27) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392530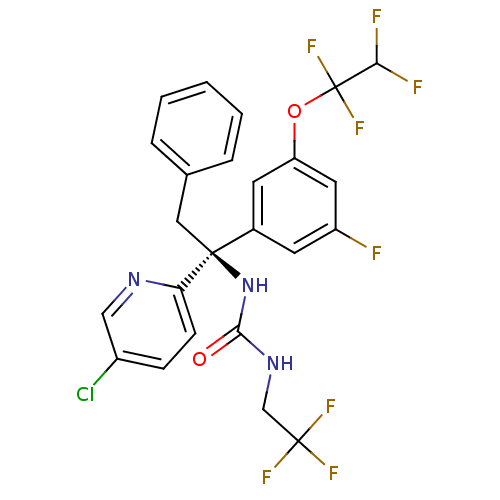 (CHEMBL2152183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516398 (CHEMBL4457286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50104843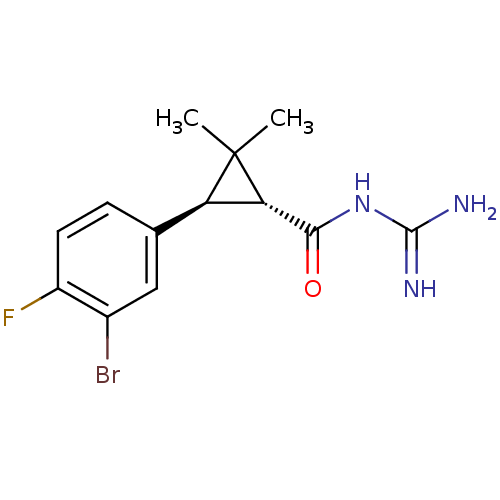 (CHEMBL419578 | N-[3-(3-Bromo-4-fluoro-phenyl)-2,2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Screened in AP1 cells expressing human NHE-1 for sodium hydrogen exchange activity | J Med Chem 44: 3302-10 (2001) BindingDB Entry DOI: 10.7270/Q2H41QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50094519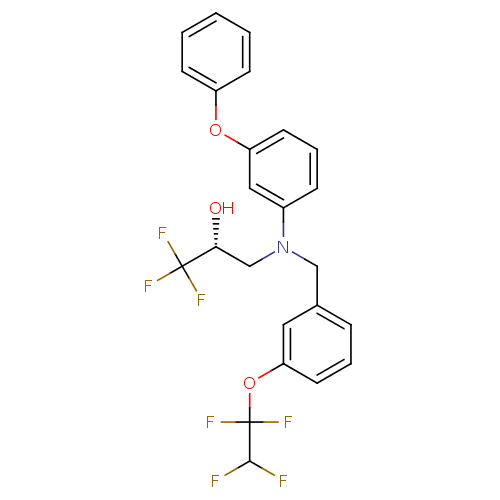 ((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human CETP by scintillation proximity assay | Bioorg Med Chem Lett 18: 2640-4 (2008) Article DOI: 10.1016/j.bmcl.2008.03.030 BindingDB Entry DOI: 10.7270/Q2V40W3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546545 (CHEMBL4753754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK1 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546540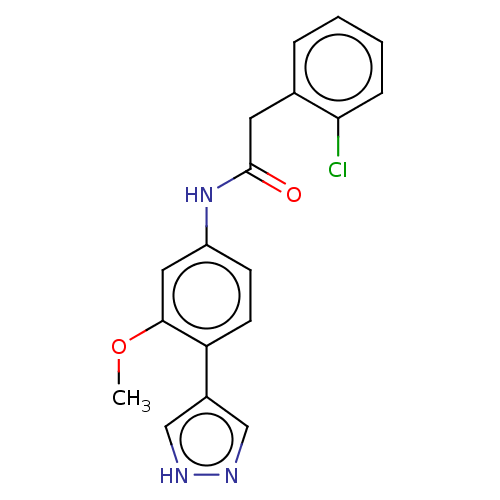 (CHEMBL4756156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM205027 (US9249096, 40) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546542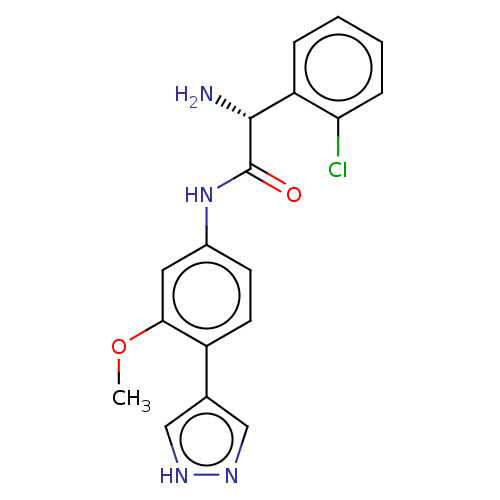 (CHEMBL4780269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK2 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50506506 (CHEMBL4465833) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50506505 (CHEMBL4460376) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546533 (CHEMBL4764401) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK1 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM251931 (US9458110, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ROCK1 (unknown origin) using FITC-AHA-AKRRRLSSLRA-OH peptide substrate by caliper assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127495 BindingDB Entry DOI: 10.7270/Q2J969Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM187290 (US9169240, 30) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Homo sapiens (Human)) | BDBM50104848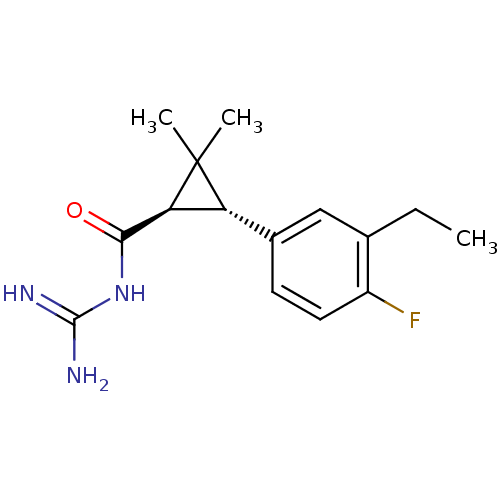 (CHEMBL112903 | N-[3-(3-Ethyl-4-fluoro-phenyl)-2,2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Screened in AP1 cells expressing human NHE-1 for sodium hydrogen exchange activity | J Med Chem 44: 3302-10 (2001) BindingDB Entry DOI: 10.7270/Q2H41QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 703 total ) | Next | Last >> |