

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50137724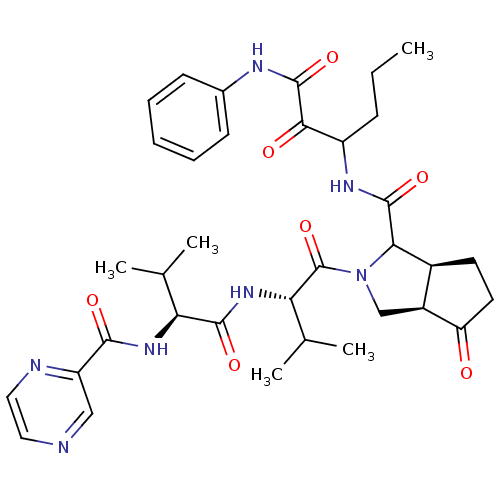 ((3aR,5S)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyraz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137721 (2-(3-{[(1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137713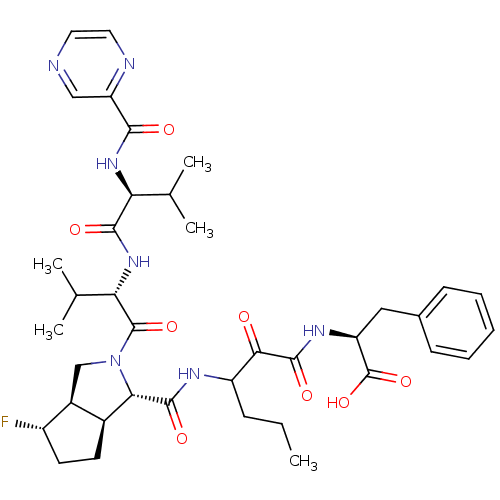 ((S)-2-((S)-3-{[(1S,5S,6R)-4-Fluoro-2-((S)-3-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137718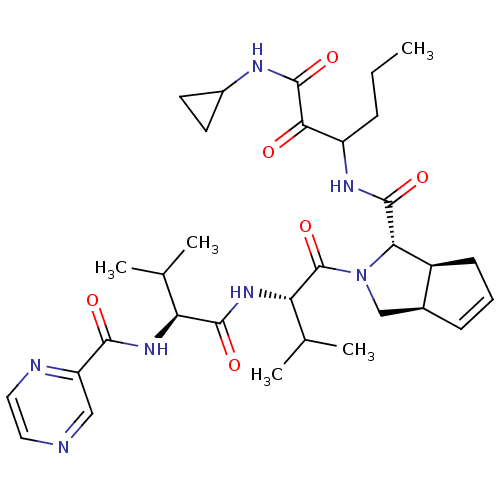 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137722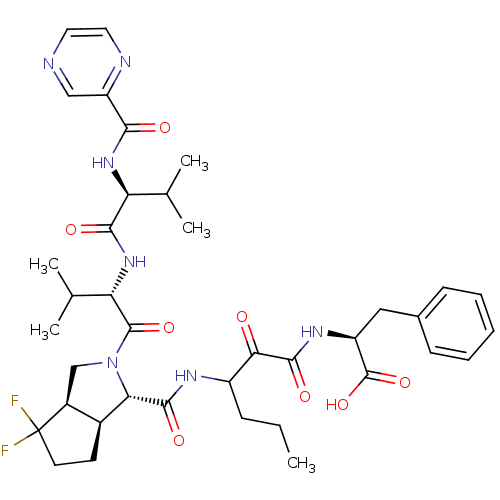 ((S)-2-(3-{[(1S,5S,6R)-4,4-Difluoro-2-((S)-3-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137717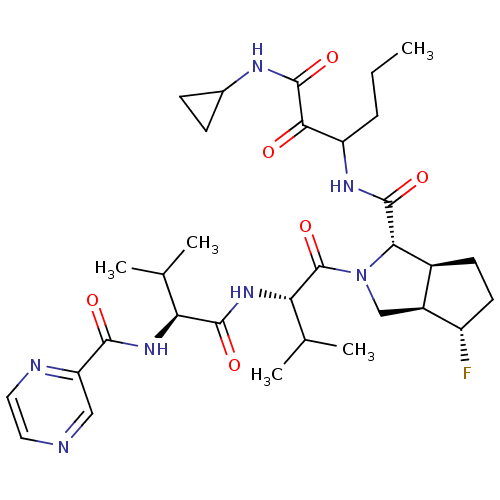 ((1S,5S,6R)-4-Fluoro-2-((S)-3-methyl-2-{(S)-3-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137727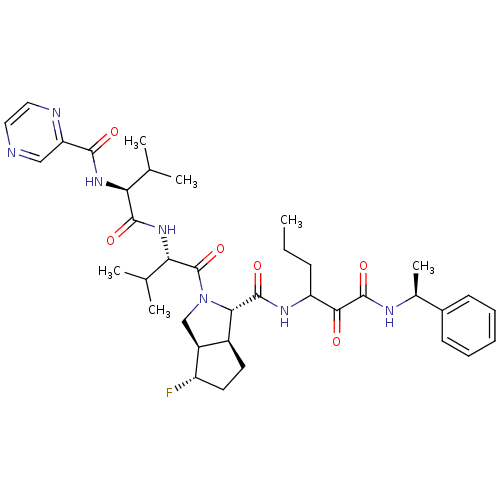 ((1S,5S,6R)-4-Fluoro-2-((S)-3-methyl-2-{(S)-3-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137723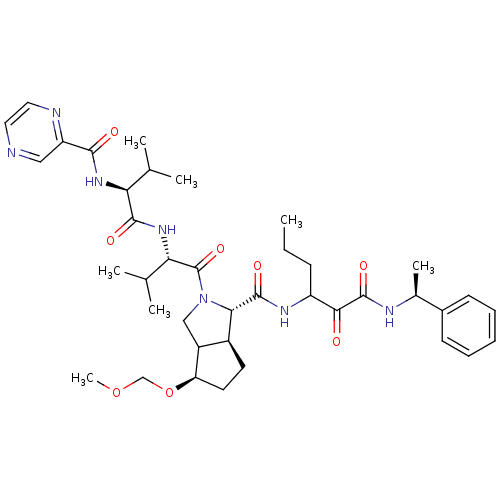 ((1S,5S)-4-Methoxymethoxy-2-((S)-3-methyl-2-{(S)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity against Protease in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137719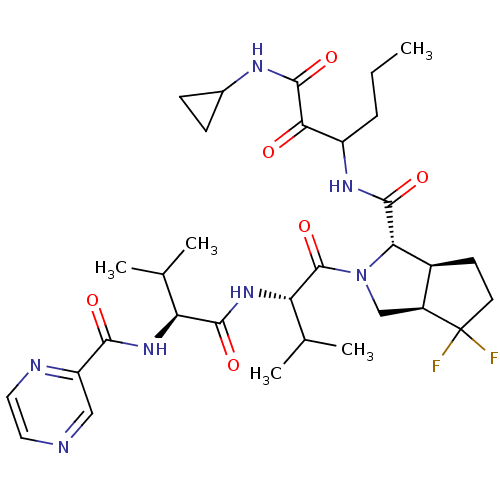 ((1S,5S,6R)-4,4-Difluoro-2-((S)-3-methyl-2-{(S)-3-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137725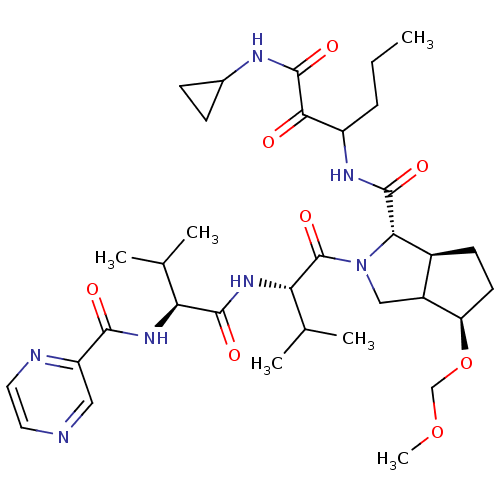 ((1S,5S)-4-Methoxymethoxy-2-((S)-3-methyl-2-{(S)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity against Protease in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137728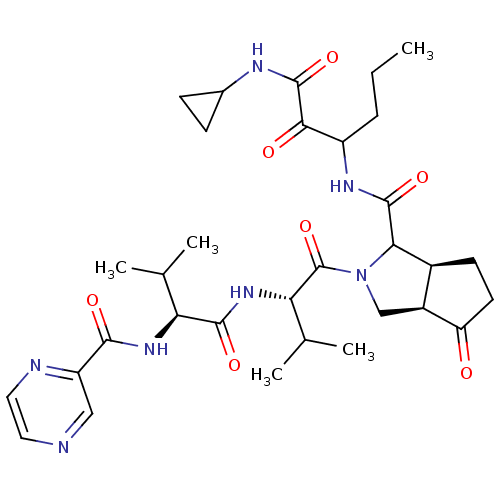 ((5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyrazi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Cytotoxic activity against Protease in rat liver Huh-7 cells | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137714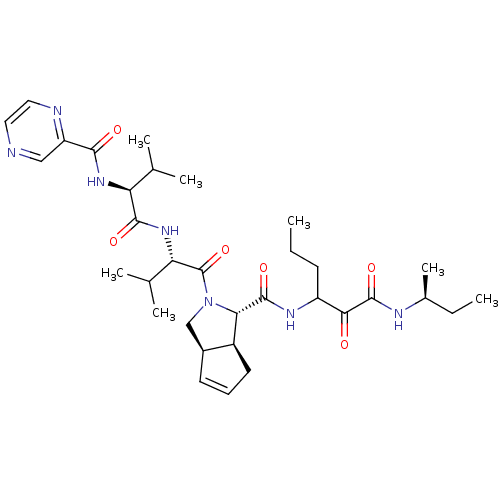 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137716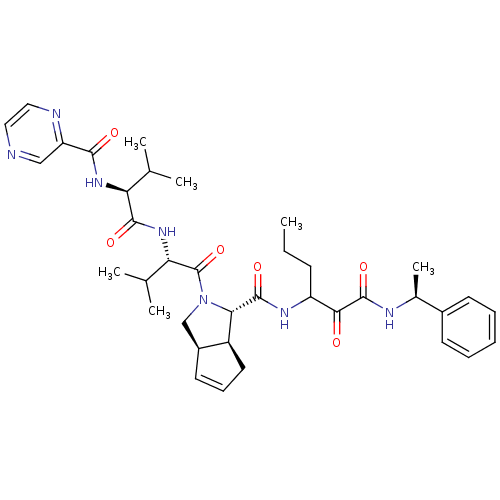 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137726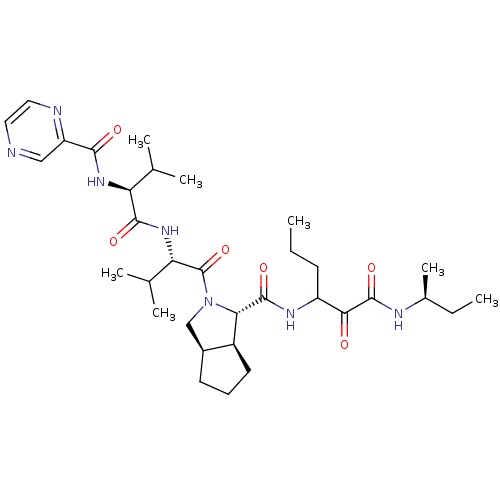 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50137715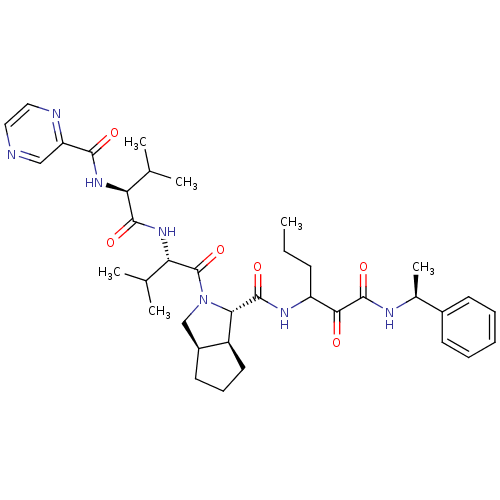 ((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Binding affinity towards Protease using PNA assay in rats | Bioorg Med Chem Lett 14: 251-6 (2003) BindingDB Entry DOI: 10.7270/Q24B30R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM821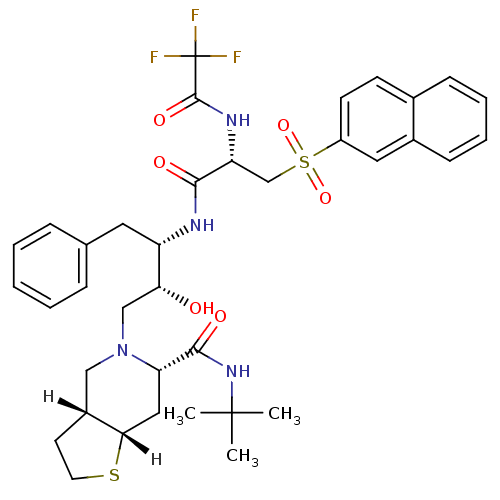 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM823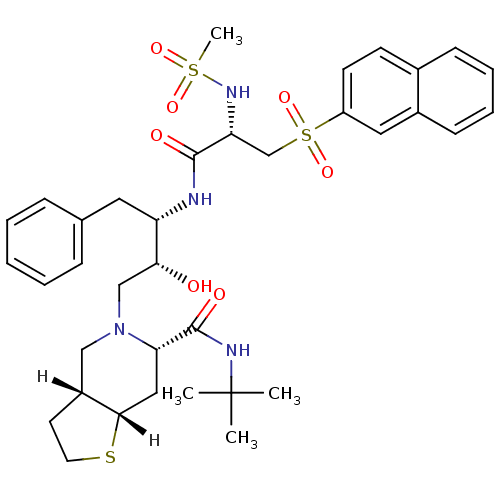 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM817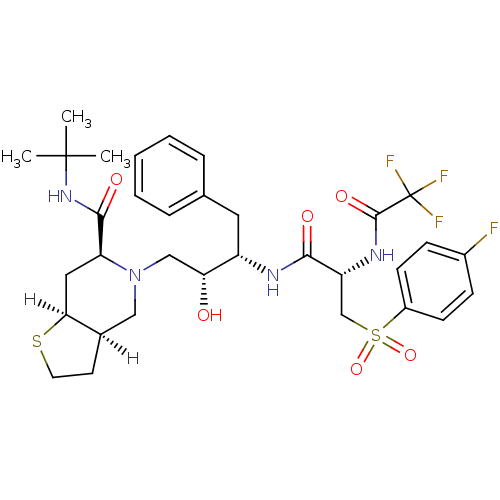 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM821 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM817 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM823 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM825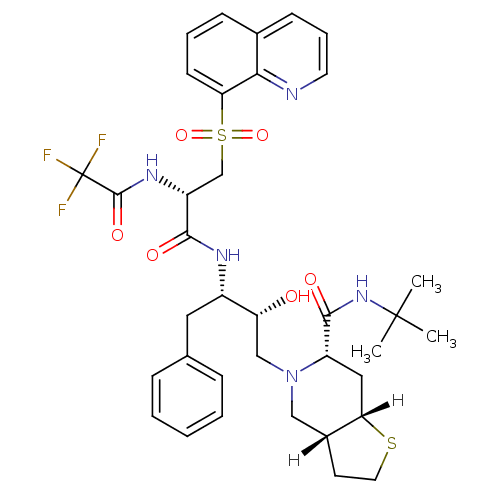 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-4-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM825 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-4-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM819 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM819 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM318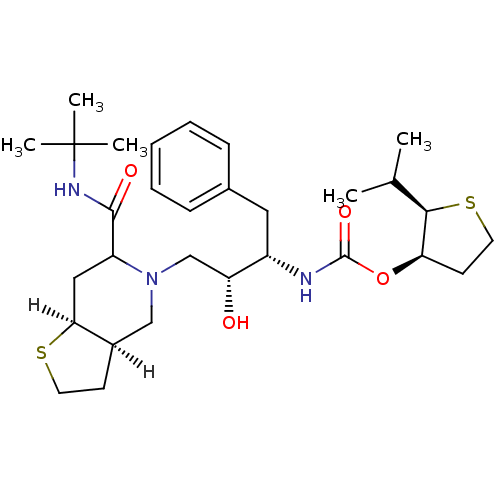 ((2R,3R)-2-(propan-2-yl)thiolan-3-yl N-[(2S,3R)-4-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285971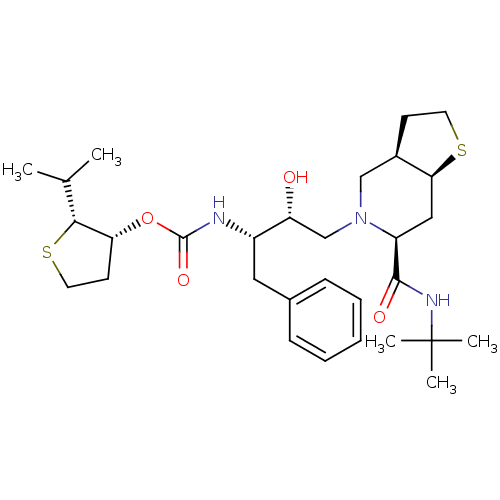 (CHEMBL95546 | [(1S,2R)-1-Benzyl-3-((3aR,6S,7aS)-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM306 ((2S,3S)-2-methyloxolan-3-yl N-[(2R,3R)-4-[(3aR,7aS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285969 (CHEMBL99336 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM313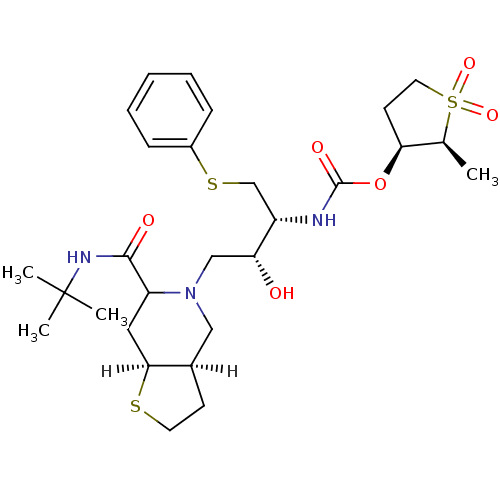 ((2S,3S)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285976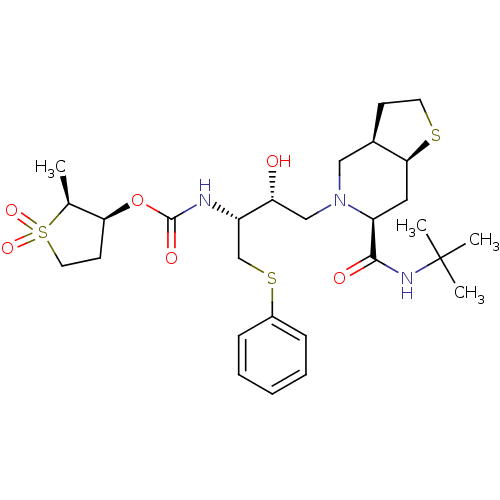 (CHEMBL321949 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM824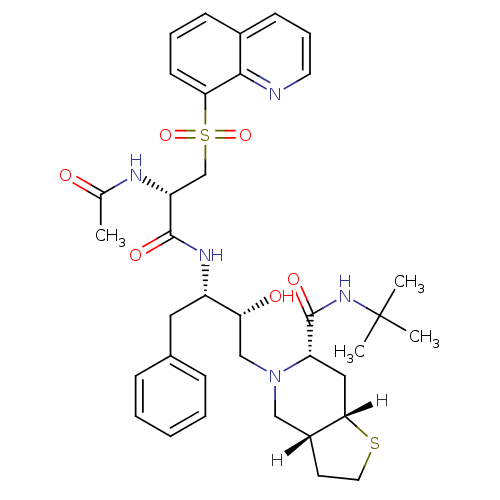 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM824 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM322 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285980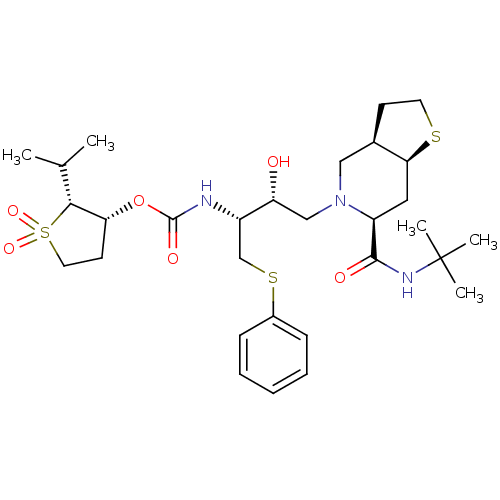 (CHEMBL97585 | LY-326188 | [(1R,2R)-3-((3aR,6S,7aS)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM820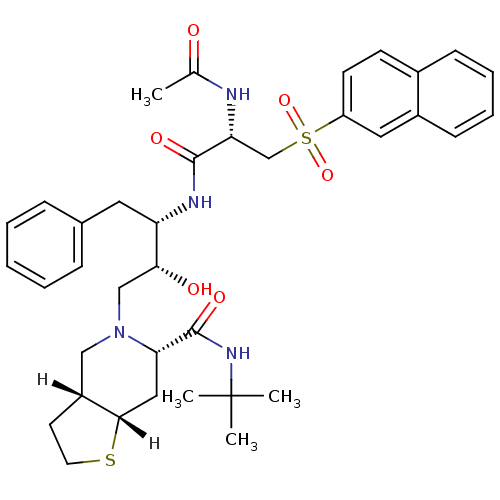 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM811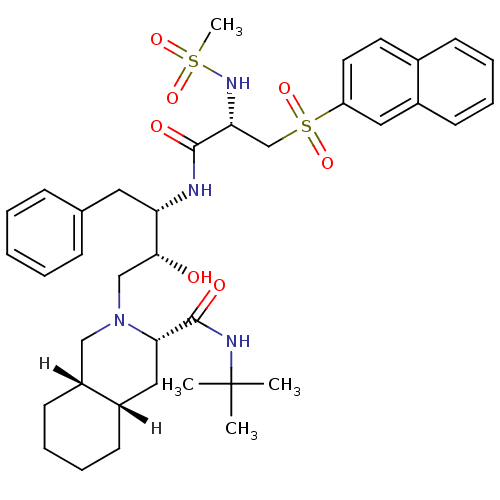 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM811 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM820 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-3-[(2S)-2-ace...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM807 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-3-[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM807 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-3-[(4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM826 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | |
Lilly Research Laboratories | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM826 ((3aR,6S,7aS)-N-tert-butyl-5-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 5.5 | 22 | |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2897-902 (1995) BindingDB Entry DOI: 10.7270/Q22F7KMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285977 (CHEMBL99214 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM314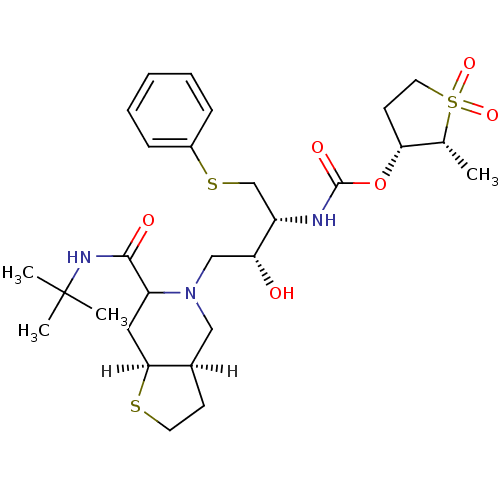 ((2R,3R)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285982 (CHEMBL316717 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM321 ((2S,3S)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |