

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50402202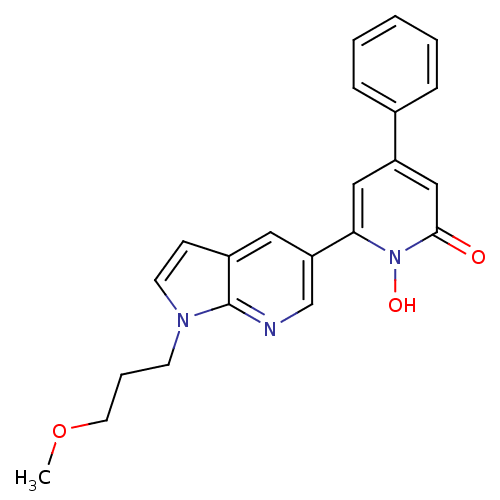 (CHEMBL2203964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092825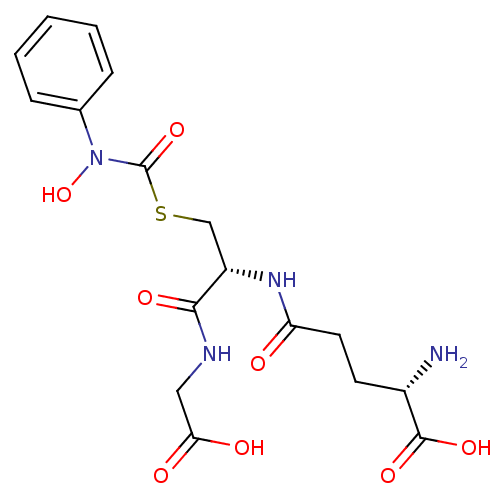 (CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50517464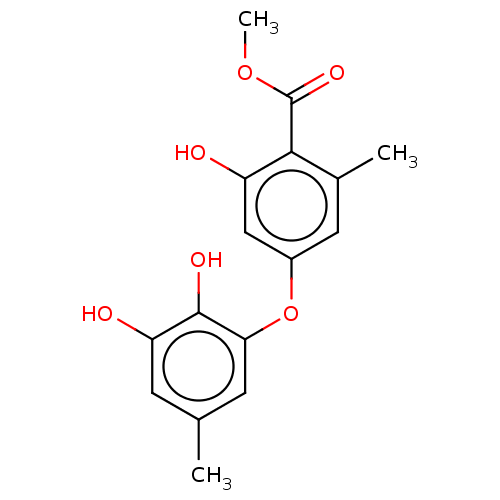 (CHEMBL1234300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human GLO1 | J Med Chem 62: 1609-1625 (2019) Article DOI: 10.1021/acs.jmedchem.8b01868 BindingDB Entry DOI: 10.7270/Q2251NJC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to human Zn2+-HDAC8 assessed as loss of activity by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Clostridium botulinum BoNT/A using SNAP-25 (141-206) as substrate by HPLC analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546871 (CHEMBL4745069) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Reversible-time dependent inhibition of human wild type HDAC8 by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50048539 (CHEMBL3309328) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of recombinant Clostridium botulinum N-terminal 6His-tagged BoNT/A (Met1 to Phe425 residues) catalytic domain expressed in Es... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546870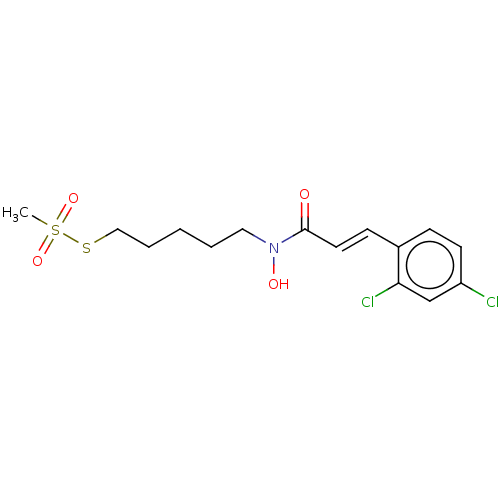 (CHEMBL4790141) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057418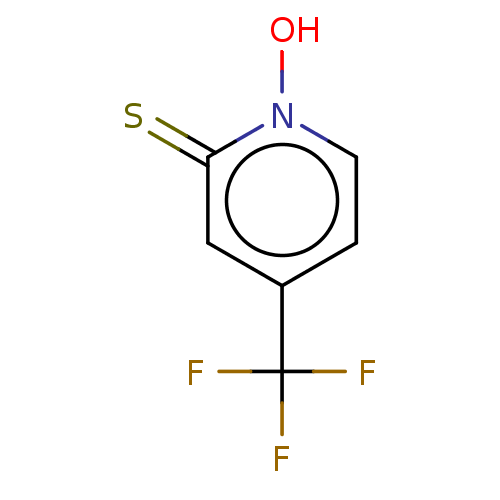 (CHEMBL3326435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546872 (CHEMBL4761825) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546874 (CHEMBL4795025) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057411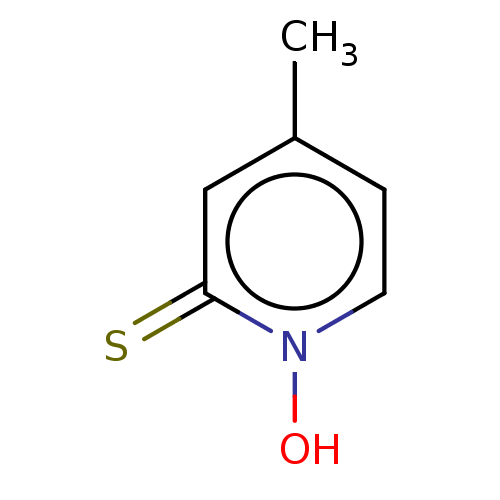 (CHEMBL3326430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546876 (CHEMBL4787837) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546880 (CHEMBL4799810) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057412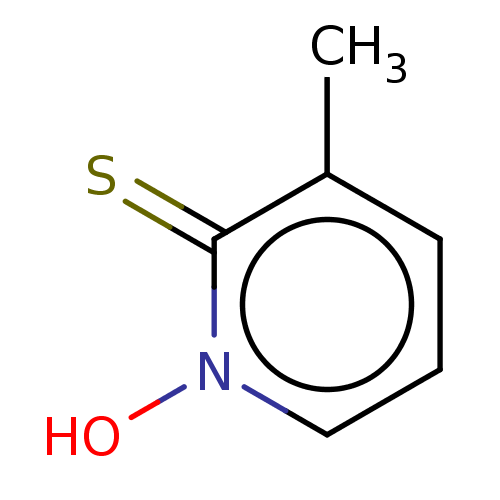 (CHEMBL3326429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546875 (CHEMBL4746123) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate by me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50048539 (CHEMBL3309328) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057406 (CHEMBL3326434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546878 (CHEMBL4790780) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546879 (CHEMBL4787587) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546877 (CHEMBL4743480) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide flp6 as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057410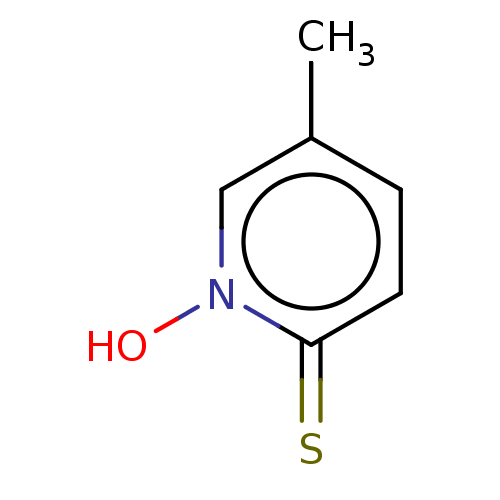 (CHEMBL3326431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057417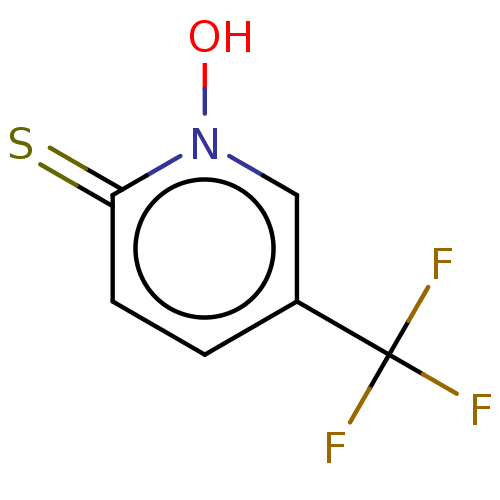 (CHEMBL3326436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM60985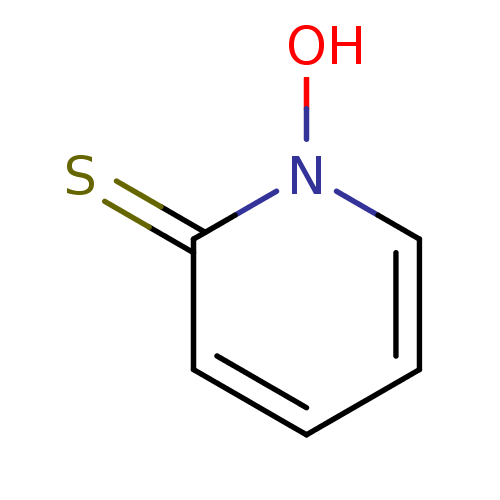 (1-hydroxy-2-pyridinethione | 1-hydroxypyridine-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057414 (CHEMBL3326439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057408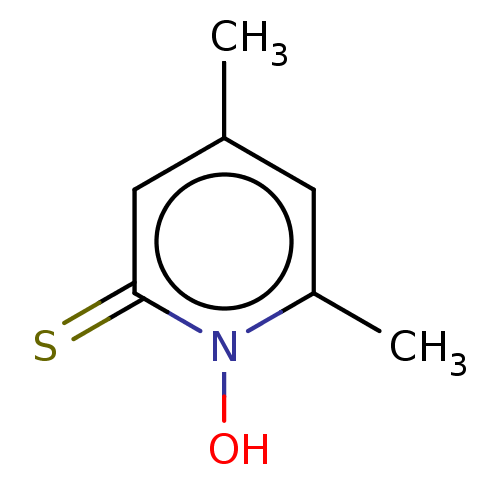 (CHEMBL3326433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50546881 (CHEMBL4761785) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Irreversible inhibition of Clostridium botulinum BoNT/A light chain expressed in Escherichia coli BL21 (DE3) using SNAPtide as substrate preincubated... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057415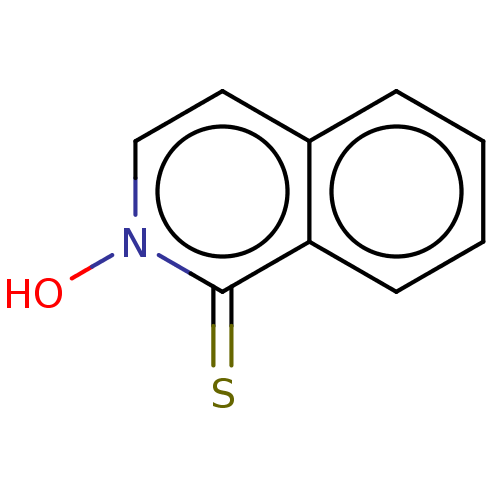 (CHEMBL3326438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057409 (CHEMBL3326432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50057416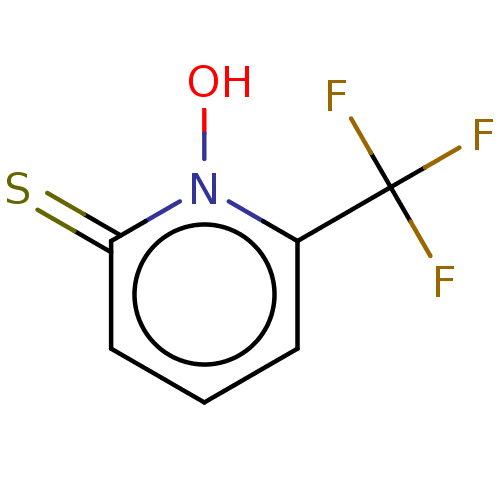 (CHEMBL3326437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase using p-nitrophenylacetate as substrate preincubated for 10 mins before substrate addition by spectrophotometr... | J Med Chem 57: 7126-35 (2014) Article DOI: 10.1021/jm500984b BindingDB Entry DOI: 10.7270/Q20V8FD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509161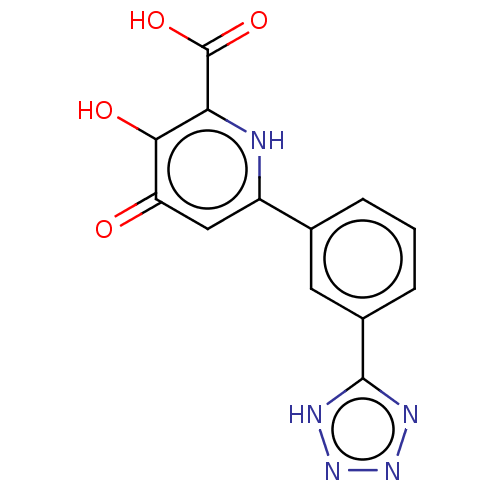 (CHEMBL4563716) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509155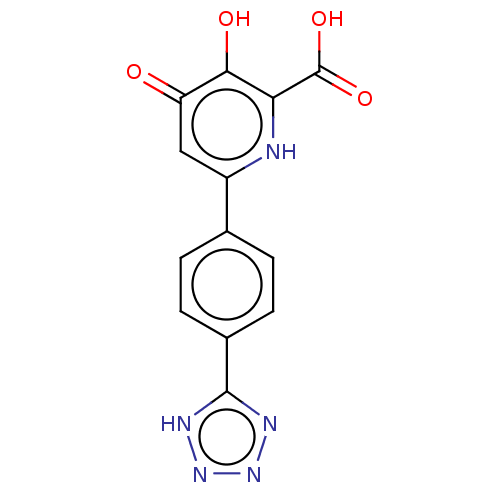 (CHEMBL4440123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509159 (CHEMBL4588964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509169 (CHEMBL4545631) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509165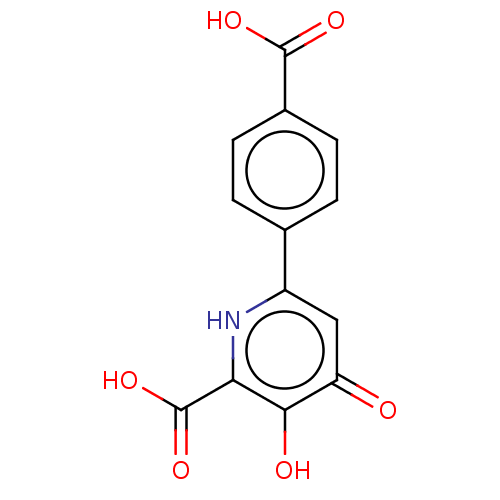 (CHEMBL4459575) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509169 (CHEMBL4545631) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509167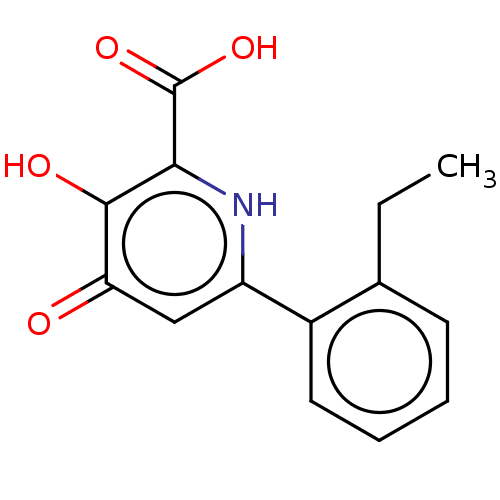 (CHEMBL4539596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509161 (CHEMBL4563716) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509159 (CHEMBL4588964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509167 (CHEMBL4539596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509165 (CHEMBL4459575) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509155 (CHEMBL4440123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509170 (CHEMBL4440338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509160 (CHEMBL4570983) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509160 (CHEMBL4570983) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509170 (CHEMBL4440338) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509166 (CHEMBL4550646) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509157 (CHEMBL4530222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polymerase acidic protein (Hepatitis C virus) | BDBM50509157 (CHEMBL4530222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Influenza A virus RNA-dependent RNA polymerase PA N-terminal endonuclease using [6-FAM]AATCGCAGGCAGCACTC[TAM] substrate and measured ov... | J Med Chem 62: 9438-9449 (2019) Article DOI: 10.1021/acs.jmedchem.9b00747 BindingDB Entry DOI: 10.7270/Q27M0C7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 562 total ) | Next | Last >> |