

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525149 (US10988505, Comparative #1) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzymatic assays and Ki determination were performed at room temperature in an assay buffer containing 50 mM Tris-HCl, 150 mM NaCl and 500 μg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236490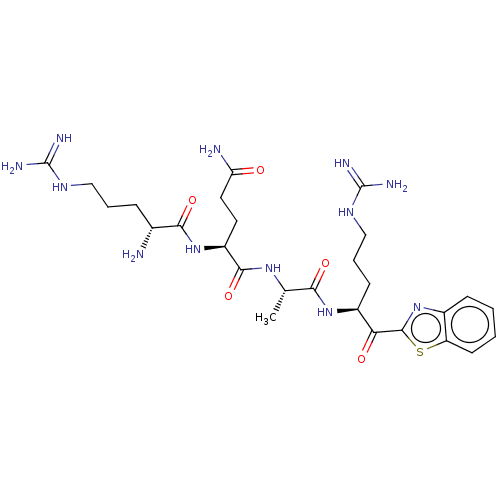 (US9365853, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0110 | -62.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271367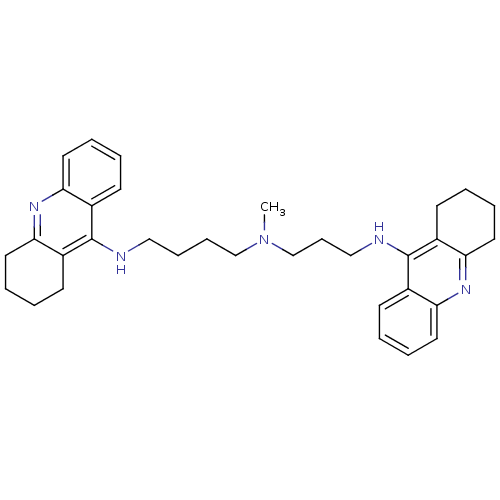 (CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50005193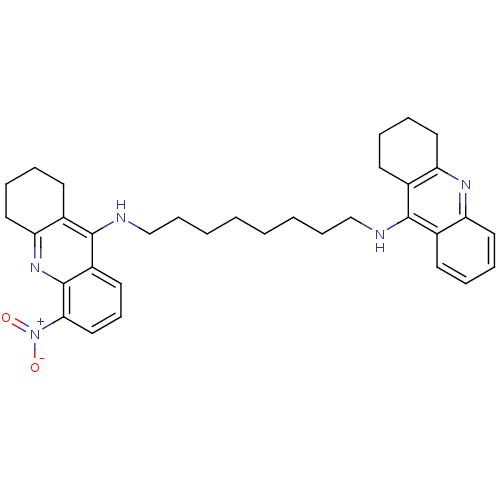 (CHEMBL3099496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032696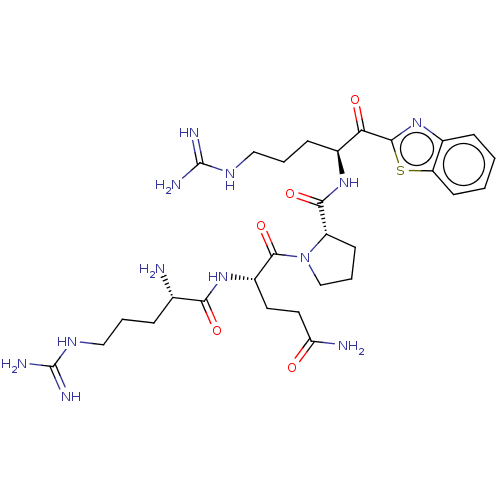 (CHEMBL3354674) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190273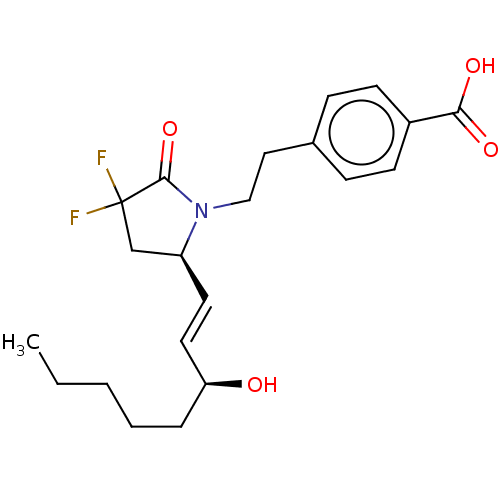 (US9180116, 21C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | 0.220 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM236493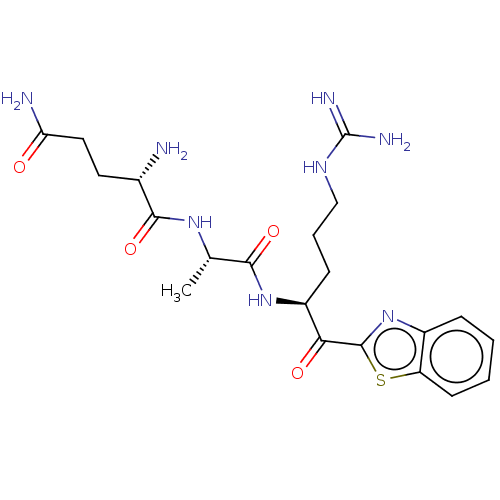 (US10988505, Comparative #2 | US9365853, 4) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzymatic assays and Ki determination were performed at room temperature in an assay buffer containing 50 mM Tris-HCl, 150 mM NaCl and 500 μg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236493 (US10988505, Comparative #2 | US9365853, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0880 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420336 (CHEMBL2089123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032702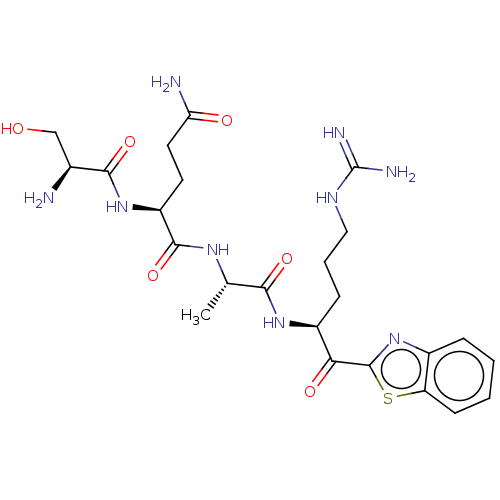 (CHEMBL3352840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190282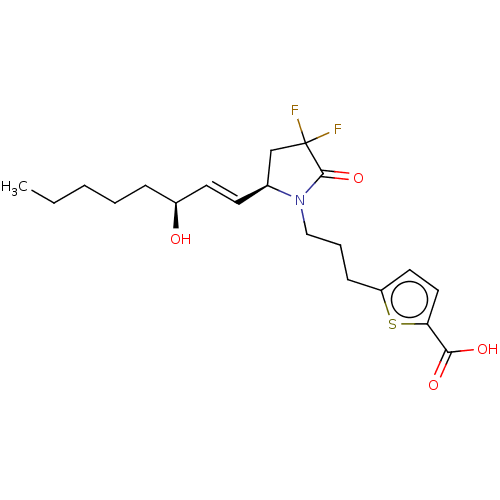 (US9180116, 33C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | 0.280 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190272 (US9180116, 12D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | n/a | 0.320 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525151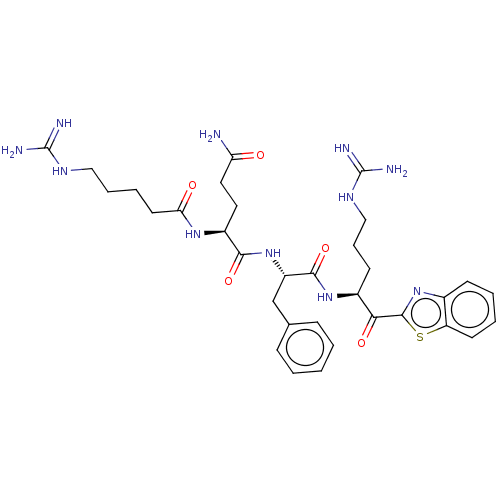 (US10988505, Example 1) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzymatic assays and Ki determination were performed at room temperature in an assay buffer containing 50 mM Tris-HCl, 150 mM NaCl and 500 μg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822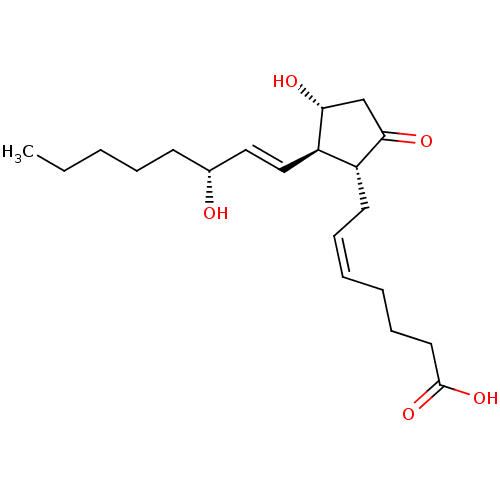 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032699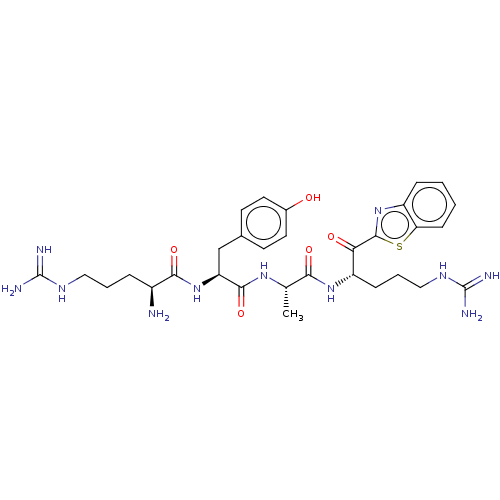 (CHEMBL3354677) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032701 (CHEMBL3354679) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032697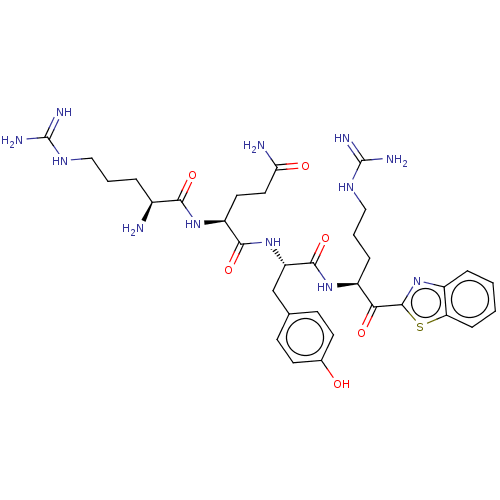 (CHEMBL3354675) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50541723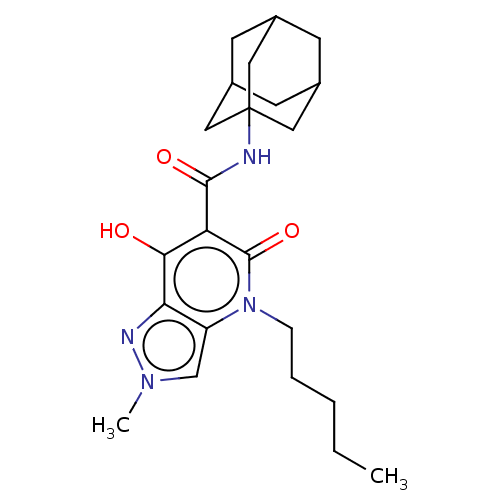 (CHEMBL4649122) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from recombinant human CB2R expressed in HEK293 cell membranes | J Med Chem 63: 7369-7391 (2020) Article DOI: 10.1021/acs.jmedchem.0c00595 BindingDB Entry DOI: 10.7270/Q2N01B3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270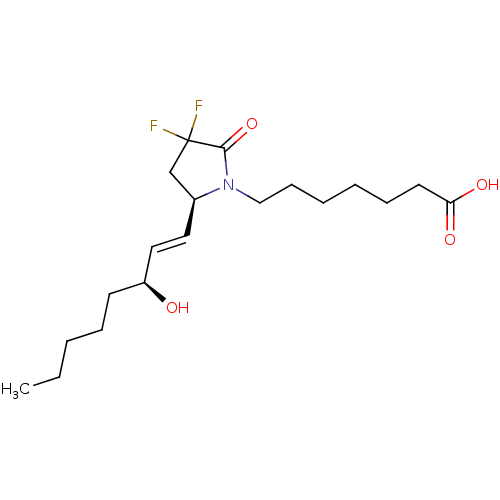 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | 0.570 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032698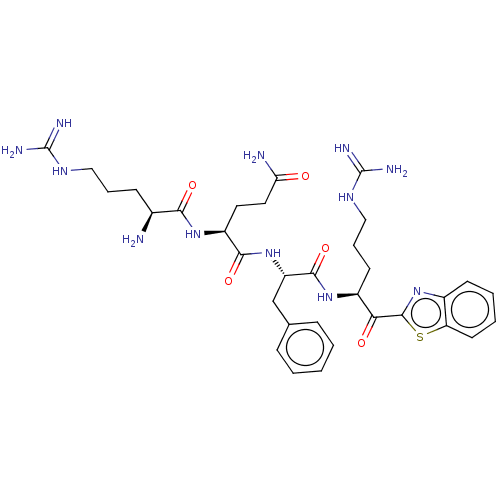 (CHEMBL3354676 | N-0130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005192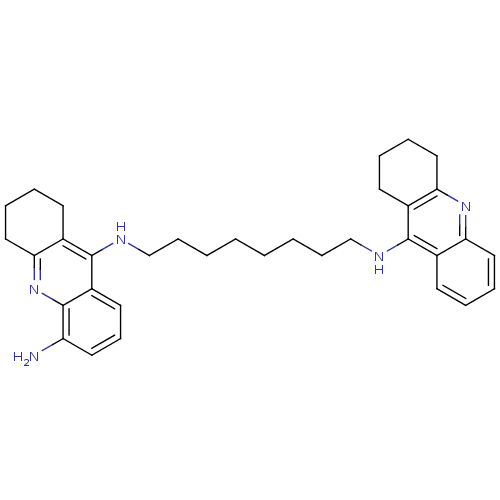 (CHEMBL3099497) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190275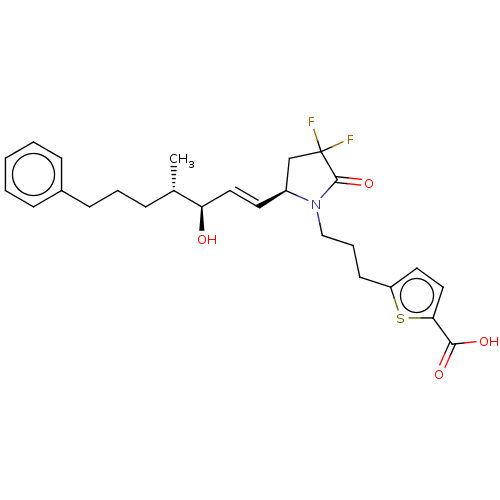 (US9180116, 28C | US9180116, 28H) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | 0.740 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101858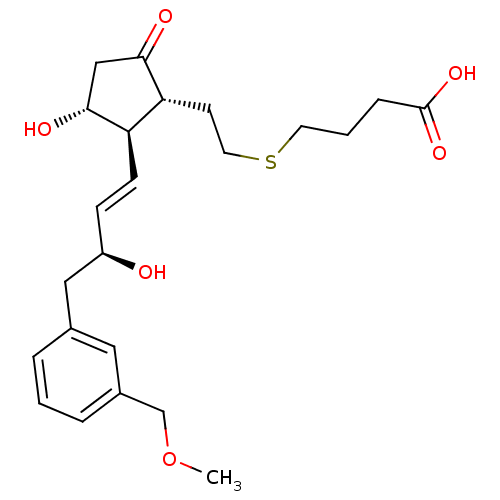 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236918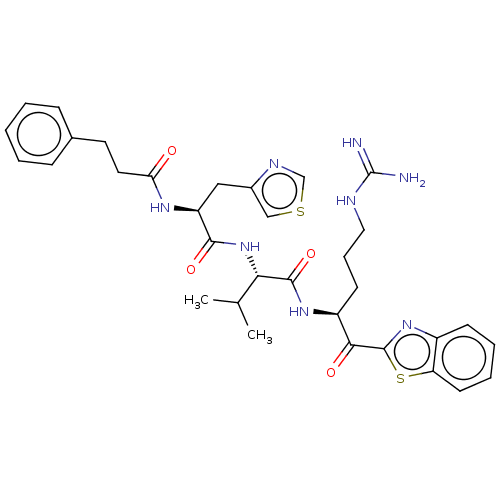 (CHEMBL4081543) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal V5-His-tagged matriptase-2 expressed in Drosophila S2 cells using Boc-Gln-Ala-ArgAMC as substrate measured... | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190267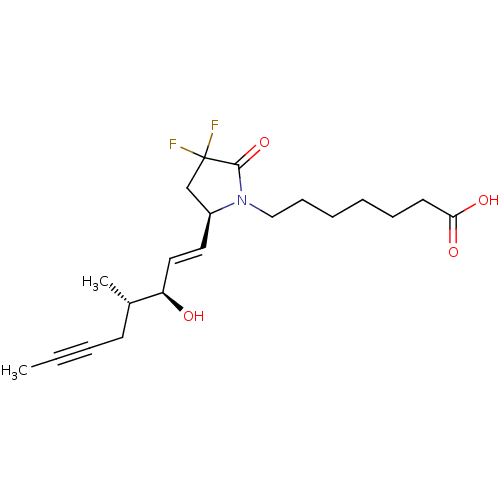 (US9180116, 1F) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032705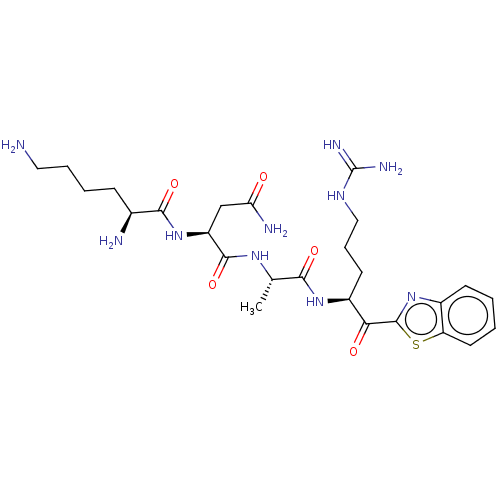 (CHEMBL3354682) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM525153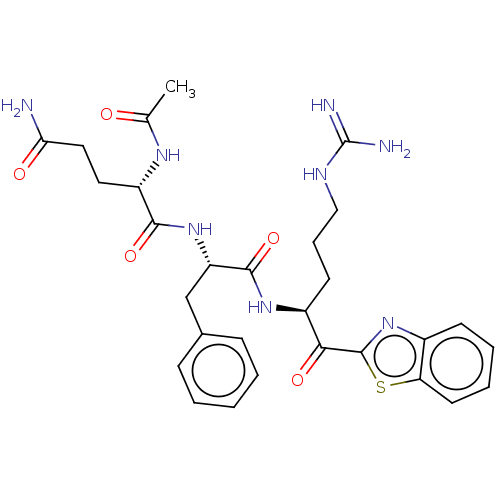 (Ac-QFR-kbt | N-0386 | US10988505, Example 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.506 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Enzymatic assays and Ki determination were performed at room temperature in an assay buffer containing 50 mM Tris-HCl, 150 mM NaCl and 500 μg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2X351MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50356552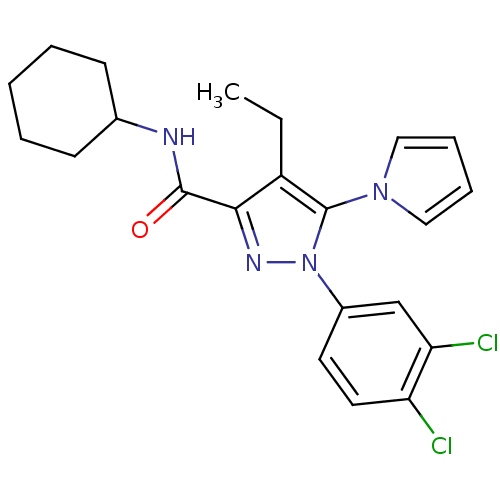 (CHEMBL1909860) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells | Eur J Med Chem 46: 5641-53 (2011) Article DOI: 10.1016/j.ejmech.2011.09.037 BindingDB Entry DOI: 10.7270/Q2P55NXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032700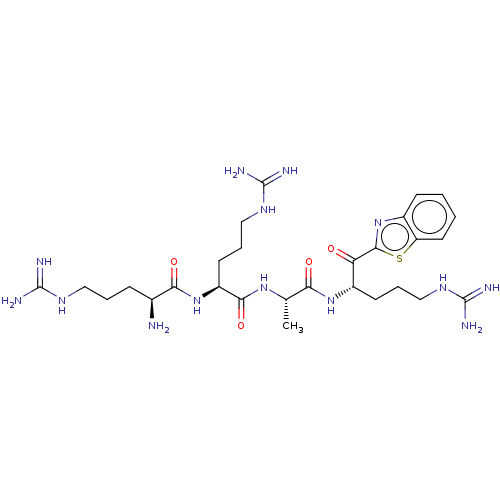 (CHEMBL3354678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032697 (CHEMBL3354675) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005193 (CHEMBL3099496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50271367 (CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190269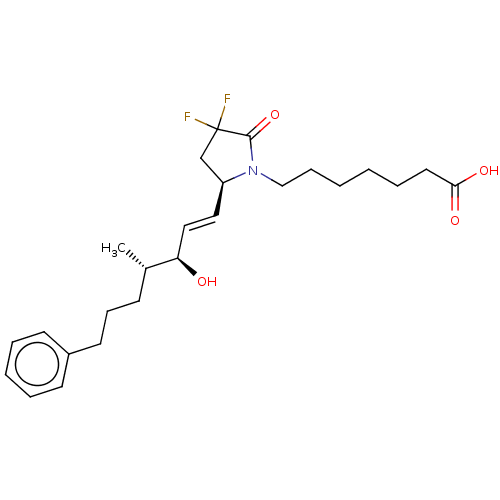 (US9180116, 6D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.890 | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032710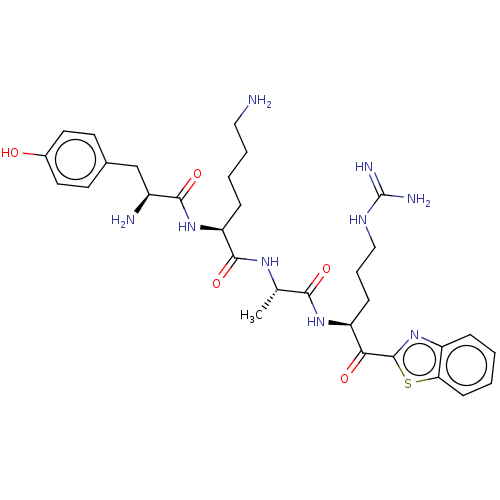 (CHEMBL3354687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50356548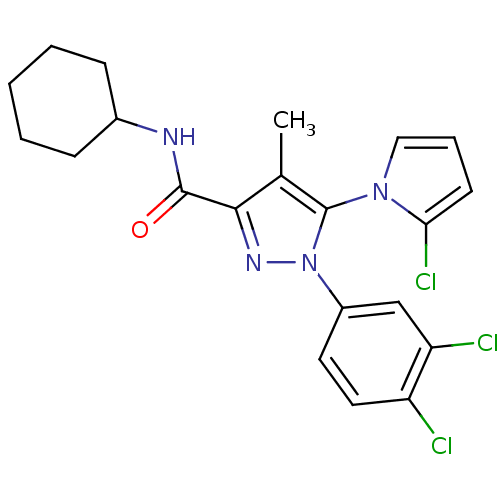 (CHEMBL1909856) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells | Eur J Med Chem 46: 5641-53 (2011) Article DOI: 10.1016/j.ejmech.2011.09.037 BindingDB Entry DOI: 10.7270/Q2P55NXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50541729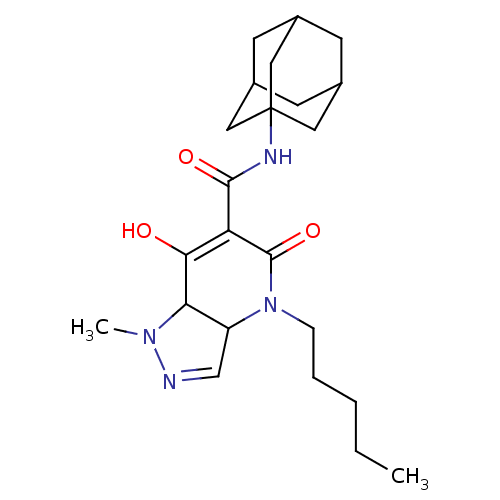 (CHEMBL4637535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from recombinant human CB2R expressed in HEK293 cell membranes | J Med Chem 63: 7369-7391 (2020) Article DOI: 10.1021/acs.jmedchem.0c00595 BindingDB Entry DOI: 10.7270/Q2N01B3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032704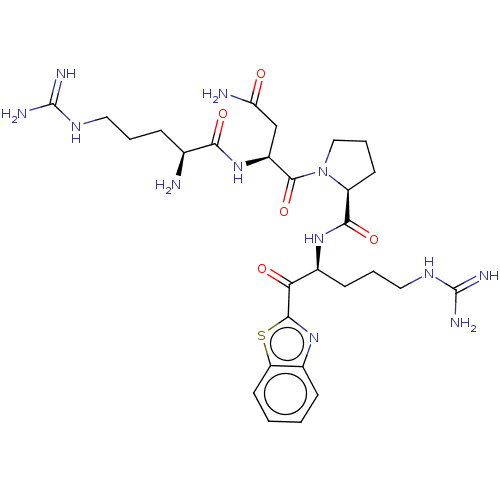 (CHEMBL3354681) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50048364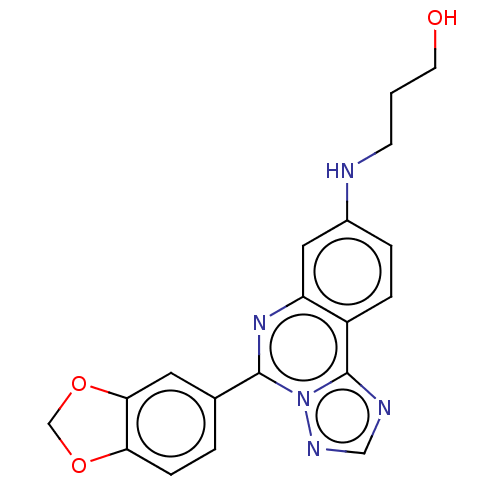 (CHEMBL3309997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Hsp90 (unknown origin) by FP displacement assay | Bioorg Med Chem 22: 4135-50 (2014) Article DOI: 10.1016/j.bmc.2014.05.056 BindingDB Entry DOI: 10.7270/Q2S46TMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032706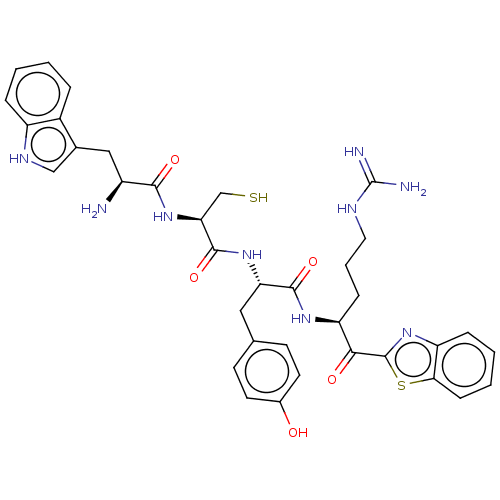 (CHEMBL3354683) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human hepsin expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior to ... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236924 (CHEMBL4093487) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-L-Fucosidase of human placenta | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190274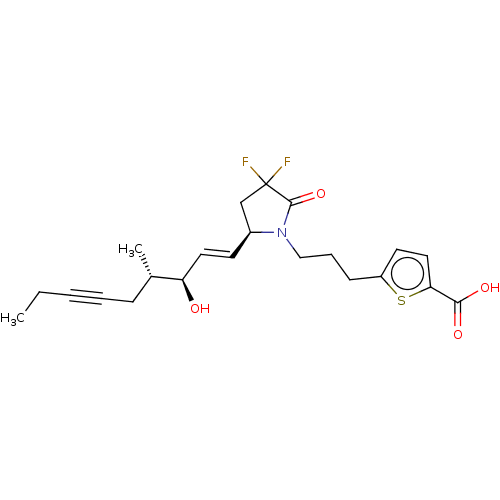 (US9180116, 24D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | 3.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50236911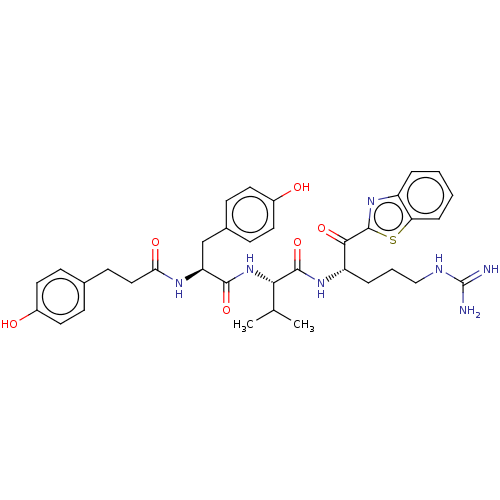 (CHEMBL4103740) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibitory activity against alpha-L-Fucosidase of bovine epididymis expressed as Ki | Eur J Med Chem 129: 110-123 (2017) Article DOI: 10.1016/j.ejmech.2017.02.006 BindingDB Entry DOI: 10.7270/Q2GB269P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50356550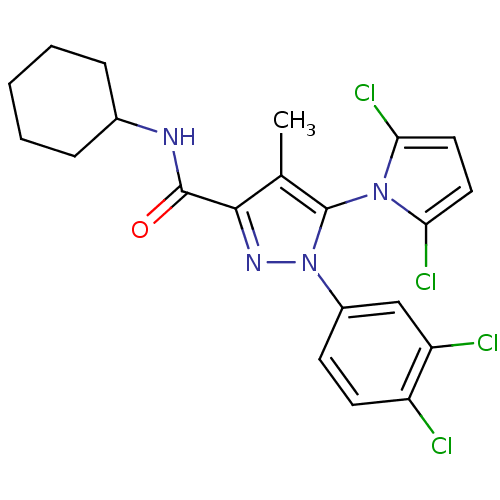 (CHEMBL1909858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human CB2 receptor expressed in HEK cells | Eur J Med Chem 46: 5641-53 (2011) Article DOI: 10.1016/j.ejmech.2011.09.037 BindingDB Entry DOI: 10.7270/Q2P55NXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3005 total ) | Next | Last >> |