

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454862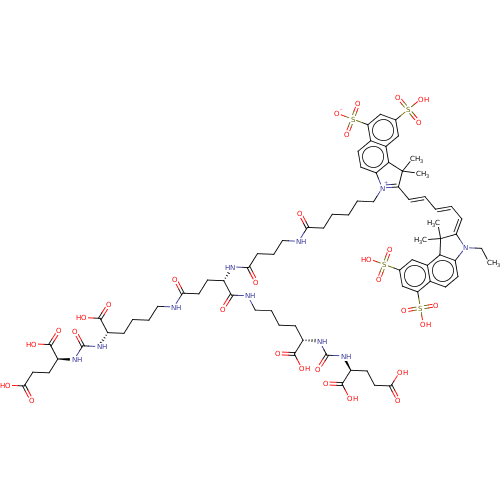 (CHEMBL4211875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454861 (CHEMBL4206989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180197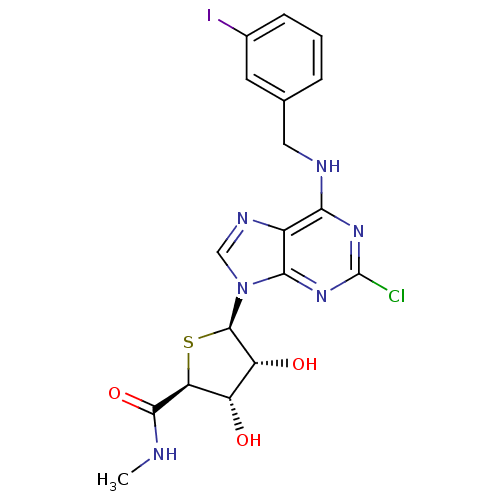 ((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454860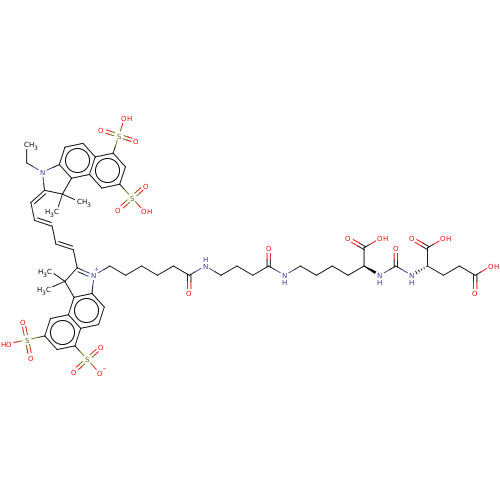 (CHEMBL4202635) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50293031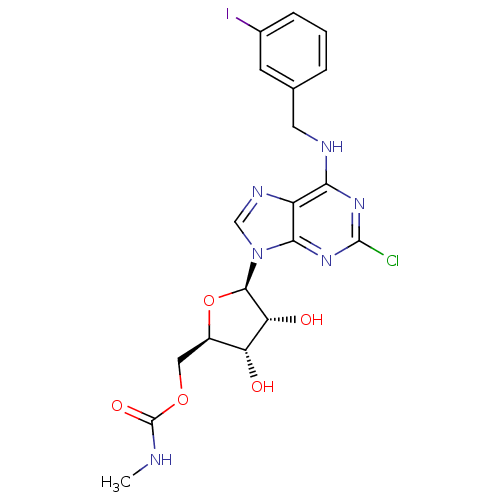 (2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoylade...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Binding affinity to human adenosine A3 receptor | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339076 ((2R,3R,4S)-2-(6-amino-2-(hex-1-ynyl)-9H-purin-9-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363172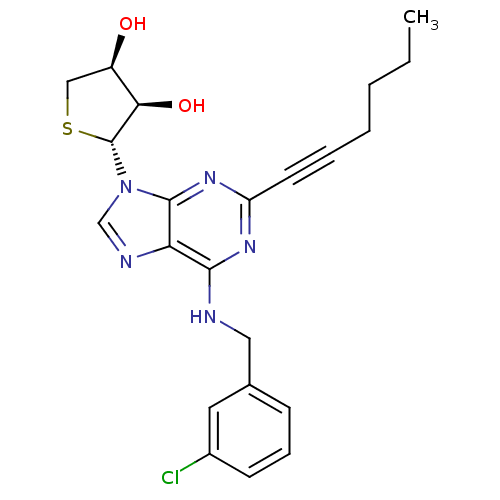 (CHEMBL1946295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363173 (CHEMBL1946296) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363169 (CHEMBL1946299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363174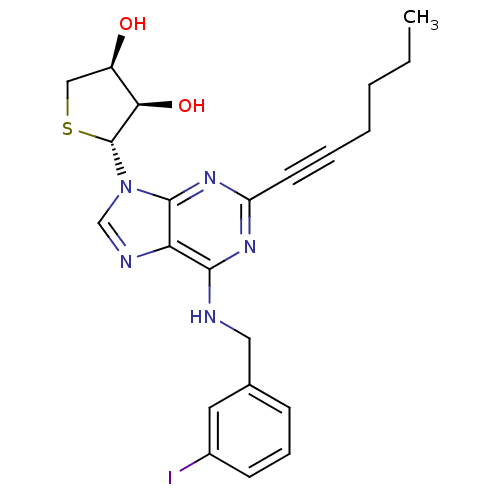 (CHEMBL1946297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363168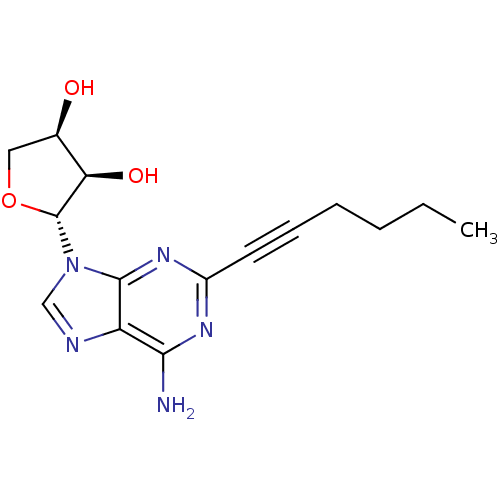 (CHEMBL1945563) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363178 (CHEMBL1946291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50339077 ((2R,3R,4S)-2-(6-amino-2-((E)-hex-1-enyl)-9H-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363170 (CHEMBL1946301) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363168 (CHEMBL1945563) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363180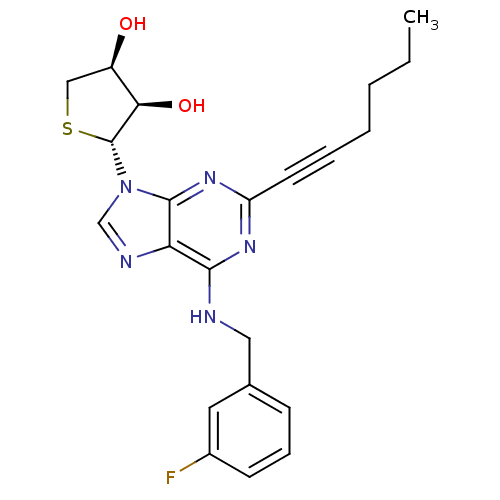 (CHEMBL1946294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363177 (CHEMBL1946290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363175 (CHEMBL1946298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363175 (CHEMBL1946298) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363179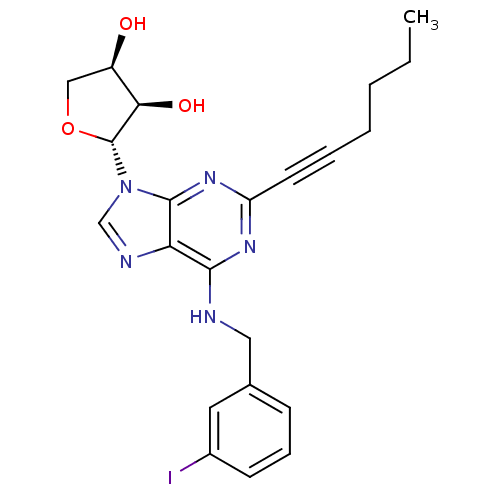 (CHEMBL1946292) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50363169 (CHEMBL1946299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50363170 (CHEMBL1946301) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50363176 (CHEMBL1945564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50363168 (CHEMBL1945563) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human recombinant adenosine A1 receptor expressed in CHO cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363171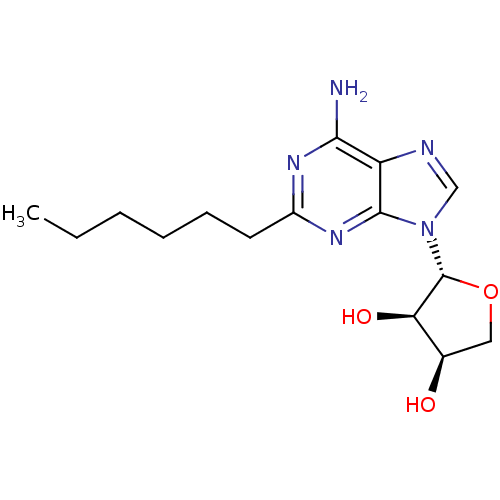 (CHEMBL1946293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363172 (CHEMBL1946295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363173 (CHEMBL1946296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50363174 (CHEMBL1946297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in HEK293 cells after 60 mins by gamma counting | J Med Chem 55: 342-56 (2012) Article DOI: 10.1021/jm201229j BindingDB Entry DOI: 10.7270/Q2VQ334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50251131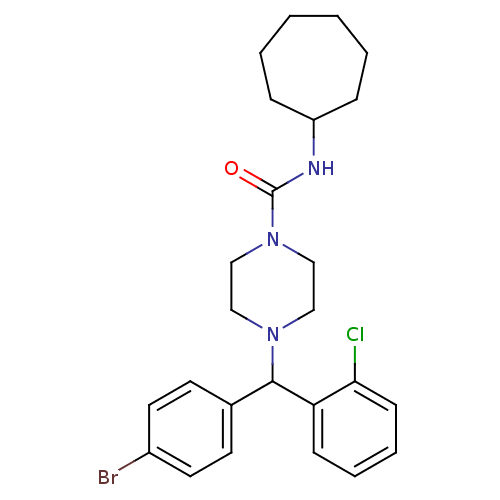 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-cycloh...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50251130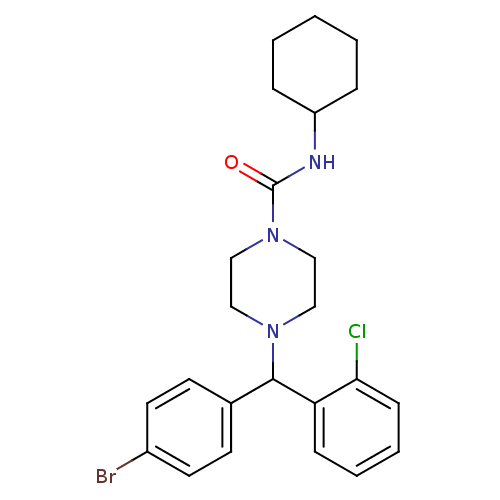 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-Ncyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242549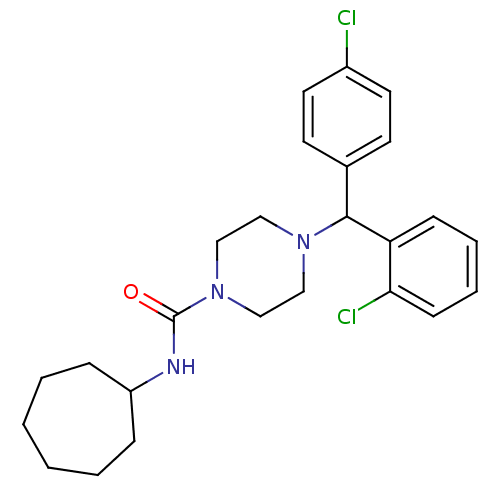 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242548 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50251131 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-cycloh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242557 (4-((4-Chlorophenyl)(2,4-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50251130 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-Ncyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242582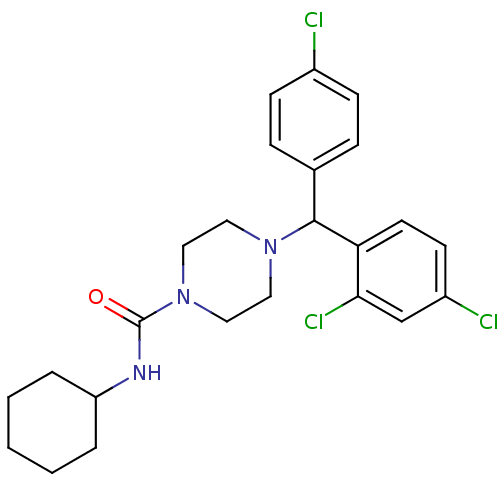 (4-((4-Chlorophenyl)(2,4-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242566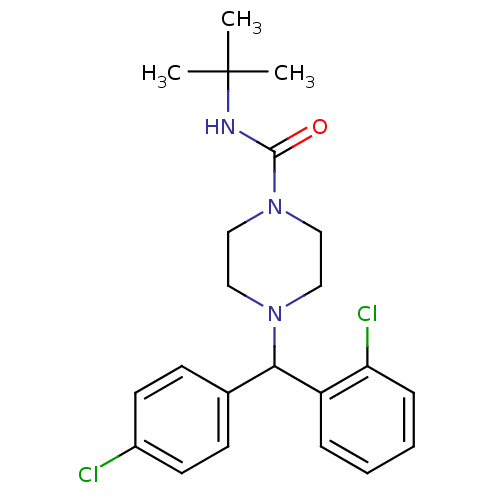 (CHEMBL519584 | N-tert-Butyl-4-((2-chlorophenyl)(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50251132 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-(cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242548 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-cyclo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50354185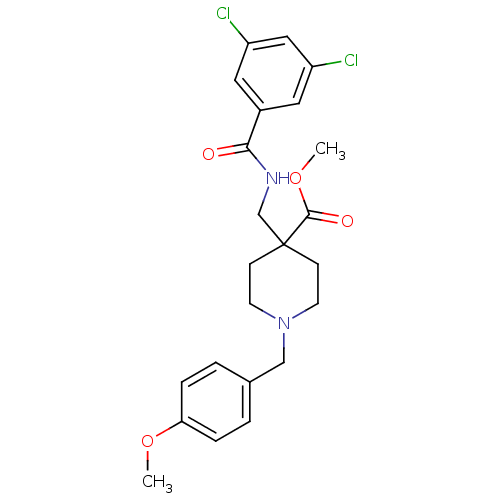 (CHEMBL1836249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp assay | Bioorg Med Chem Lett 21: 5910-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.087 BindingDB Entry DOI: 10.7270/Q2VD6ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242601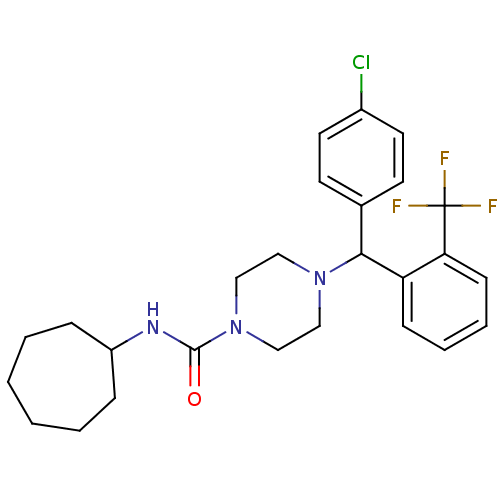 (4-((4-Chlorophenyl)(2-(trifluoromethyl)phenyl)-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50251132 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-(cyclo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242568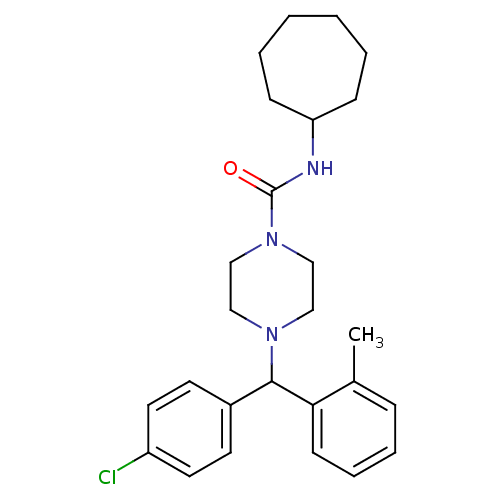 (4-((4-Chlorophenyl)(o-tolyl)methyl)-N-cycloheptylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242537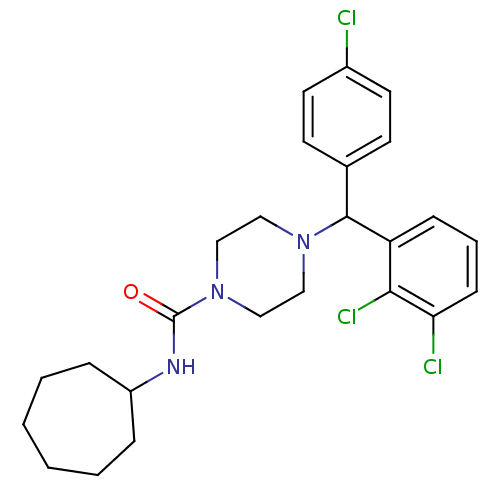 (4-((4-Chlorophenyl)(2,3-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50354183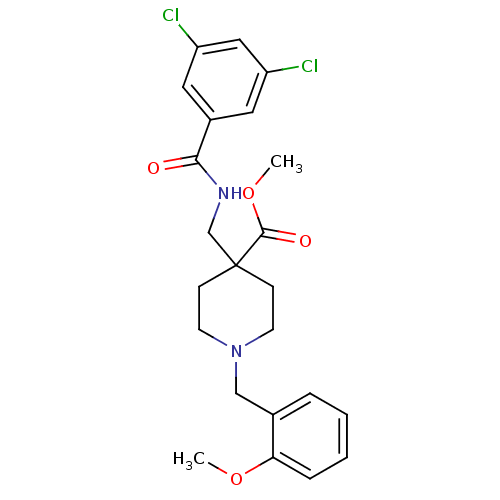 (CHEMBL1836247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp assay | Bioorg Med Chem Lett 21: 5910-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.087 BindingDB Entry DOI: 10.7270/Q2VD6ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242568 (4-((4-Chlorophenyl)(o-tolyl)methyl)-N-cycloheptylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242600 (4-((4-Chlorophenyl)(2-(trifluoromethyl)phenyl)-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |