

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were determined ... | J Med Chem 46: 4405-18 (2003) Article DOI: 10.1021/jm020578e BindingDB Entry DOI: 10.7270/Q2TT4P78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12693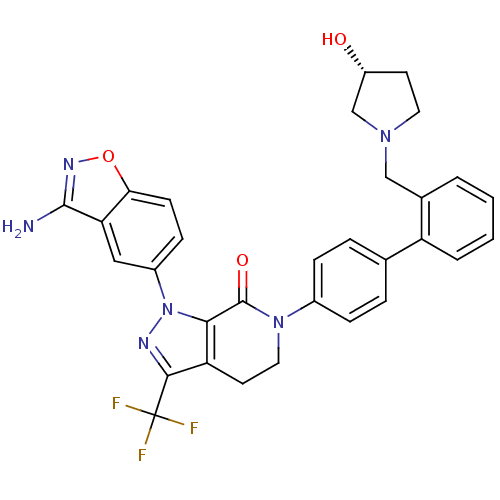 (1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12681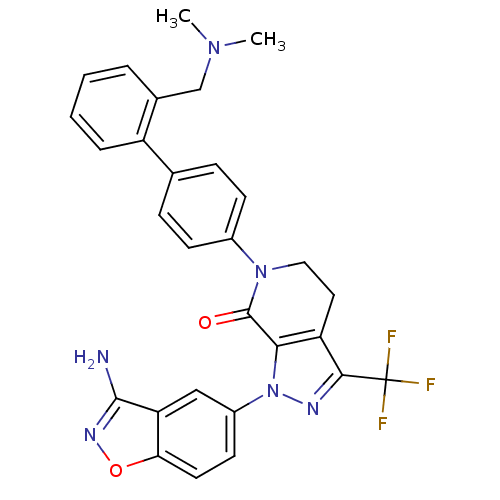 (1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12659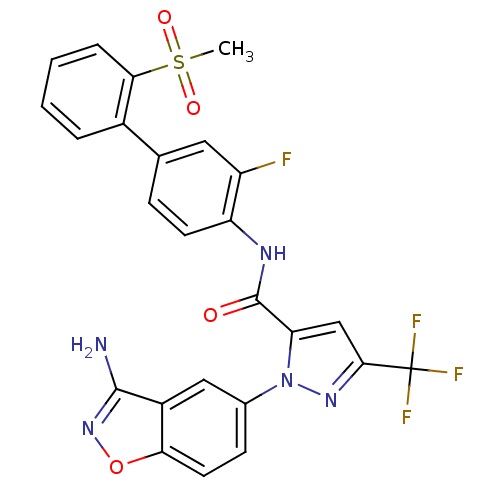 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212159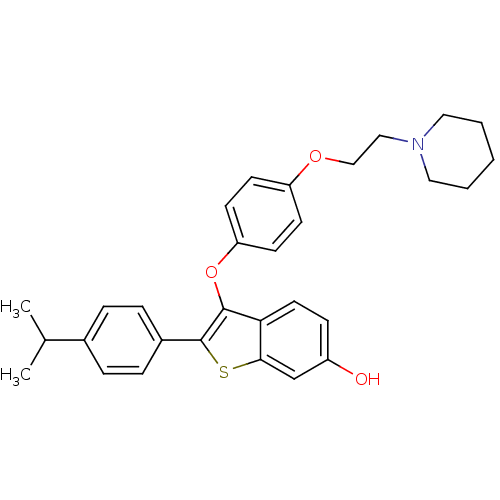 (2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12661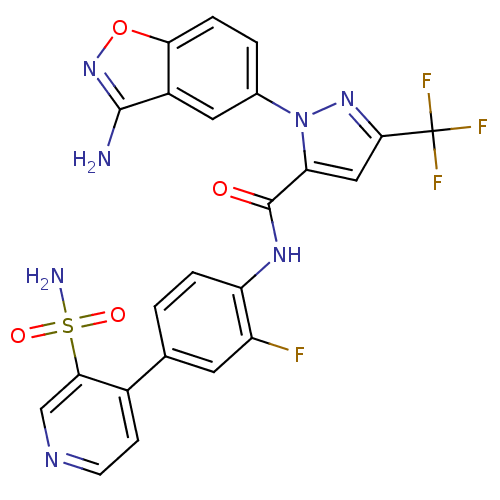 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12748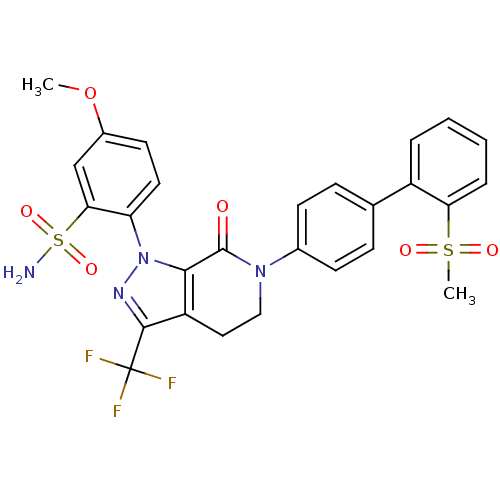 (2-{6-[4-(2-methanesulfonylphenyl)phenyl]-7-oxo-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657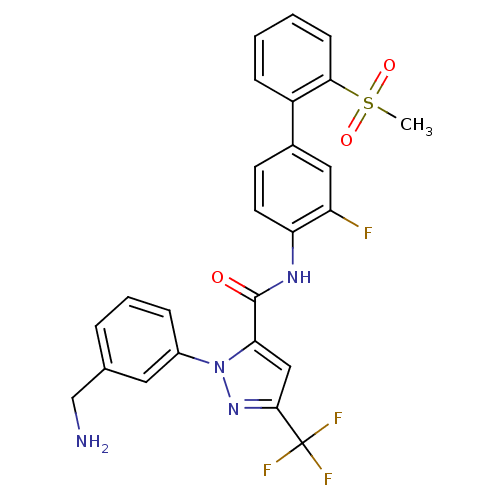 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212148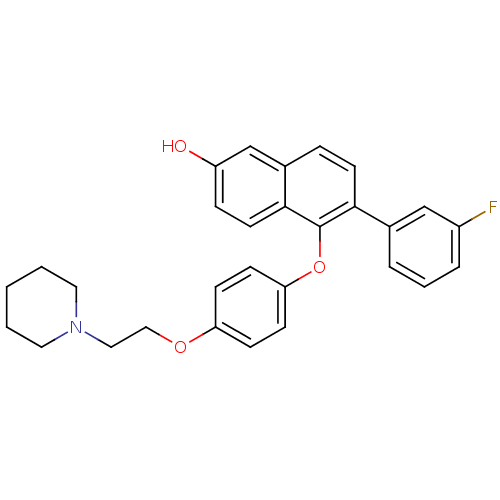 (6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12660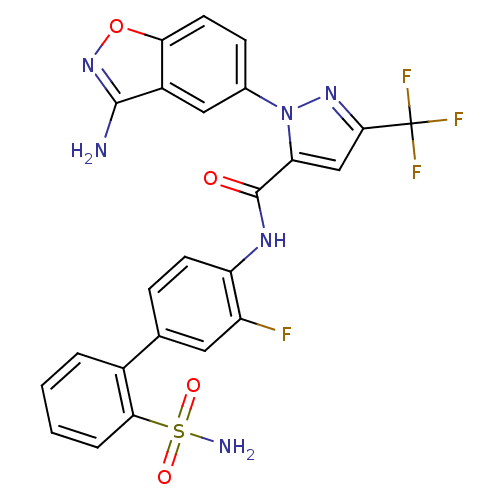 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322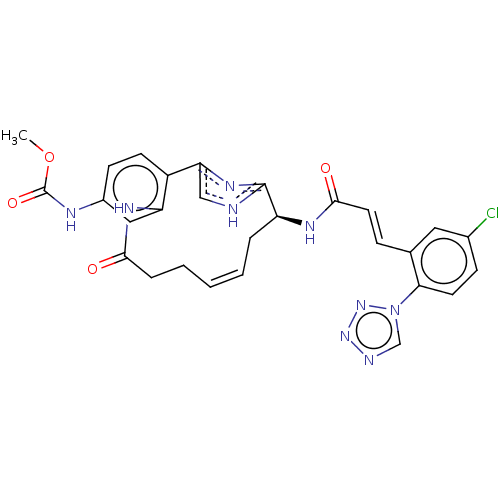 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12675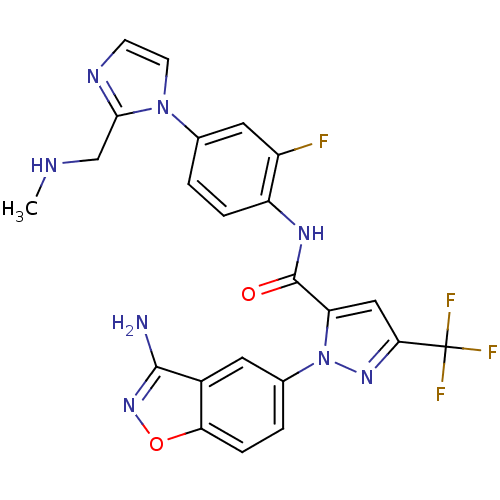 (1-(3-amino-1,2-benzoxazol-5-yl)-N-(2-fluoro-4-{2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212148 (6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12746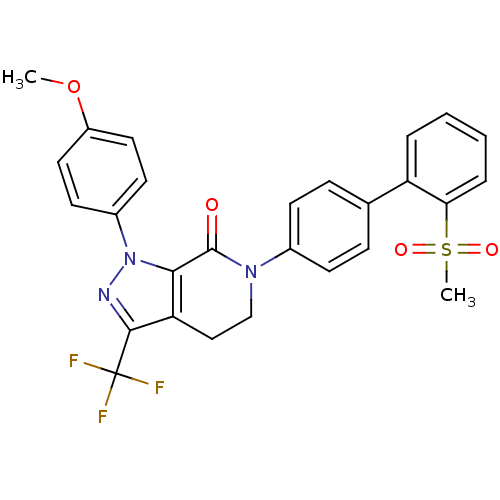 (6-[4-(2-methanesulfonylphenyl)phenyl]-1-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514438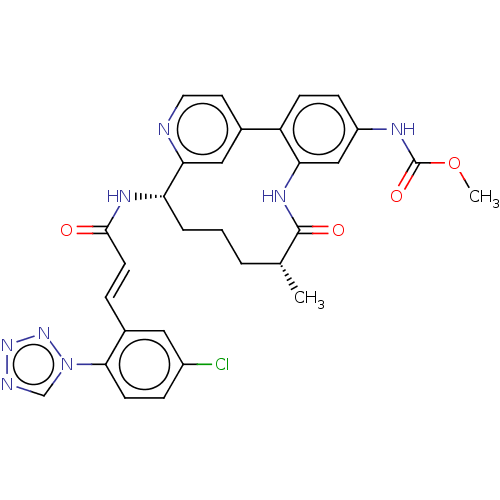 (CHEMBL4439729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12747 (2-{6-[4-(2-methanesulfonylphenyl)phenyl]-7-oxo-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12733 (3-[6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269186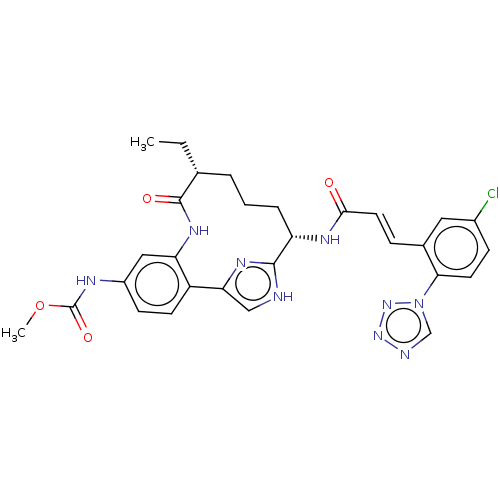 (CHEMBL4089185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12749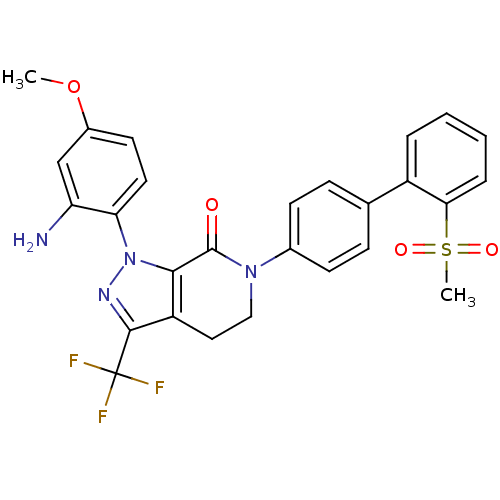 (1-(2-amino-4-methoxyphenyl)-6-[4-(2-methanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50514437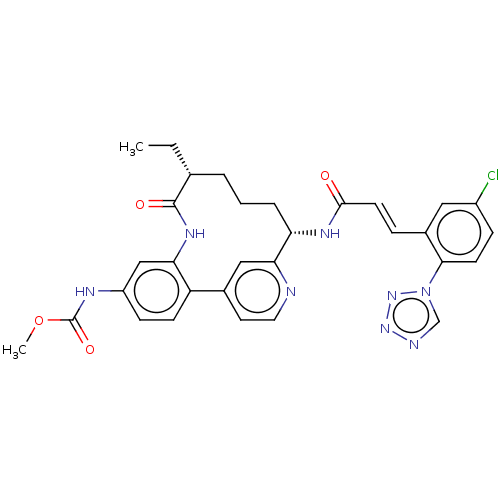 (CHEMBL4444690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212157 (6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212149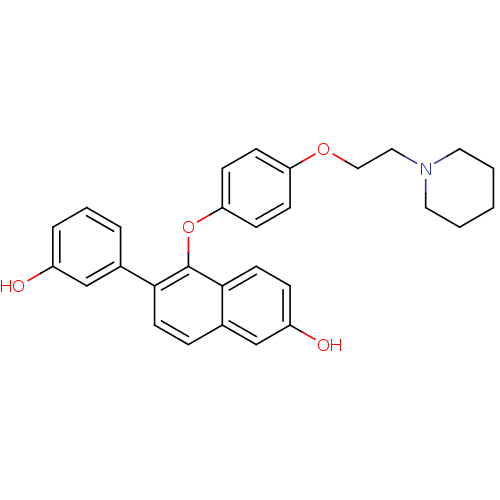 (6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50336931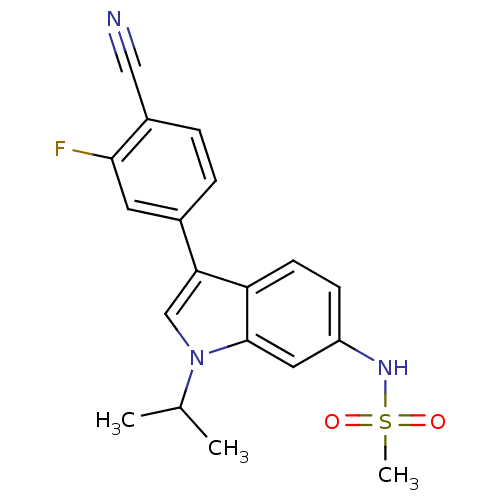 (CHEMBL1672547 | N-(3-(4-cyano-3-fluorophenyl)-1-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells | ACS Med Chem Lett 2: 148-153 (2011) Article DOI: 10.1021/ml100220b BindingDB Entry DOI: 10.7270/Q2Z038G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212160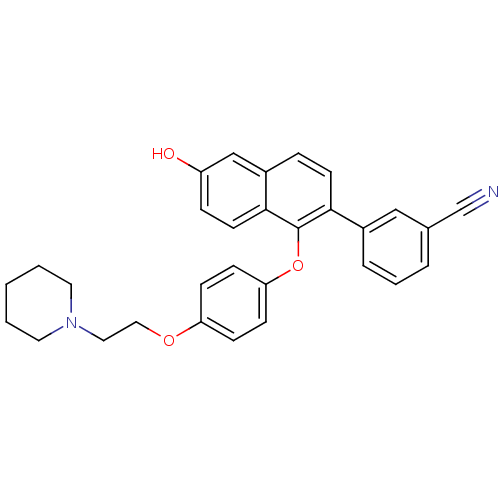 (3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19967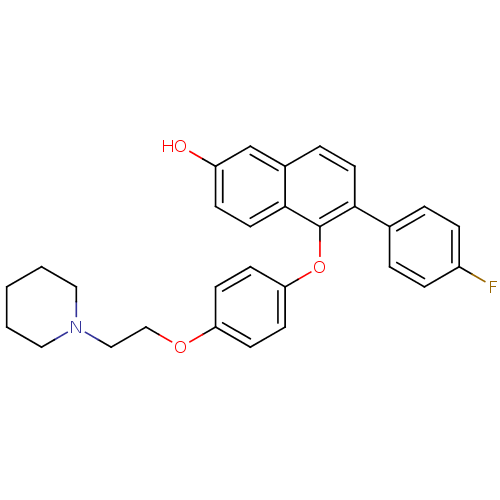 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586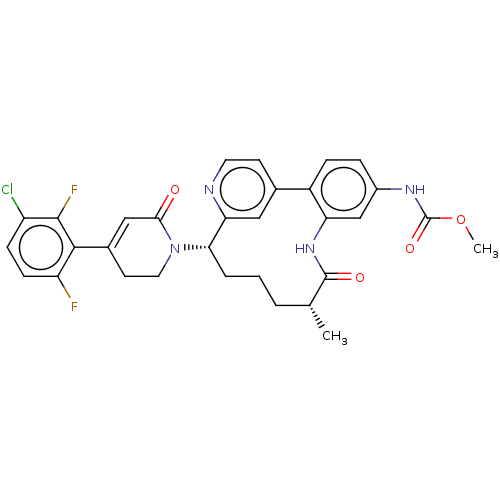 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762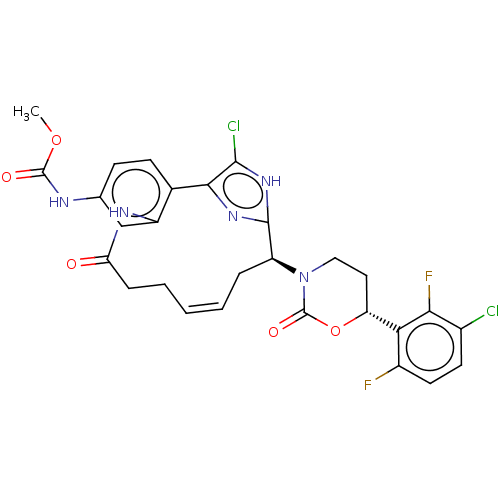 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19967 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212157 (6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50336933 (CHEMBL1672541 | N-(3-(4-cyano-3-methylphenyl)-1-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells | ACS Med Chem Lett 2: 148-153 (2011) Article DOI: 10.1021/ml100220b BindingDB Entry DOI: 10.7270/Q2Z038G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228078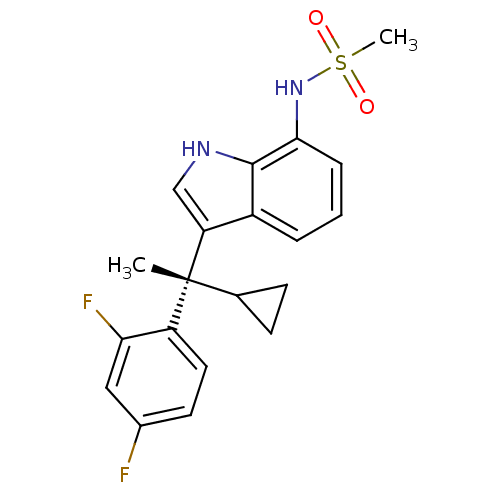 ((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.319 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from human mineralocorticoid receptor expressed in HEK293 cells | ACS Med Chem Lett 2: 148-153 (2011) Article DOI: 10.1021/ml100220b BindingDB Entry DOI: 10.7270/Q2Z038G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50336930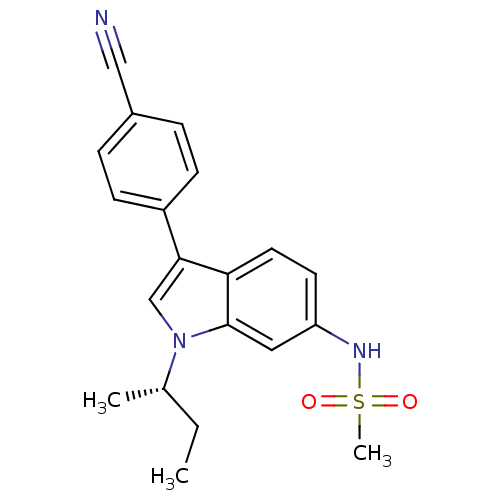 ((S)-N-(1-sec-butyl-3-(4-cyanophenyl)-1H-indol-6-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from human progesterone receptor expressed in HEK293 cells | ACS Med Chem Lett 2: 148-153 (2011) Article DOI: 10.1021/ml100220b BindingDB Entry DOI: 10.7270/Q2Z038G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212147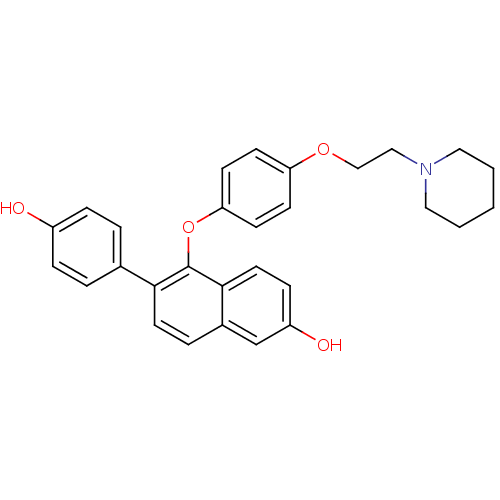 (6-(4-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM18982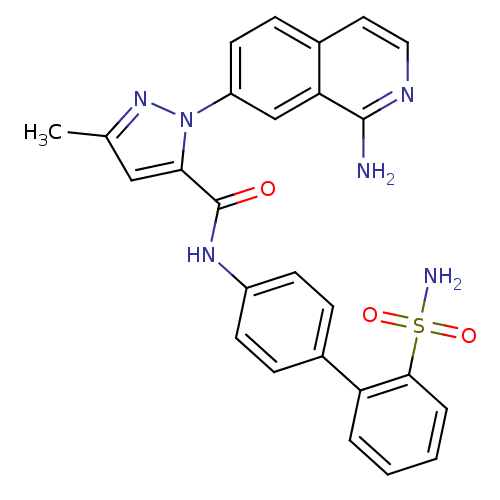 (1-(1-aminoisoquinolin-7-yl)-3-methyl-N-[4-(2-sulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were determined ... | J Med Chem 46: 4405-18 (2003) Article DOI: 10.1021/jm020578e BindingDB Entry DOI: 10.7270/Q2TT4P78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212160 (3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12740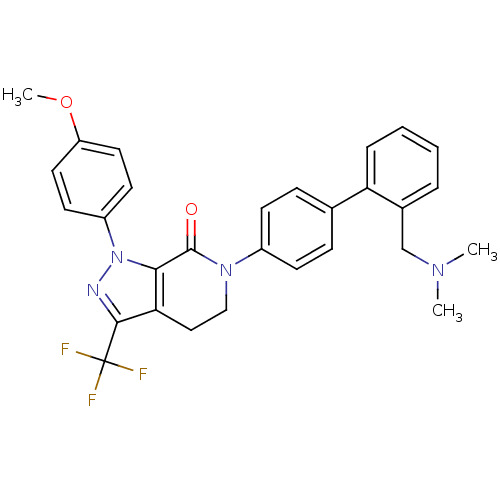 (6-(4-{2-[(dimethylamino)methyl]phenyl}phenyl)-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 5584-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.027 BindingDB Entry DOI: 10.7270/Q2Z899NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335990 (4-(2-(3-(3-chlorophenyl)- 4,5-dihydroisoxazole-5- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212149 (6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12677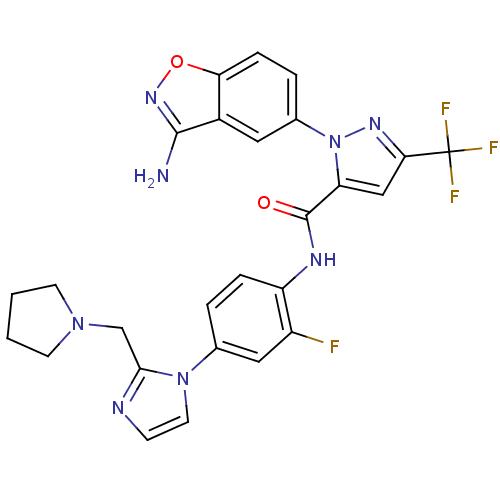 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269195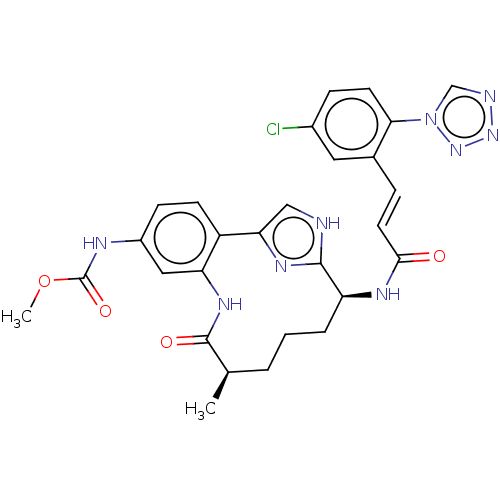 (CHEMBL4101766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | J Med Chem 63: 784-803 (2020) Article DOI: 10.1021/acs.jmedchem.9b01768 BindingDB Entry DOI: 10.7270/Q2J67M9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212153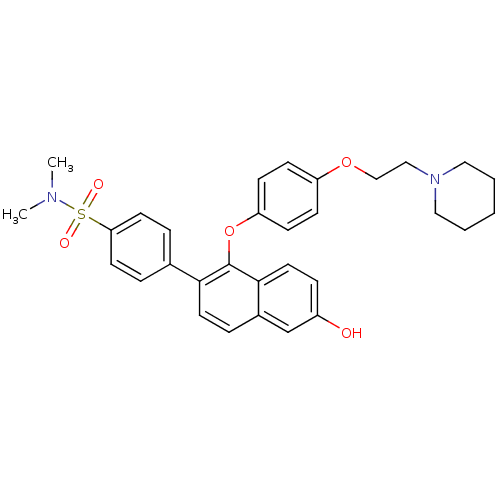 (4-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335779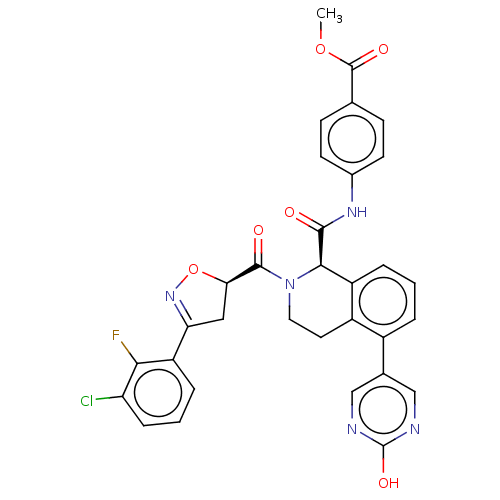 (4-(2-(1-(3-chloro-2- fluorophenyl)-1H-1,2,3- triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19966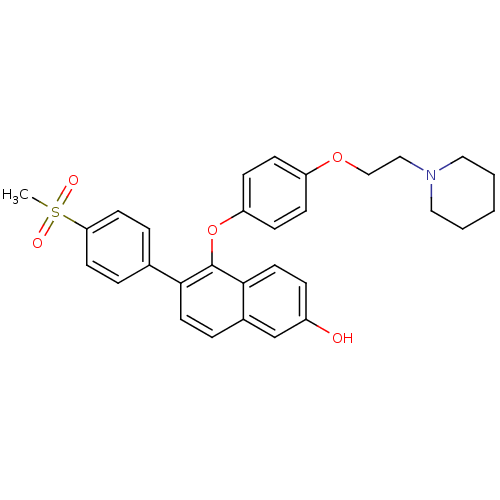 (6-(4-methanesulfonylphenyl)-5-{4-[2-(piperidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212147 (6-(4-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212155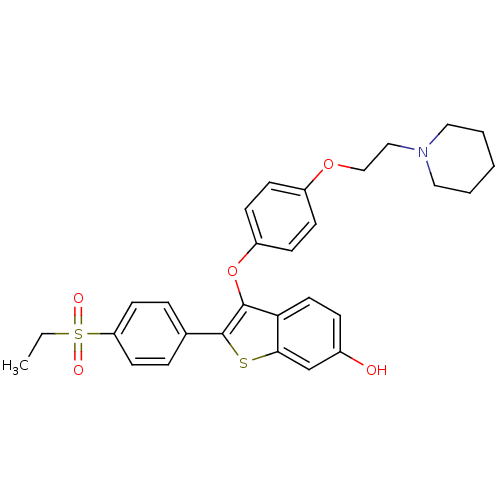 (2-(4-(ethylsulfonyl)phenyl)-3-(4-(2-(piperidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541581 (CHEMBL4646341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12662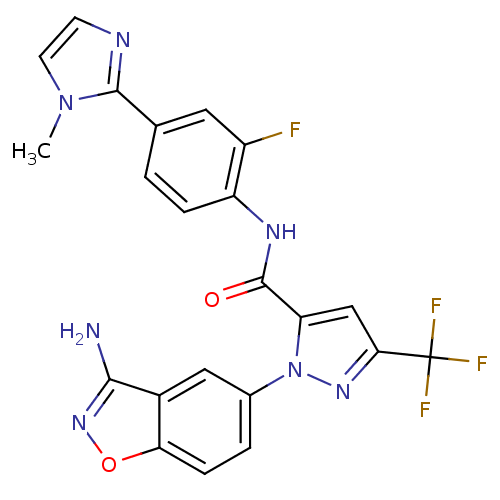 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.510 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12666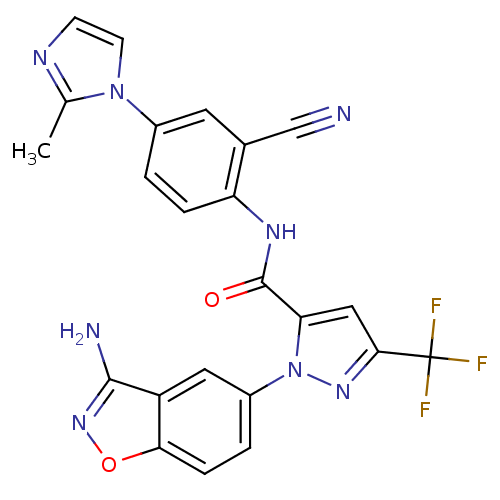 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.520 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | J Med Chem 48: 1729-44 (2005) Article DOI: 10.1021/jm0497949 BindingDB Entry DOI: 10.7270/Q2BK19K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335984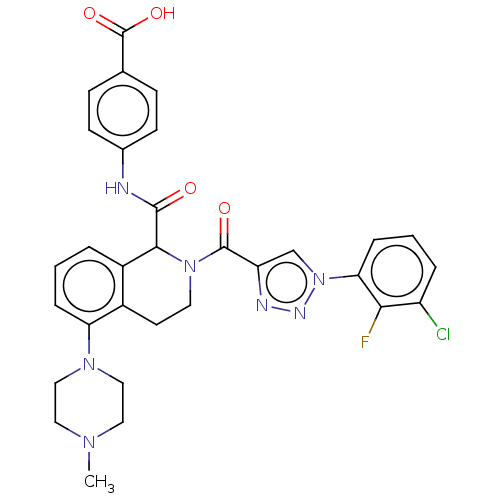 (4-(2-(1-(3-chloro-2- fluorophenyl)-1H-1,2,3- triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3334 total ) | Next | Last >> |