

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516404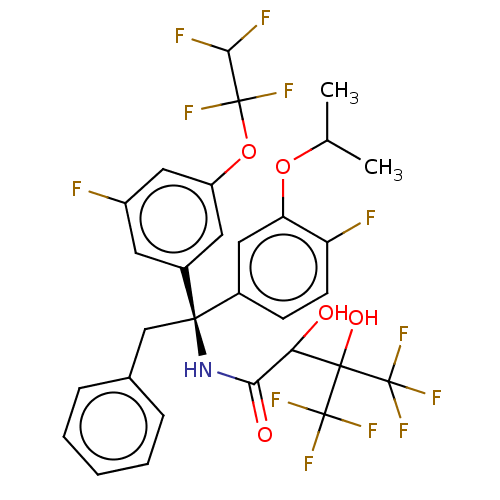 (CHEMBL4439823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178701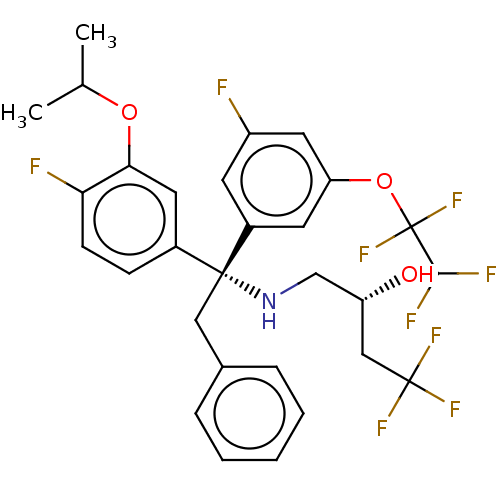 (CHEMBL3814374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178698 (CHEMBL3813720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178699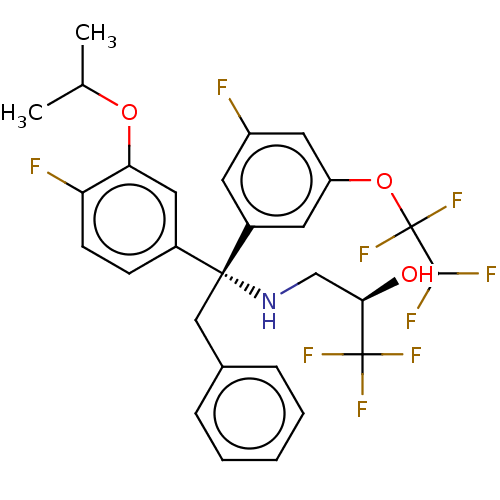 (CHEMBL3813836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516410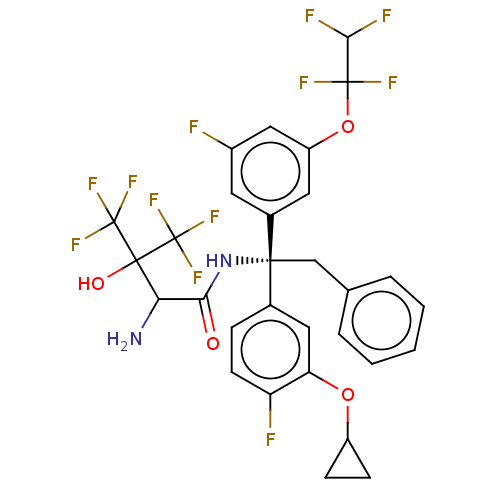 (CHEMBL4574163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178702 (CHEMBL3814963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178700 (CHEMBL3814418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516398 (CHEMBL4457286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM205027 (US9249096, 40) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516406 (CHEMBL4469874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516405 (CHEMBL4552555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458638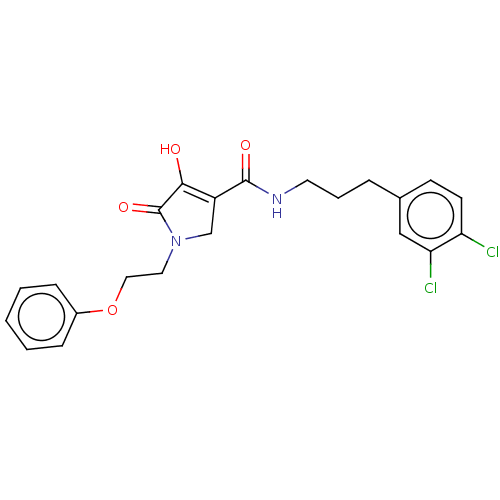 (CHEMBL4203395) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516400 (CHEMBL4439856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178691 (CHEMBL3814359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458637 (CHEMBL4210998) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516402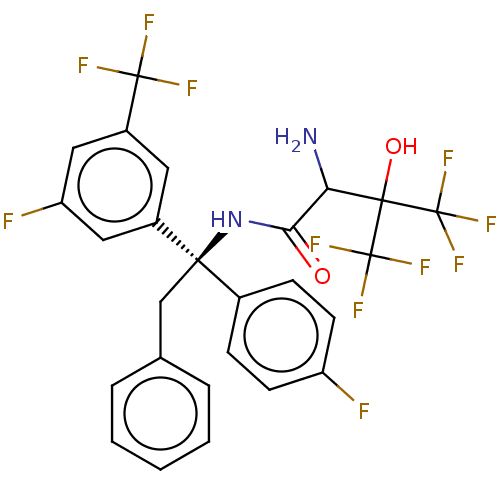 (CHEMBL4473304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312718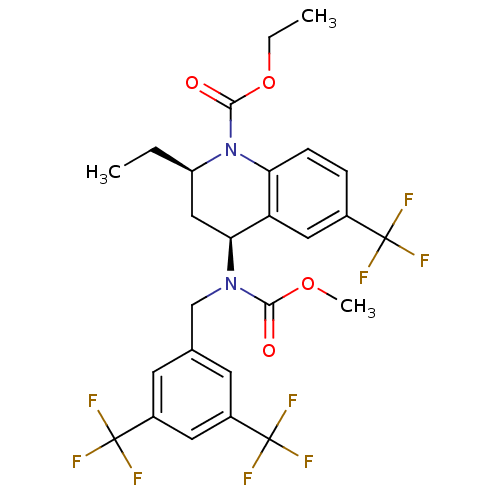 (CHEMBL479527 | torcetrapib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178697 (CHEMBL3813777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50458647 (CHEMBL4212095) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human HL expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followed by substrate a... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506741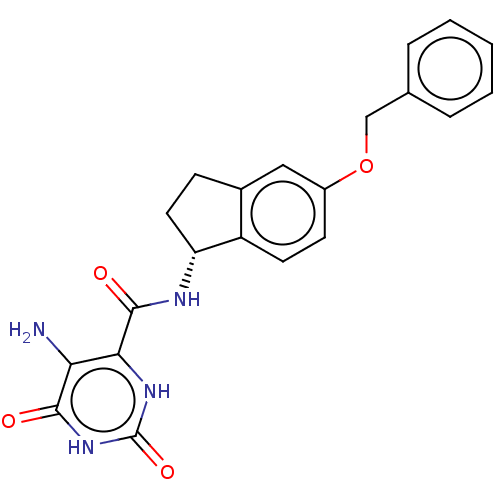 (CHEMBL4513722) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458645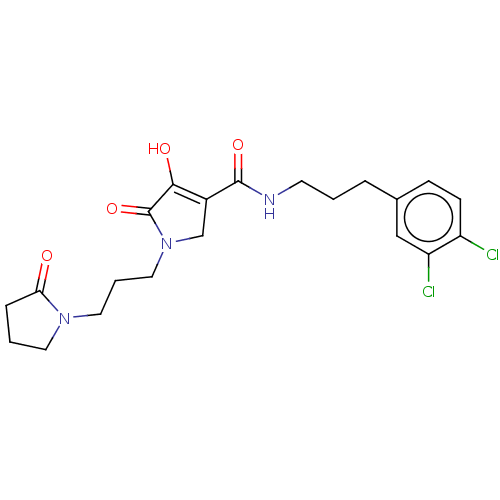 (CHEMBL4215063) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458642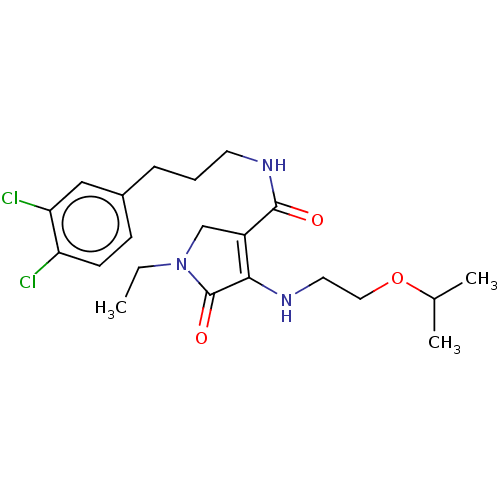 (CHEMBL4218352) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506738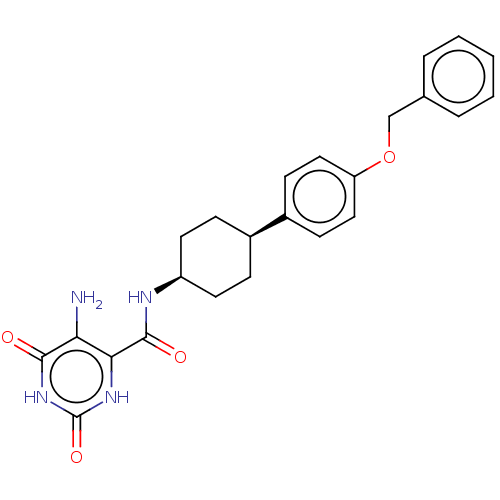 (CHEMBL4558644) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178692 (CHEMBL3814646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458636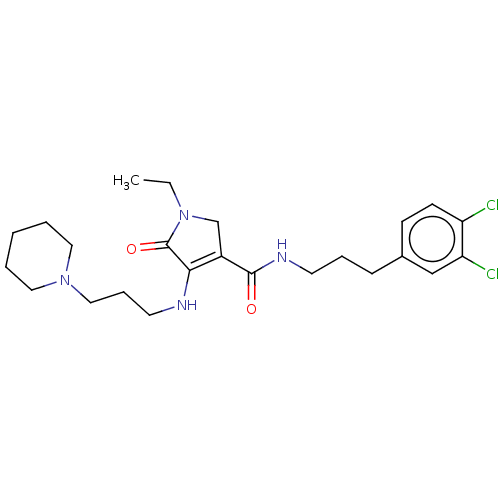 (CHEMBL4206624) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516408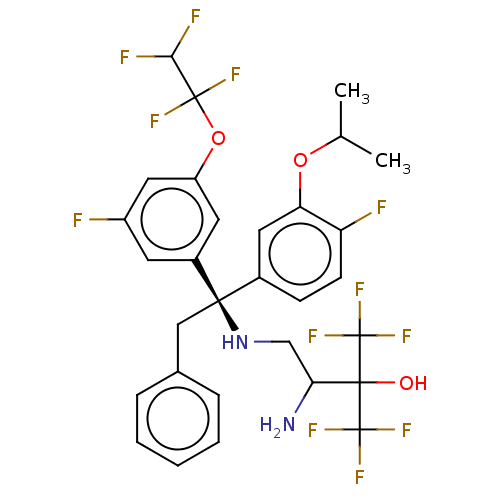 (CHEMBL4468692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506731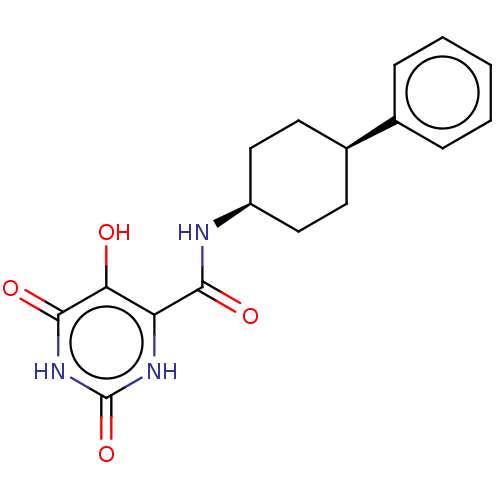 (CHEMBL4439500) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458644 (CHEMBL4214459) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458646 (CHEMBL4207746) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516401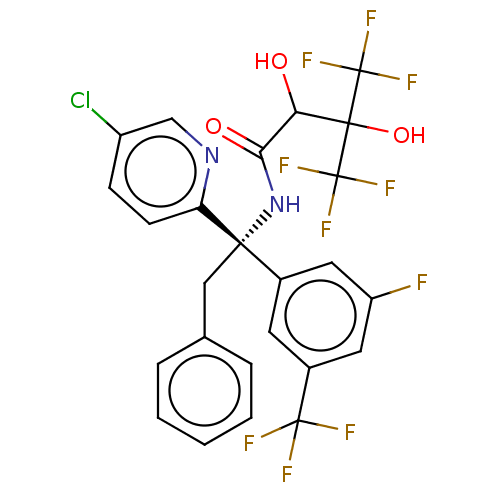 (CHEMBL4475826) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458639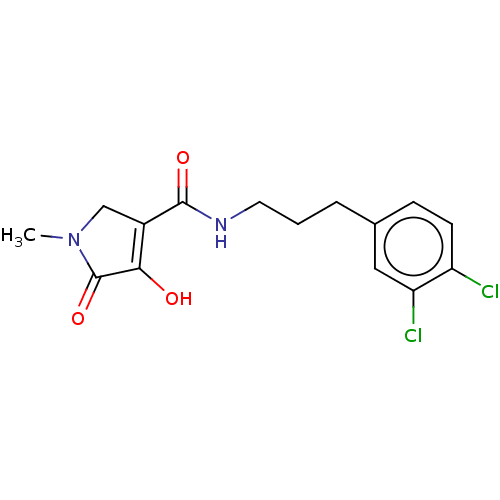 (CHEMBL4207216) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458640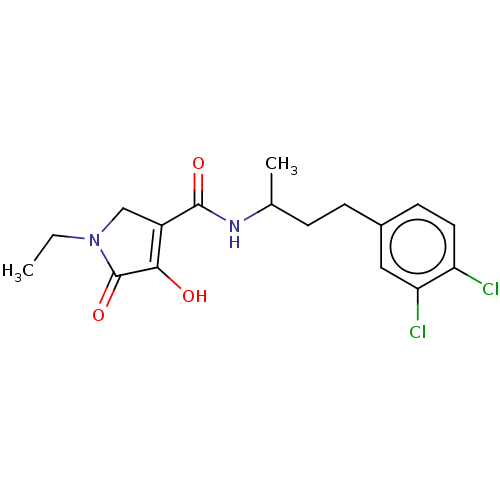 (CHEMBL4203934) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178701 (CHEMBL3814374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of CETP in human whole plasma assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to LDL/VLDL | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506735 (CHEMBL4583457) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506734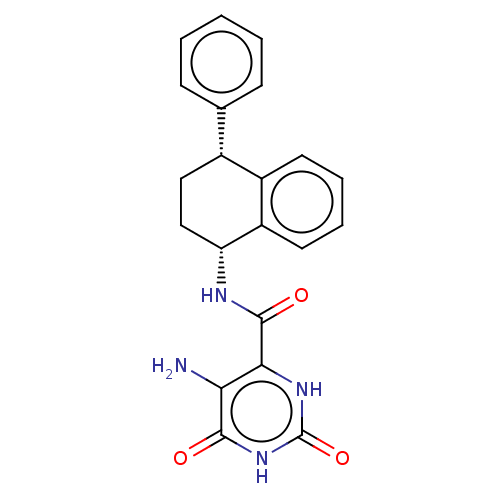 (CHEMBL4586520) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178693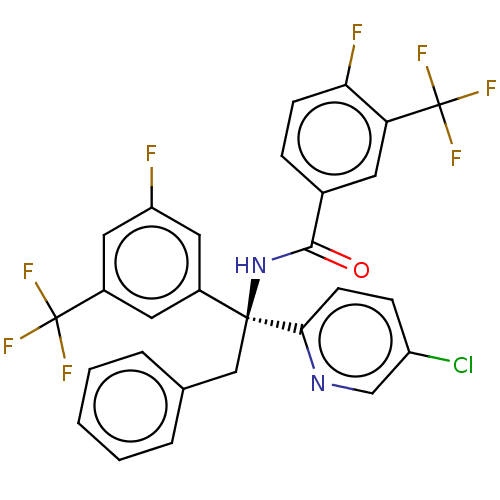 (CHEMBL3814330) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516399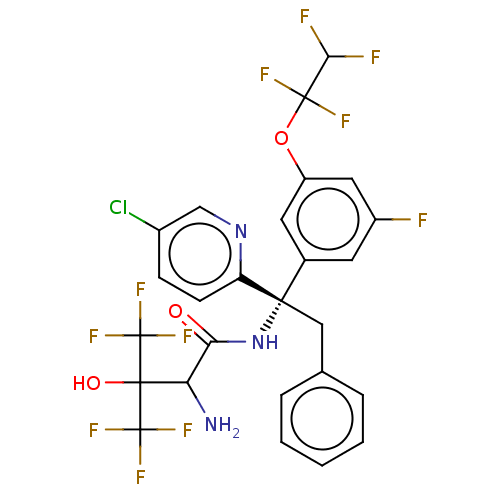 (CHEMBL4448253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516399 (CHEMBL4448253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178699 (CHEMBL3813836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of CETP in human whole plasma assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to LDL/VLDL | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516398 (CHEMBL4457286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CETP in human whole plasma assessed as reduction in [3H]-CE/HDL transfer incubated for 2.5 hrs by topcount scintillation counting assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516405 (CHEMBL4552555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CETP in human whole plasma assessed as reduction in [3H]-CE/HDL transfer incubated for 2.5 hrs by topcount scintillation counting assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458650 (CHEMBL4212990) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458643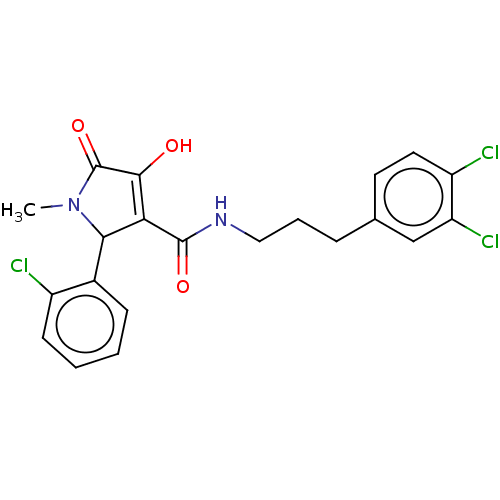 (CHEMBL4206109) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506737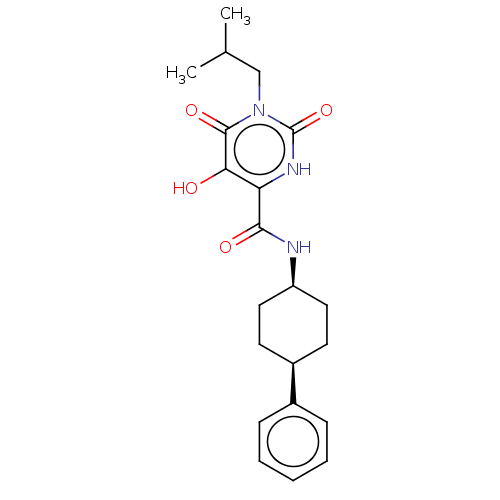 (CHEMBL4555254) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50458647 (CHEMBL4212095) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of endothelial lipase in human HT1080 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by substrat... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516410 (CHEMBL4574163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CETP in human whole plasma assessed as reduction in [3H]-CE/HDL transfer incubated for 2.5 hrs by topcount scintillation counting assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50458639 (CHEMBL4207216) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human HL expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followed by substrate a... | ACS Med Chem Lett 9: 673-678 (2018) Article DOI: 10.1021/acsmedchemlett.8b00138 BindingDB Entry DOI: 10.7270/Q2JS9T2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506742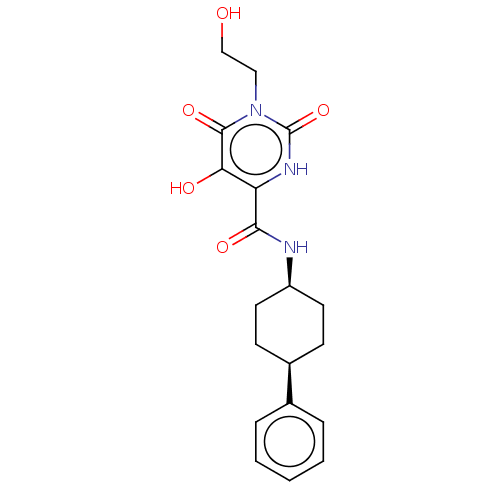 (CHEMBL4589512) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506732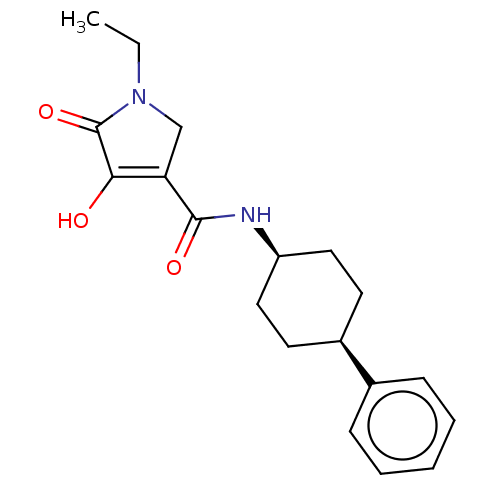 (CHEMBL4592858) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human endothelial lipase expressed in African green monkey COS7 cells using HDL as substrate pretreated for 10 mins followe... | Bioorg Med Chem Lett 28: 3721-3725 (2018) Article DOI: 10.1016/j.bmcl.2018.10.022 BindingDB Entry DOI: 10.7270/Q2JS9TQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516404 (CHEMBL4439823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CETP in human whole plasma assessed as reduction in [3H]-CE/HDL transfer incubated for 2.5 hrs by topcount scintillation counting assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |