

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069330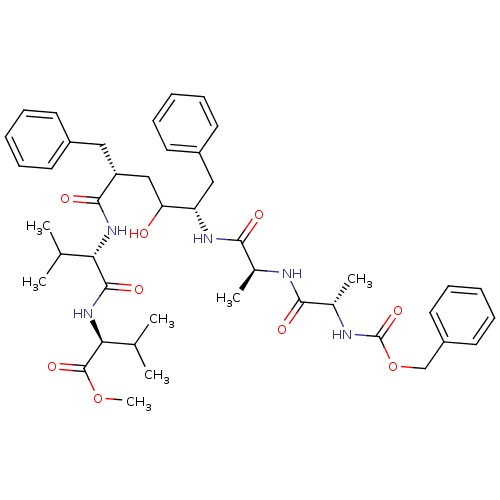 ((S)-2-((S)-2-{(2R,5S)-2-Benzyl-5-[(S)-2-((S)-2-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Protease was determined | Bioorg Med Chem Lett 8: 699-704 (1999) BindingDB Entry DOI: 10.7270/Q20K27QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59224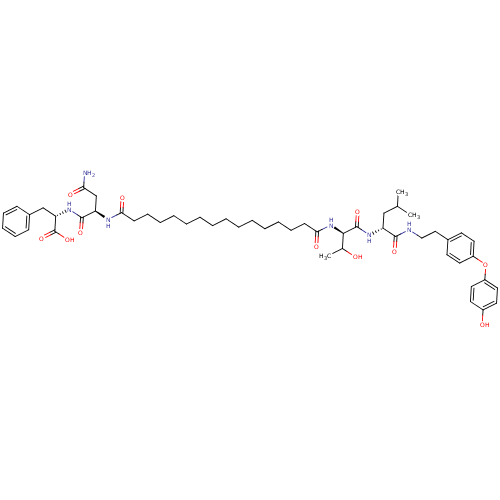 (Pepstatin analog, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | 360 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163700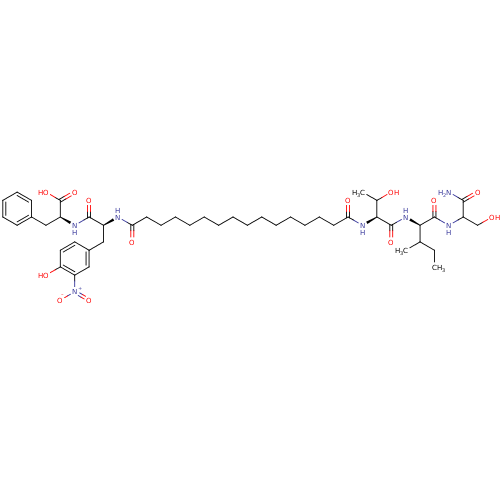 ((S)-2-[(S)-2-(15-{(S)-1-[(R)-1-(1-Carbamoyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163699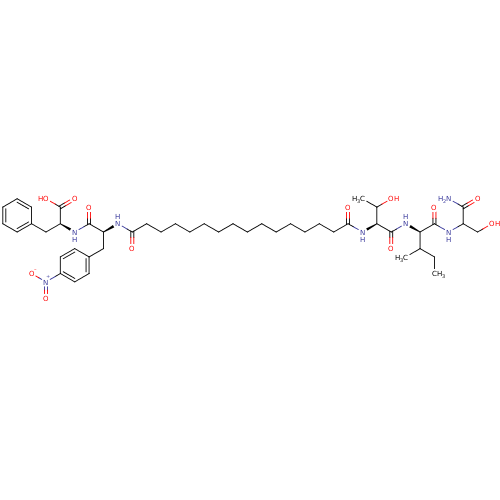 ((S)-2-[(S)-2-(15-{(S)-1-[(R)-1-(1-Carbamoyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163688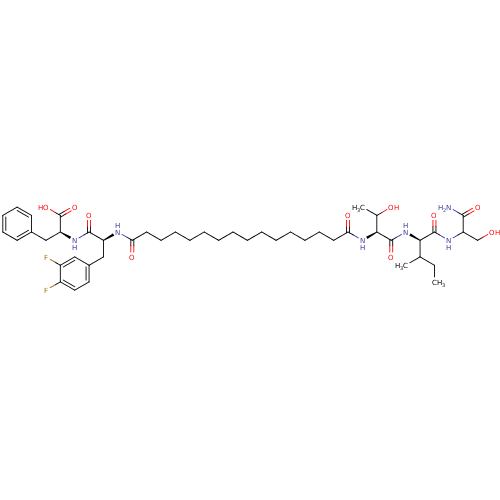 ((S)-2-[(S)-2-(15-{(S)-1-[(R)-1-(1-Carbamoyl-2-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163711 ((S)-2-[(R)-2-(4-Benzyloxy-phenyl)-2-(15-{(S)-1-[(R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370636 (CHEMBL1791327) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370640 (CHEMBL1791339) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370653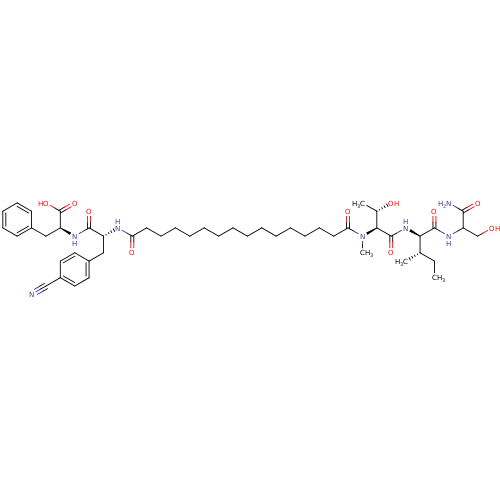 (CHEMBL1791328) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370638 (CHEMBL1791330) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370648 (CHEMBL1791340) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59223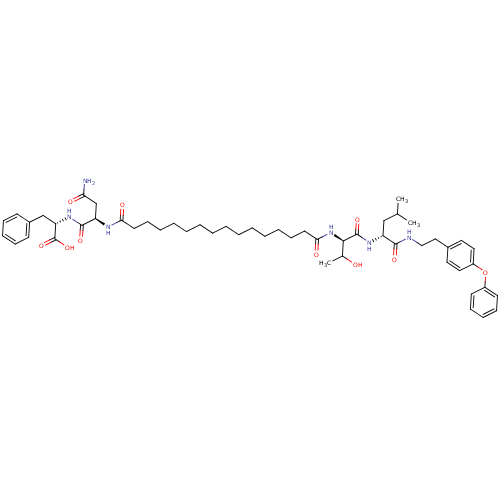 (Pepstatin analog, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | 730 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370650 (CHEMBL1791331) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59213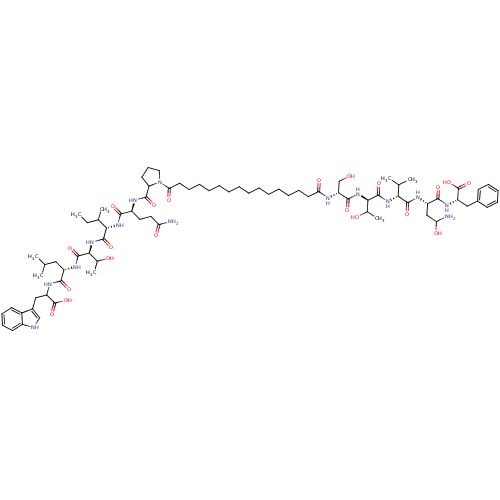 (Pepstatin analog, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | 350 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370413 (CHEMBL1791290) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370413 (CHEMBL1791290) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus-1 protease; (Dimerization inhibitor) | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163694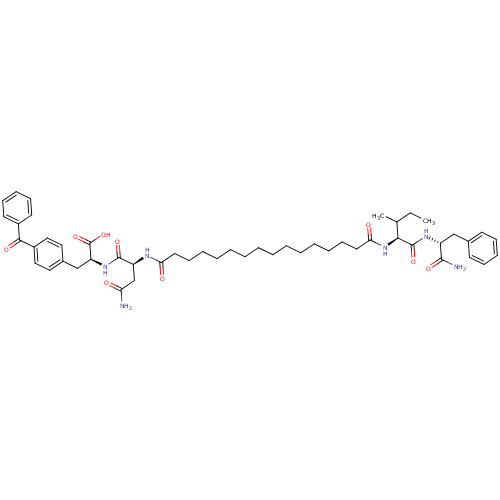 ((S)-3-(4-Benzoyl-phenyl)-2-((S)-3-carbamoyl-2-{15-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163710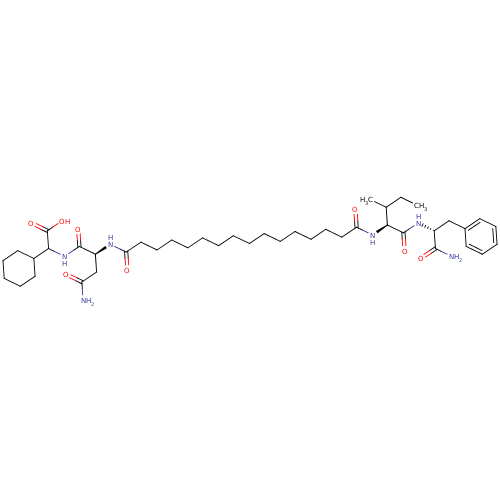 (((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamoyl-2-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163717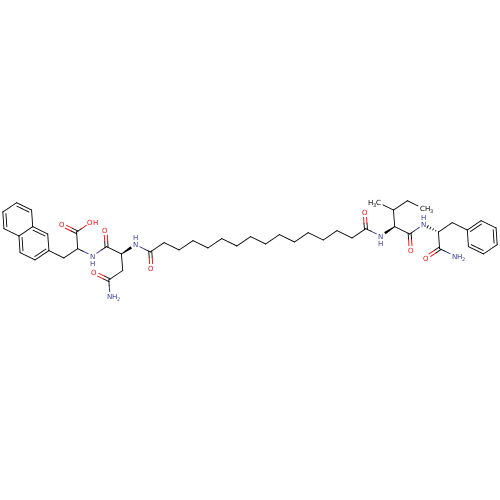 (2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamoyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163696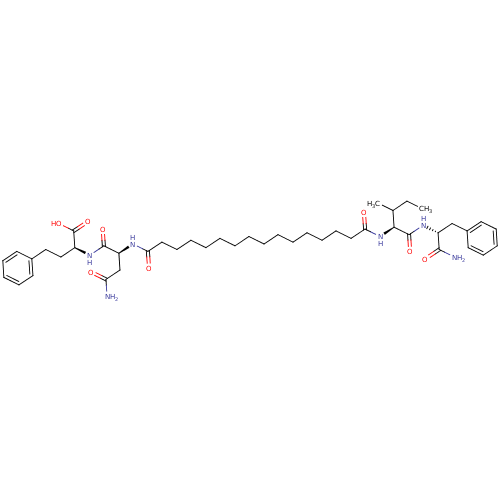 ((S)-2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370643 (CHEMBL1791333) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163707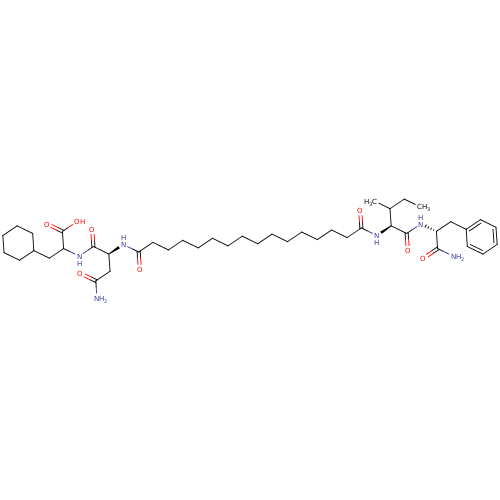 (2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamoyl-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370654 (CHEMBL1791341) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370645 (CHEMBL1791342) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163692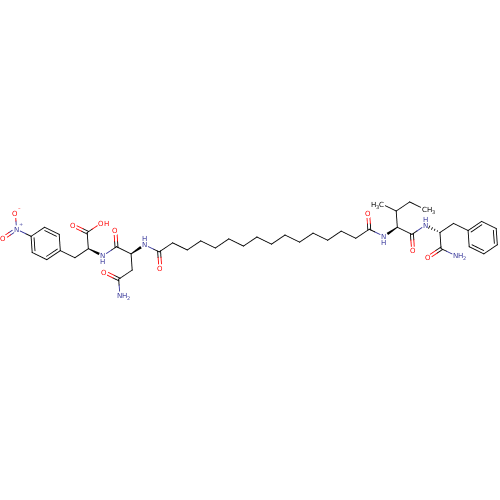 ((S)-2-((S)-3-Carbamoyl-2-{15-[(S)-1-((R)-1-carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370641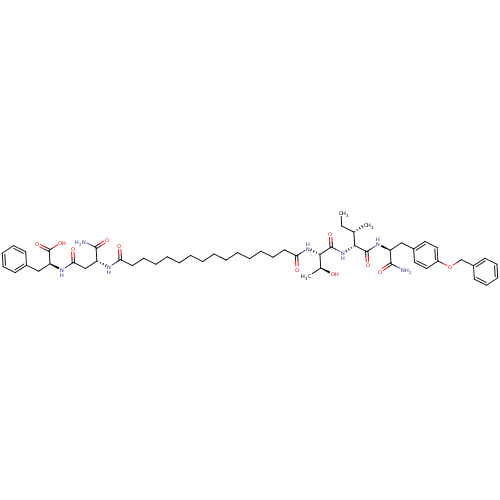 (CHEMBL1791318) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59220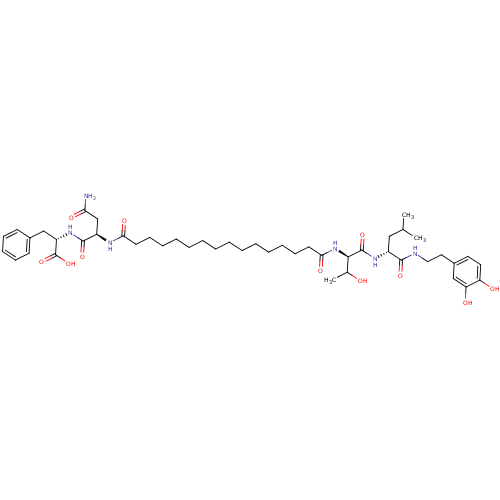 (Pepstatin analog, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59222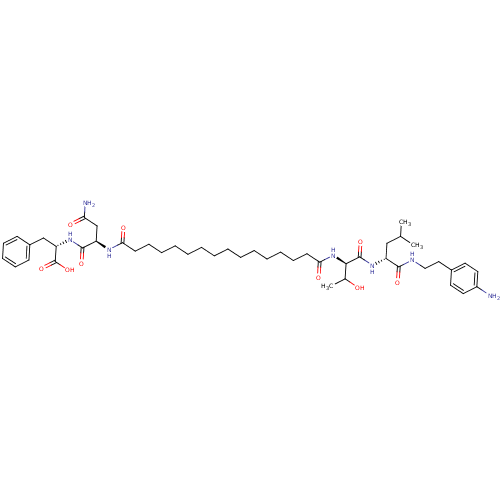 (Pepstatin analog, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59219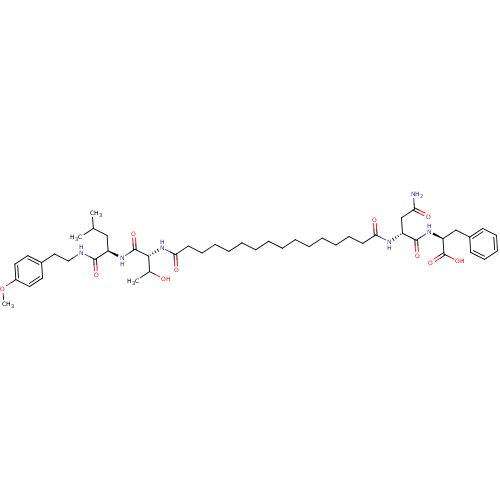 (Pepstatin analog, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59216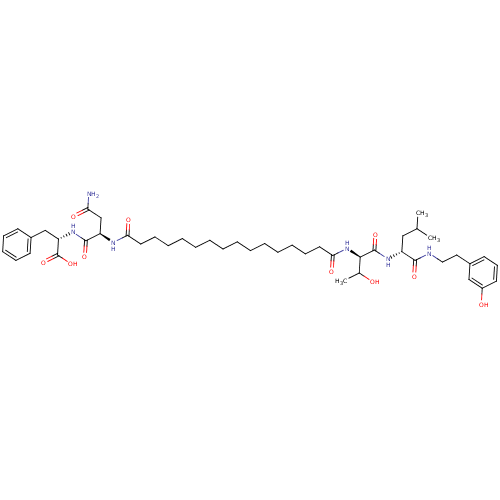 (Pepstatin analog, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.13E+3 | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479598 (CHEMBL478672) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59217 (Pepstatin analog, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479600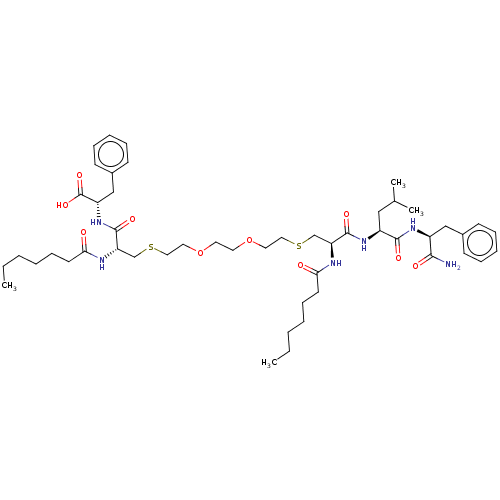 (CHEMBL504512) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50215900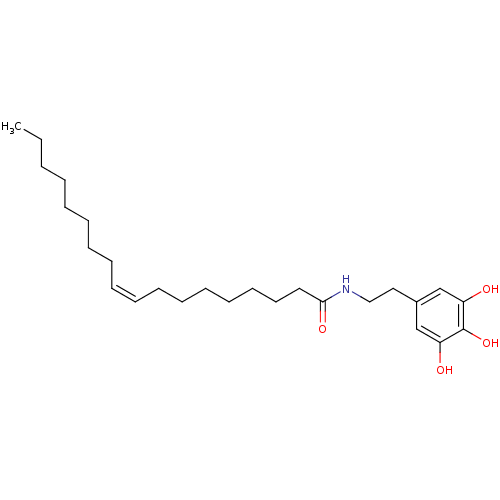 (CHEMBL251733 | N-(3,4,5-trihydroxyphenethyl)oleami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of anthrax lethal factor by FRET assay | Bioorg Med Chem Lett 17: 4575-8 (2007) Article DOI: 10.1016/j.bmcl.2007.05.089 BindingDB Entry DOI: 10.7270/Q2W958X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50375787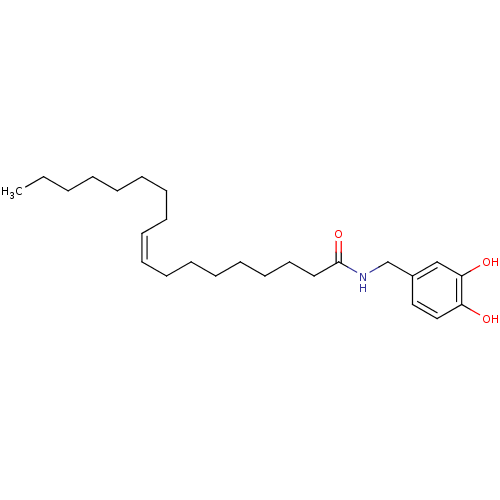 (CHEMBL403034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor by FRET assay | Bioorg Med Chem Lett 18: 2467-70 (2008) Article DOI: 10.1016/j.bmcl.2008.02.044 BindingDB Entry DOI: 10.7270/Q27M08T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479594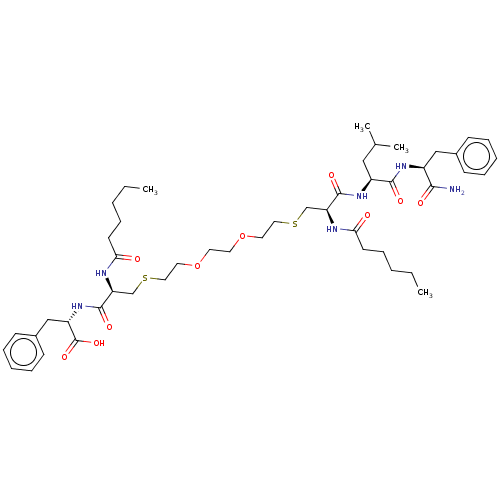 (CHEMBL510483) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59214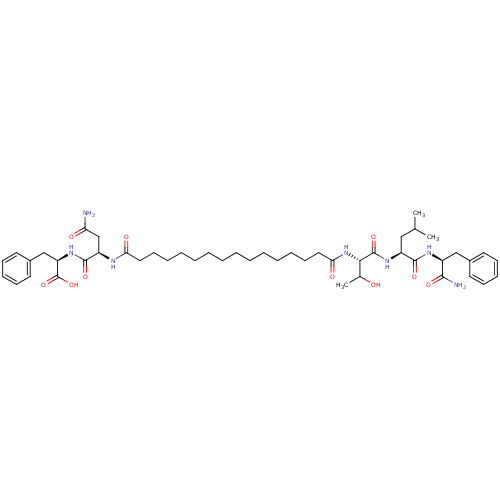 (Pepstatin analog, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50370412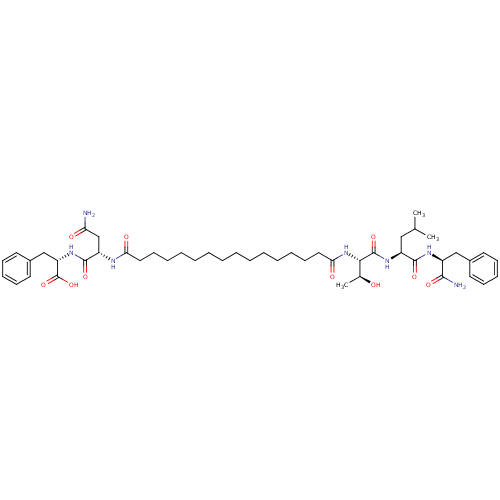 (CHEMBL449777) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50370412 (CHEMBL449777) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50215899 (CHEMBL250711 | N-oleoyl-dopamine | Oleoyl dopamine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of anthrax lethal factor by FRET assay | Bioorg Med Chem Lett 17: 4575-8 (2007) Article DOI: 10.1016/j.bmcl.2007.05.089 BindingDB Entry DOI: 10.7270/Q2W958X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50163697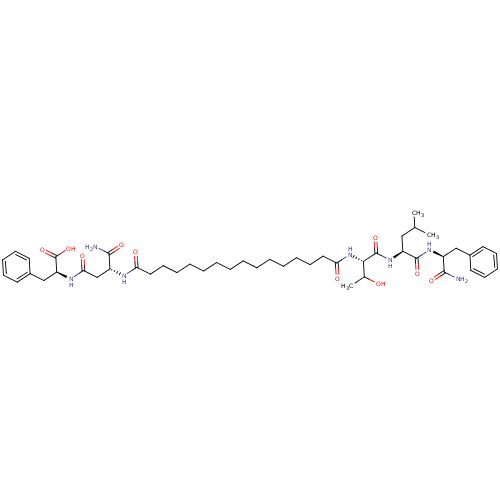 ((S)-2-[(R)-3-Carbamoyl-3-(15-{(S)-1-[(S)-1-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus -1 protease | J Med Chem 48: 2239-42 (2005) Article DOI: 10.1021/jm049581j BindingDB Entry DOI: 10.7270/Q2H70GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50069332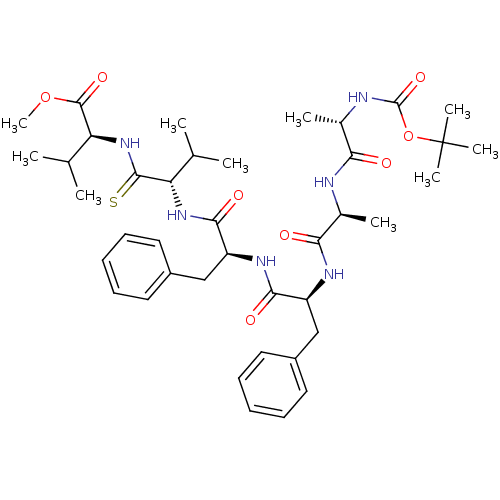 ((S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-tert-Buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity was evaluated for competitive inhibition of HIV-1 Protease | Bioorg Med Chem Lett 8: 699-704 (1999) BindingDB Entry DOI: 10.7270/Q20K27QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50150552 ((E)-3-{(S)-1-[((S)-Carbamoyl-{11-[(S)-1-((S)-1-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity towards Human immunodeficiency virus-1 protease | Bioorg Med Chem Lett 14: 4297-300 (2004) Article DOI: 10.1016/j.bmcl.2004.05.081 BindingDB Entry DOI: 10.7270/Q2XG9RW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50215901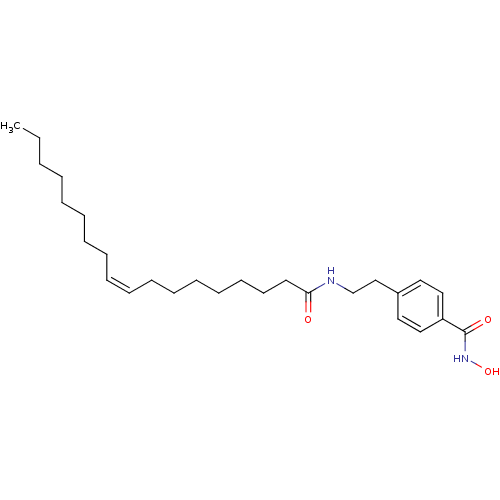 (CHEMBL251734 | N-hydroxy-4-(2-oleamidoethyl)benzam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of anthrax lethal factor by FRET assay | Bioorg Med Chem Lett 17: 4575-8 (2007) Article DOI: 10.1016/j.bmcl.2007.05.089 BindingDB Entry DOI: 10.7270/Q2W958X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50375786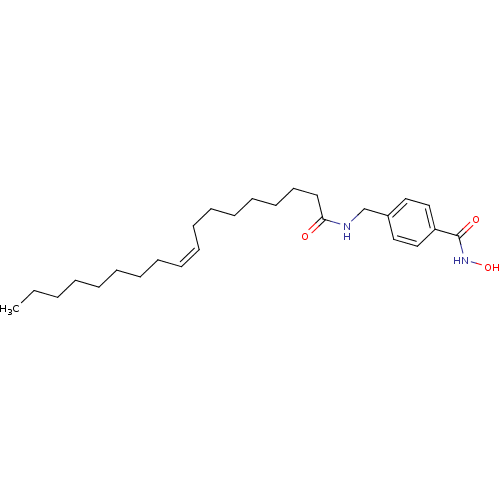 (CHEMBL271701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor by FRET assay | Bioorg Med Chem Lett 18: 2467-70 (2008) Article DOI: 10.1016/j.bmcl.2008.02.044 BindingDB Entry DOI: 10.7270/Q27M08T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479603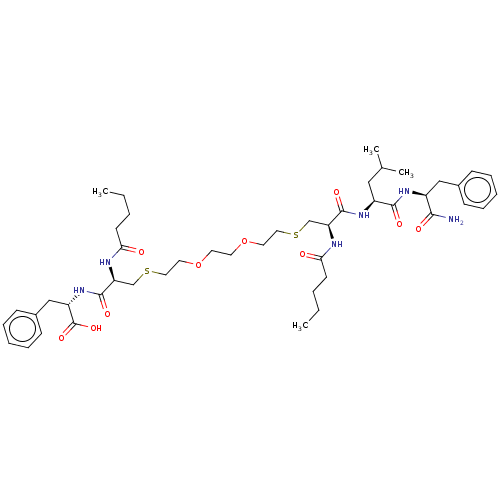 (CHEMBL446001) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479593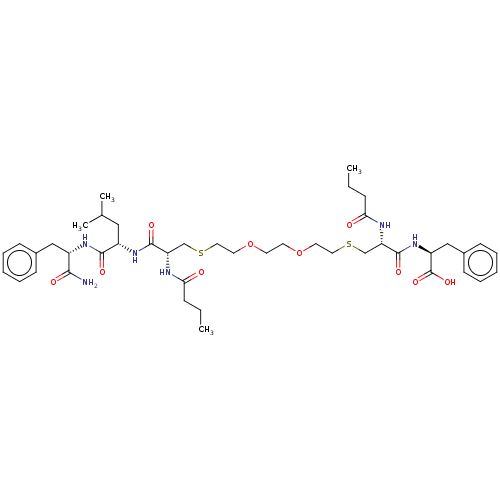 (CHEMBL498854) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479592 (CHEMBL450301) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by competitive inhibition assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479599 (CHEMBL508493) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50479596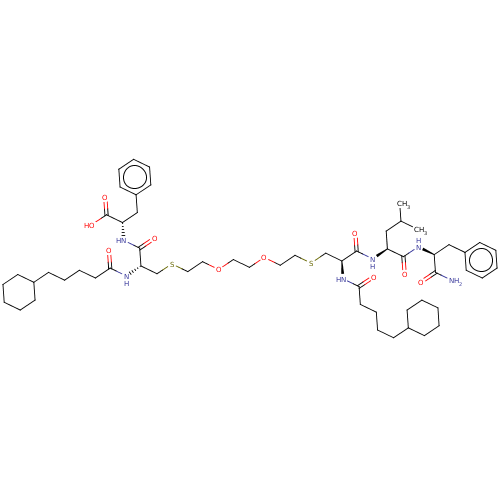 (CHEMBL454643) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization by Zhang-Poorman kinetic assay | Bioorg Med Chem 17: 967-76 (2009) Article DOI: 10.1016/j.bmc.2008.02.060 BindingDB Entry DOI: 10.7270/Q2QN69K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 208 total ) | Next | Last >> |