

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316673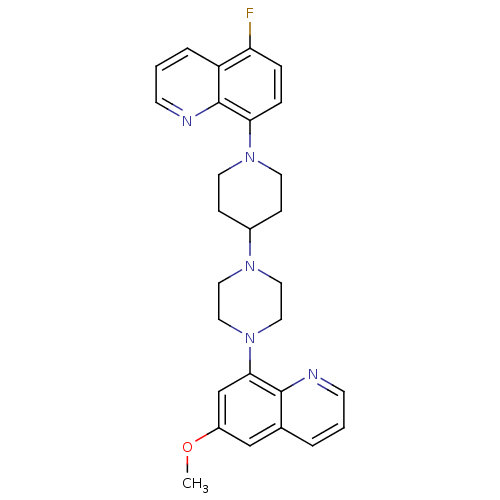 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316677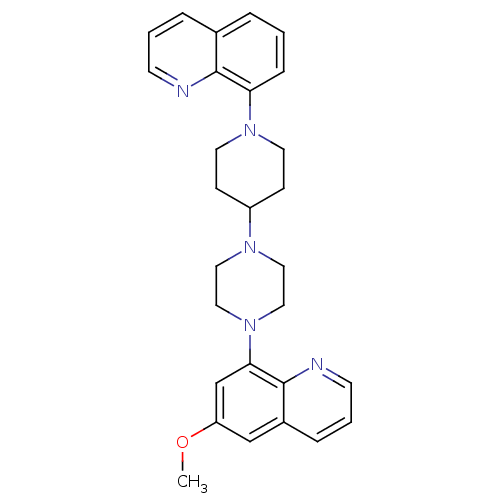 (6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316680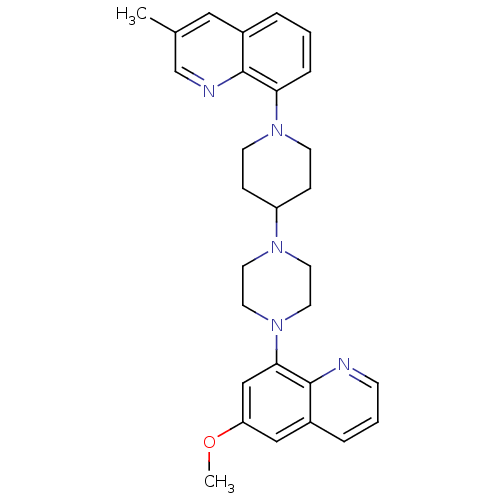 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316684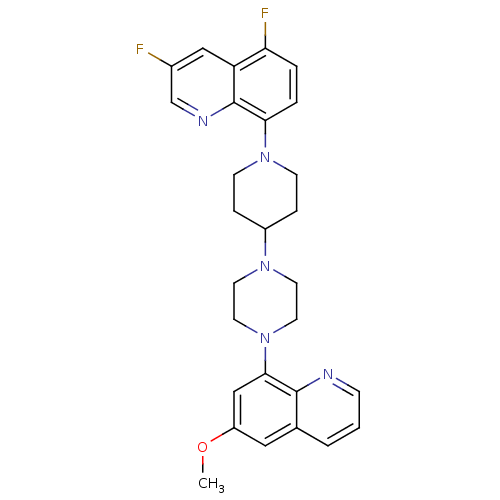 (3,5-Difluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316688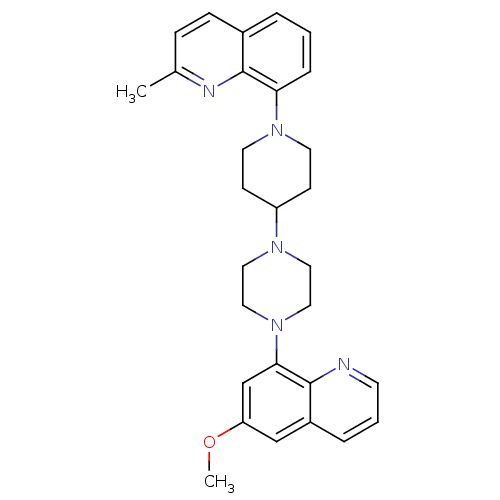 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316690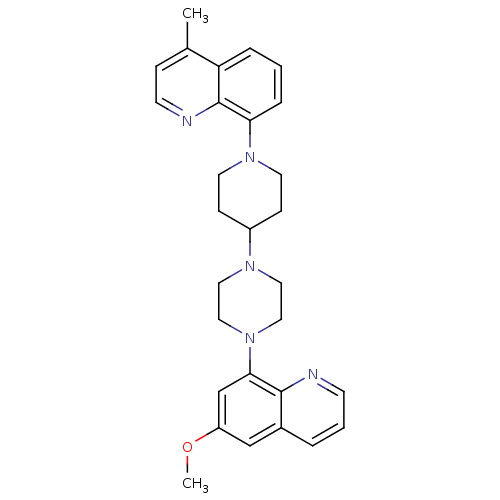 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316682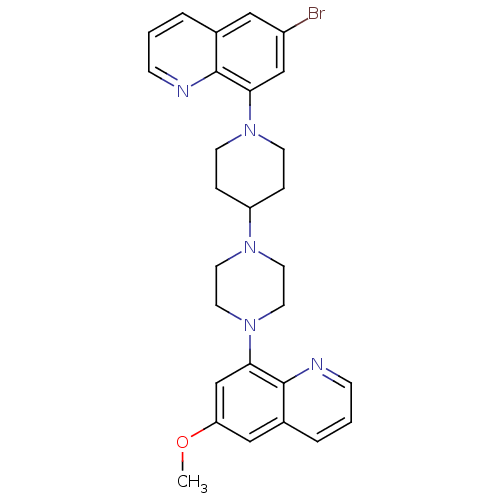 (6-Bromo-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316678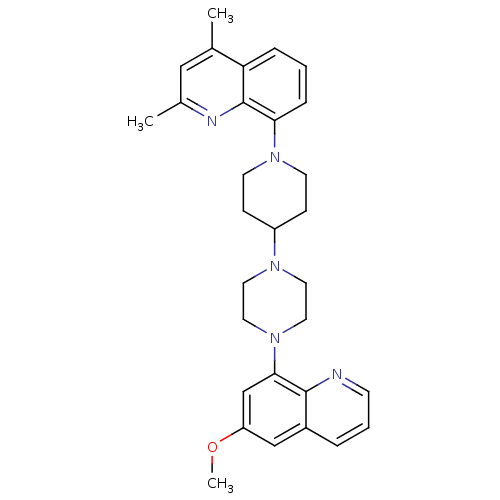 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316679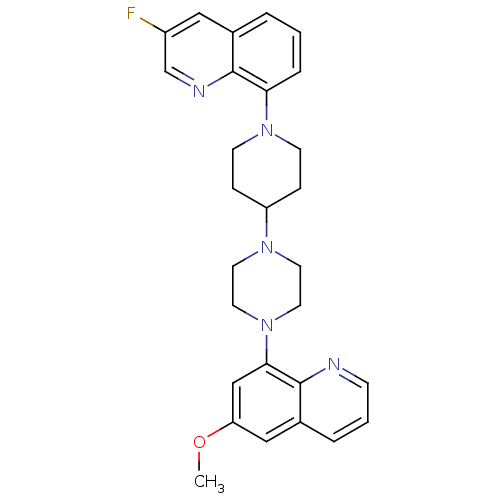 (3-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316683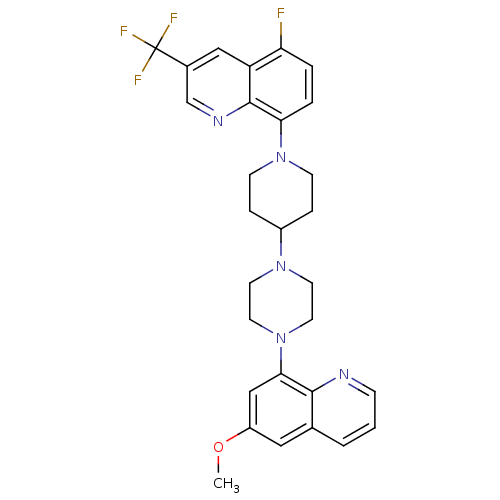 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316686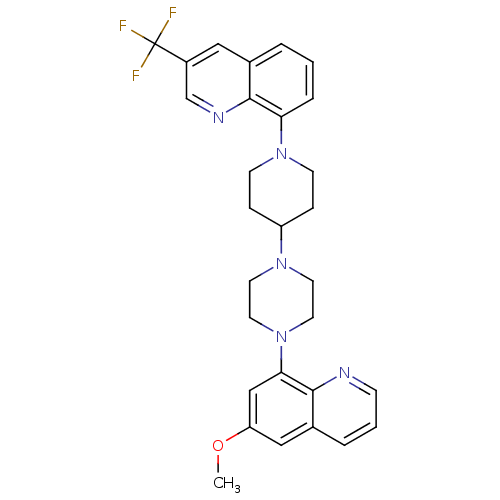 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316685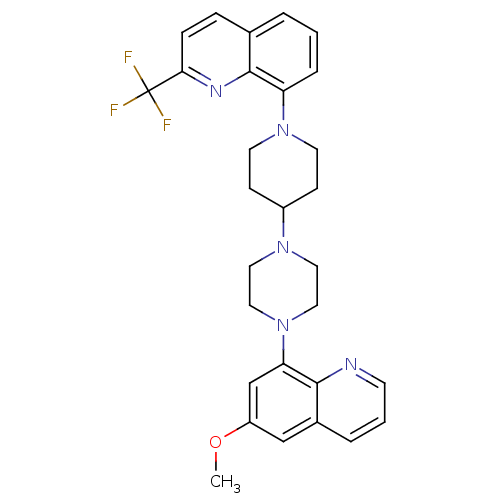 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316681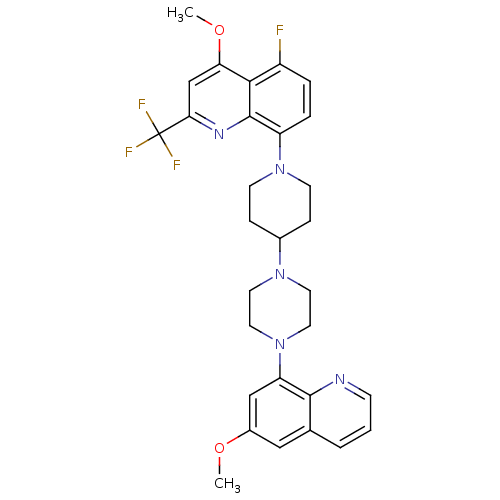 (5-Fluoro-4-methoxy-8-{4-[4-(6-methoxyquinolin-8-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316687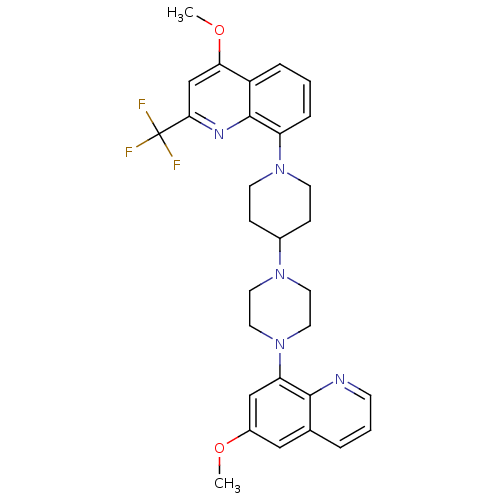 (4-Methoxy-8-{4-[4-(6-methoxyquinolin-8-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316675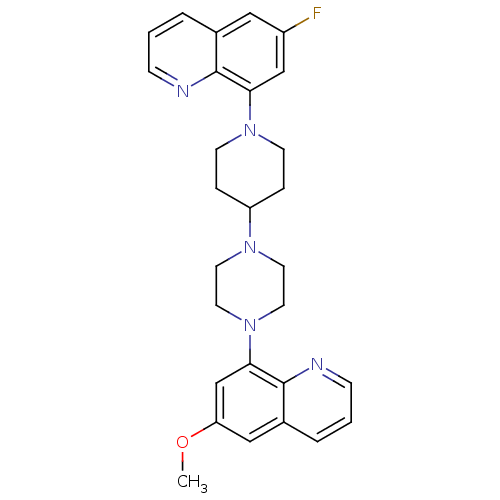 (6-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316676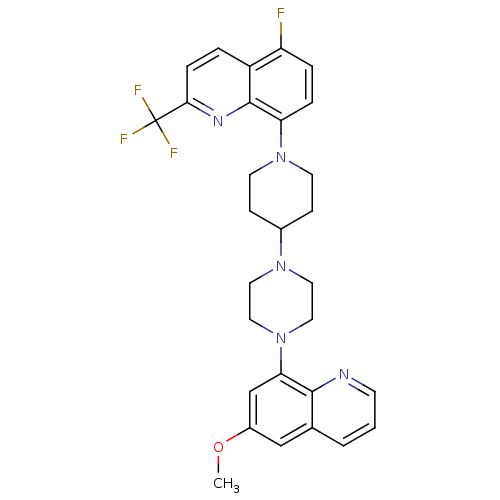 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316674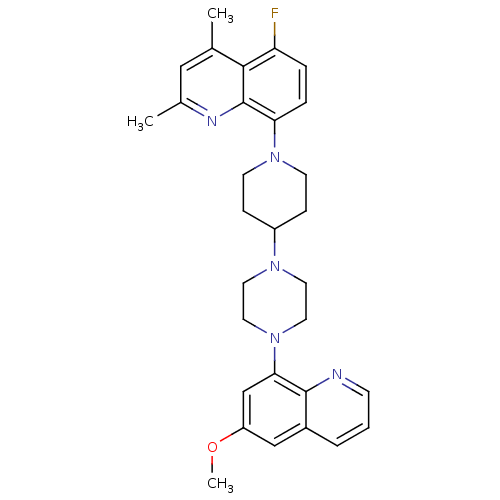 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316689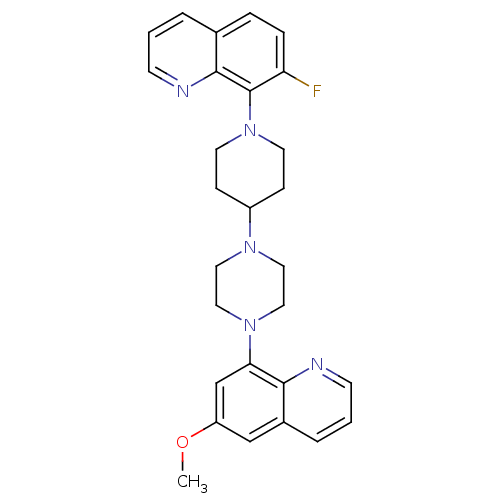 (7-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35221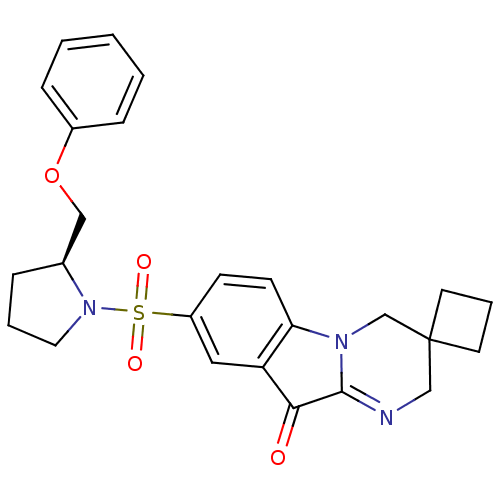 (pyrimidoindolone, 25b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316690 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35223 (pyrimidoindolone, 25d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35202 (pyrimidoindolone, 11k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316673 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316674 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35220 (pyrimidoindolone, 25a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316677 (6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35203 (pyrimidoindolone, 11l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.43 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316678 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35206 (pyrimidoindolone, 14a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.99 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316679 (3-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316680 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM19730 (3,3-dimethyl-8-{[(2S)-2-(phenoxymethyl)pyrrolidine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316676 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35204 (pyrimidoindolone, 11m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.18 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35199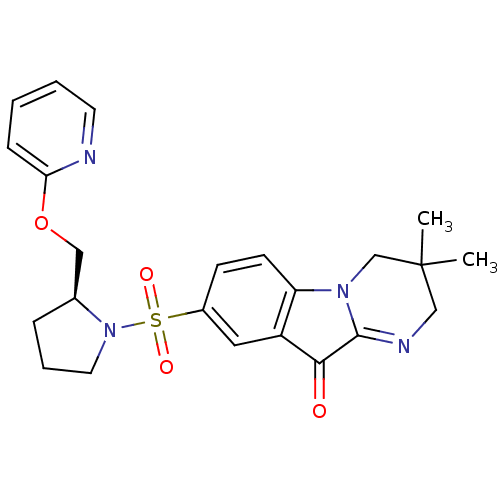 (pyrimidoindolone, 11h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316681 (5-Fluoro-4-methoxy-8-{4-[4-(6-methoxyquinolin-8-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316682 (6-Bromo-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35205 (pyrimidoindolone, 11n) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.1 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35200 (pyrimidoindolone, 11i) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35192 (pyrimidoindolone, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.8 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35201 (pyrimidoindolone, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35224 (pyrimidoindolone, 25e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13.6 | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35193 (pyrimidoindolone, 11b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35207 (pyrimidoindolone, 14b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | 7.2 | 23 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM35222 (pyrimidoindolone, 25c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | 25 |
Wyeth Research | Assay Description Caspase-3 was assayed at 23 deg C (room temp) in 96-well plates using the internally quenched tetrapeptide substrate. Enzymatic cleavage between the ... | Bioorg Med Chem 17: 7755-68 (2009) Article DOI: 10.1016/j.bmc.2009.09.036 BindingDB Entry DOI: 10.7270/Q2BG2M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316683 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316684 (3,5-Difluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316685 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human 5HT1A receptor expressed in CHO cells assessed as blockade of 8-OH-DPAT-induced inhibition of forskolin-stimulated incre... | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |