 Found 841 hits with Last Name = 'connolly' and Initial = 'c'
Found 841 hits with Last Name = 'connolly' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363283

(CHEMBL1945711)Show SMILES COc1ccc(cc1)C1=[N+]2C(C=C1)=Cc1c(C)c(CCC(=O)NCCCCCC(=O)NCCCn3nc(C(=O)NC4C[C@@H]5CCC[C@@H](C4)N5C)c4ccccc34)c(C)n1[B-]2(F)F |r,c:12,14,t:9,TLB:49:48:43.44.45:47.40.41| Show InChI InChI=1S/C47H59BF2N8O4/c1-31-39(32(2)57-43(31)30-37-19-23-41(58(37)48(57,49)50)33-17-20-38(62-4)21-18-33)22-24-45(60)51-25-9-5-6-16-44(59)52-26-11-27-56-42-15-8-7-14-40(42)46(54-56)47(61)53-34-28-35-12-10-13-36(29-34)55(35)3/h7-8,14-15,17-21,23,30,34-36H,5-6,9-13,16,22,24-29H2,1-4H3,(H,51,60)(H,52,59)(H,53,61)/t35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363287
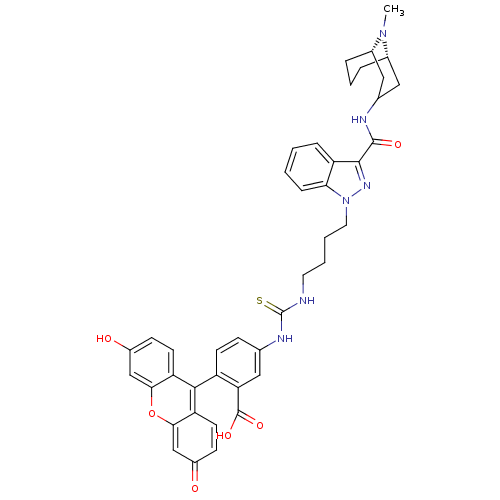
(CHEMBL1945830)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(CCCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c2ccccc12 |r,wU:2.1,6.6,TLB:0:1:3.4.5:7.8.9,(1.85,-18.97,;1.02,-20.27,;.73,-21.74,;.75,-23.57,;-.77,-23.85,;.03,-22.8,;-.02,-21.04,;-1.71,-20.98,;-2,-22.45,;-1.05,-21.7,;-2.48,-23.91,;-3.98,-24.23,;-5.02,-23.09,;-4.46,-25.7,;-3.55,-26.95,;-4.46,-28.21,;-3.69,-29.54,;-2.15,-29.54,;-1.38,-30.88,;.16,-30.88,;.93,-32.21,;2.46,-32.22,;3.19,-30.86,;3.28,-33.53,;2.55,-34.89,;1.02,-34.94,;.29,-36.29,;1.11,-37.6,;2.65,-37.55,;3.37,-36.19,;3.47,-38.85,;5.01,-38.79,;3.93,-40.31,;.39,-38.96,;1.21,-40.26,;2.74,-40.21,;3.55,-41.53,;2.81,-42.89,;3.62,-44.2,;1.27,-42.92,;.47,-41.62,;-1.07,-41.67,;-1.88,-40.36,;-3.4,-40.41,;-4.2,-39.1,;-5.74,-39.14,;-3.48,-37.76,;-1.95,-37.72,;-1.15,-39.02,;-5.94,-27.73,;-7.27,-28.5,;-8.6,-27.73,;-8.6,-26.19,;-7.27,-25.41,;-5.94,-26.18,)| Show InChI InChI=1S/C42H42N6O6S/c1-47-26-7-6-8-27(47)20-25(19-26)44-40(51)39-31-9-2-3-10-35(31)48(46-39)18-5-4-17-43-42(55)45-24-11-14-30(34(21-24)41(52)53)38-32-15-12-28(49)22-36(32)54-37-23-29(50)13-16-33(37)38/h2-3,9-16,21-23,25-27,49H,4-8,17-20H2,1H3,(H,44,51)(H,52,53)(H2,43,45,55)/t26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363291

(CHEMBL1945835)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCn1nc(C(=O)NC2C[C@@H]3CCC[C@@H](C2)N3C)c2ccccc12 |r,TLB:35:34:29.30.31:33.26.27| Show InChI InChI=1S/C32H40N6O3S/c1-36(2)28-16-7-14-26-25(28)13-8-17-30(26)42(40,41)33-18-9-19-38-29-15-5-4-12-27(29)31(35-38)32(39)34-22-20-23-10-6-11-24(21-22)37(23)3/h4-5,7-8,12-17,22-24,33H,6,9-11,18-21H2,1-3H3,(H,34,39)/t23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50000483
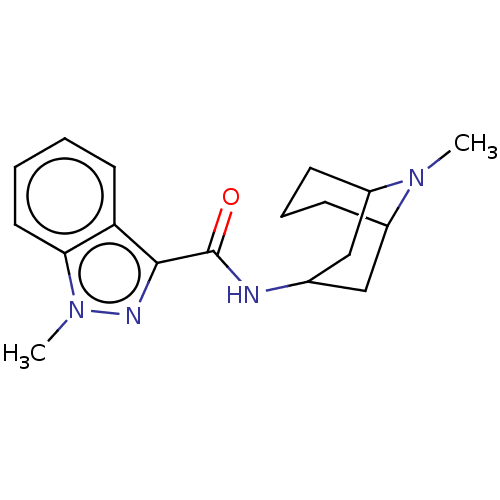
((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363285
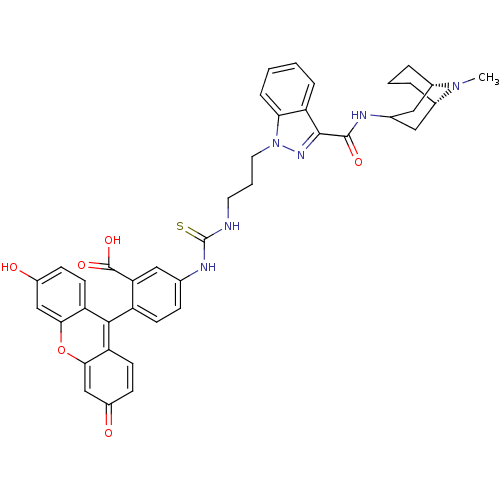
(CHEMBL1945713)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(CCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c2ccccc12 |r,wU:2.1,6.6,TLB:0:1:3.4.5:7.8.9,(1.46,5.41,;.63,4.1,;.34,2.63,;.36,.8,;-1.15,.53,;-.36,1.57,;-.41,3.34,;-2.1,3.39,;-2.39,1.93,;-1.44,2.67,;-2.87,.46,;-4.37,.14,;-5.4,1.28,;-4.85,-1.33,;-3.94,-2.58,;-4.85,-3.84,;-4.08,-5.17,;-2.54,-5.17,;-1.77,-6.51,;-.23,-6.51,;.54,-5.17,;-.23,-3.84,;2.08,-5.17,;2.85,-6.51,;2.08,-7.84,;2.85,-9.17,;4.39,-9.18,;5.16,-7.83,;4.39,-6.5,;6.7,-7.83,;7.47,-6.49,;8.19,-8.22,;5.16,-10.5,;6.7,-10.5,;7.47,-9.17,;9.02,-9.18,;9.78,-10.52,;11.32,-10.54,;8.99,-11.85,;7.46,-11.84,;6.69,-13.17,;5.15,-13.17,;4.39,-14.49,;2.85,-14.48,;2.07,-15.8,;2.1,-13.14,;2.87,-11.83,;4.4,-11.84,;-6.32,-3.36,;-7.66,-4.13,;-8.99,-3.36,;-8.99,-1.81,;-7.66,-1.04,;-6.32,-1.81,)| Show InChI InChI=1S/C41H40N6O6S/c1-46-25-6-4-7-26(46)19-24(18-25)43-39(50)38-30-8-2-3-9-34(30)47(45-38)17-5-16-42-41(54)44-23-10-13-29(33(20-23)40(51)52)37-31-14-11-27(48)21-35(31)53-36-22-28(49)12-15-32(36)37/h2-3,8-15,20-22,24-26,48H,4-7,16-19H2,1H3,(H,43,50)(H,51,52)(H2,42,44,54)/t25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363292
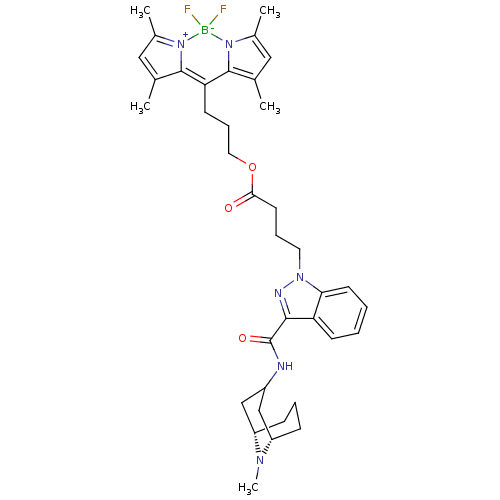
(CHEMBL1945836)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(CCCC(=O)OCCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)c2ccccc12 |r,c:27,30,33,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C37H47BF2N6O3/c1-23-19-25(3)45-35(23)31(36-24(2)20-26(4)46(36)38(45,39)40)14-10-18-49-33(47)16-9-17-44-32-15-7-6-13-30(32)34(42-44)37(48)41-27-21-28-11-8-12-29(22-27)43(28)5/h6-7,13,15,19-20,27-29H,8-12,14,16-18,21-22H2,1-5H3,(H,41,48)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363293
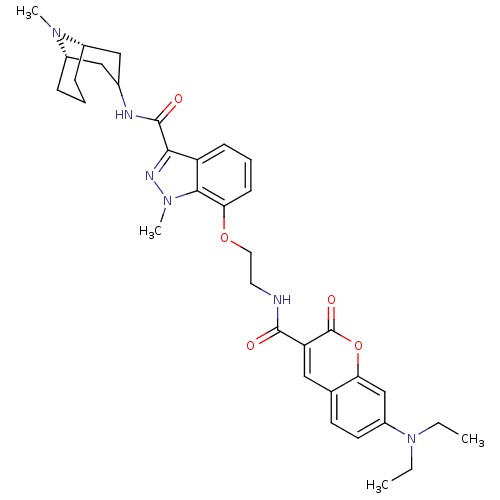
(CHEMBL1945837)Show SMILES CCN(CC)c1ccc2cc(C(=O)NCCOc3cccc4c(nn(C)c34)C(=O)NC3C[C@@H]4CCC[C@@H](C3)N4C)c(=O)oc2c1 |r,TLB:39:38:33.34.35:37.30.31| Show InChI InChI=1S/C34H42N6O5/c1-5-40(6-2)25-14-13-21-17-27(34(43)45-29(21)20-25)32(41)35-15-16-44-28-12-8-11-26-30(37-39(4)31(26)28)33(42)36-22-18-23-9-7-10-24(19-22)38(23)3/h8,11-14,17,20,22-24H,5-7,9-10,15-16,18-19H2,1-4H3,(H,35,41)(H,36,42)/t23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363286

(CHEMBL1945714)Show SMILES CCN(CC)c1ccc2cc(C(=O)NCCCCn3nc(C(=O)NC4C[C@@H]5CCC[C@@H](C4)N5C)c4ccccc34)c(=O)oc2c1 |r,TLB:33:32:27.28.29:31.24.25| Show InChI InChI=1S/C35H44N6O4/c1-4-40(5-2)27-16-15-23-19-29(35(44)45-31(23)22-27)33(42)36-17-8-9-18-41-30-14-7-6-13-28(30)32(38-41)34(43)37-24-20-25-11-10-12-26(21-24)39(25)3/h6-7,13-16,19,22,24-26H,4-5,8-12,17-18,20-21H2,1-3H3,(H,36,42)(H,37,43)/t25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363290
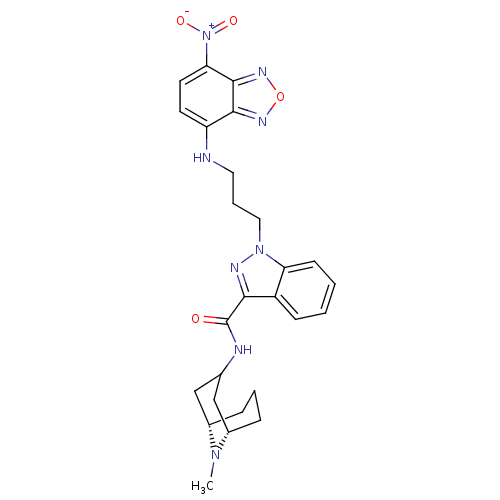
(CHEMBL1945834)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(CCCNc2ccc([N+]([O-])=O)c3nonc23)c2ccccc12 |r,TLB:0:1:3.4.5:7.8.9| Show InChI InChI=1S/C26H30N8O4/c1-32-17-6-4-7-18(32)15-16(14-17)28-26(35)23-19-8-2-3-9-21(19)33(29-23)13-5-12-27-20-10-11-22(34(36)37)25-24(20)30-38-31-25/h2-3,8-11,16-18,27H,4-7,12-15H2,1H3,(H,28,35)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM3443
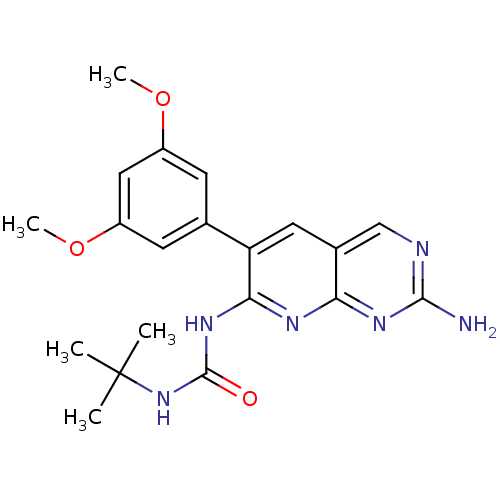
(1-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyr...)Show SMILES COc1cc(OC)cc(c1)-c1cc2cnc(N)nc2nc1NC(=O)NC(C)(C)C Show InChI InChI=1S/C20H24N6O3/c1-20(2,3)26-19(27)25-17-15(8-12-10-22-18(21)24-16(12)23-17)11-6-13(28-4)9-14(7-11)29-5/h6-10H,1-5H3,(H4,21,22,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 569-77 (1998)
BindingDB Entry DOI: 10.7270/Q2C24TZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363288
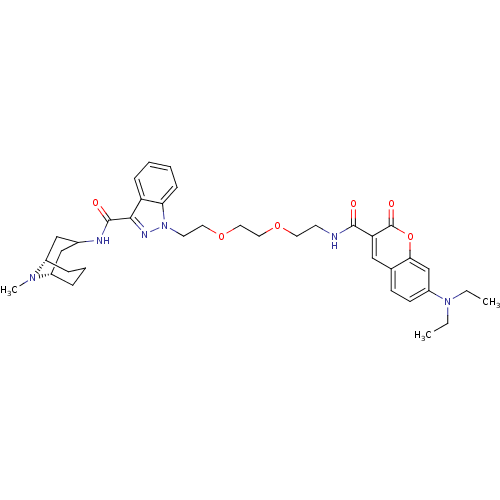
(CHEMBL1945832)Show SMILES CCN(CC)c1ccc2cc(C(=O)NCCOCCOCCn3nc(C(=O)NC4C[C@@H]5CCC[C@@H](C4)N5C)c4ccccc34)c(=O)oc2c1 |r,TLB:37:36:31.32.33:35.28.29| Show InChI InChI=1S/C37H48N6O6/c1-4-42(5-2)29-14-13-25-21-31(37(46)49-33(25)24-29)35(44)38-15-17-47-19-20-48-18-16-43-32-12-7-6-11-30(32)34(40-43)36(45)39-26-22-27-9-8-10-28(23-26)41(27)3/h6-7,11-14,21,24,26-28H,4-5,8-10,15-20,22-23H2,1-3H3,(H,38,44)(H,39,45)/t27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363294
(CHEMBL1945838)Show SMILES CCN(CC)c1ccc2cc(C(=O)NCCCOc3cccc4c(nn(C)c34)C(=O)NC3C[C@@H]4CCC[C@@H](C3)N4C)c(=O)oc2c1 |r,TLB:40:39:34.35.36:38.31.32| Show InChI InChI=1S/C35H44N6O5/c1-5-41(6-2)26-15-14-22-18-28(35(44)46-30(22)21-26)33(42)36-16-9-17-45-29-13-8-12-27-31(38-40(4)32(27)29)34(43)37-23-19-24-10-7-11-25(20-23)39(24)3/h8,12-15,18,21,23-25H,5-7,9-11,16-17,19-20H2,1-4H3,(H,36,42)(H,37,43)/t24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363284
(CHEMBL1945712)Show SMILES CCN(CC)c1ccc2cc(C(=O)NCCCn3nc(C(=O)NC4C[C@@H]5CCC[C@@H](C4)N5C)c4ccccc34)c(=O)oc2c1 |r,TLB:32:31:26.27.28:30.23.24| Show InChI InChI=1S/C34H42N6O4/c1-4-39(5-2)26-15-14-22-18-28(34(43)44-30(22)21-26)32(41)35-16-9-17-40-29-13-7-6-12-27(29)31(37-40)33(42)36-23-19-24-10-8-11-25(20-23)38(24)3/h6-7,12-15,18,21,23-25H,4-5,8-11,16-17,19-20H2,1-3H3,(H,35,41)(H,36,42)/t24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363296

(CHEMBL1946302)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(C)c2c(OCCOCCOCCNC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cccc12 |r,wU:2.1,6.6,TLB:0:1:3.4.5:7.8.9,(27.71,-22.83,;26.88,-24.13,;26.59,-25.6,;26.61,-27.43,;25.1,-27.71,;25.89,-26.66,;25.84,-24.9,;24.15,-24.84,;23.86,-26.31,;24.81,-25.56,;23.39,-27.77,;21.88,-28.09,;20.85,-26.95,;21.4,-29.56,;22.32,-30.81,;21.4,-32.07,;21.88,-33.53,;19.93,-31.59,;18.6,-32.36,;18.6,-33.9,;19.93,-34.67,;19.93,-36.21,;21.26,-36.98,;22.6,-36.21,;23.93,-36.98,;25.27,-36.21,;26.6,-36.98,;27.93,-36.21,;29.27,-36.98,;30.6,-36.21,;30.63,-34.67,;31.93,-36.99,;33.27,-36.24,;34.58,-37.02,;35.93,-36.27,;35.94,-34.73,;34.61,-33.94,;33.27,-34.7,;34.62,-32.4,;33.29,-31.62,;35.02,-30.91,;37.28,-33.97,;37.29,-32.44,;35.96,-31.64,;35.99,-30.11,;37.34,-29.36,;37.37,-27.82,;38.66,-30.15,;38.64,-31.69,;39.96,-32.47,;39.94,-34.01,;41.26,-34.78,;41.23,-36.32,;42.56,-37.11,;39.9,-37.07,;38.58,-36.28,;38.6,-34.76,;17.26,-31.59,;17.26,-30.05,;18.59,-29.27,;19.93,-30.04,)| Show InChI InChI=1S/C45H48N6O9S/c1-50-28-5-3-6-29(50)22-27(21-28)47-43(54)41-35-7-4-8-37(42(35)51(2)49-41)59-20-19-58-18-17-57-16-15-46-45(61)48-26-9-12-32(36(23-26)44(55)56)40-33-13-10-30(52)24-38(33)60-39-25-31(53)11-14-34(39)40/h4,7-14,23-25,27-29,52H,3,5-6,15-22H2,1-2H3,(H,47,54)(H,55,56)(H2,46,48,61)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363295

(CHEMBL1946154)Show SMILES CCN(CC)c1ccc2cc(C(=O)NCCOCCOCCOc3cccc4c(nn(C)c34)C(=O)NC3C[C@@H]4CCC[C@@H](C3)N4C)c(=O)oc2c1 |r,TLB:45:44:39.40.41:43.36.37| Show InChI InChI=1S/C38H50N6O7/c1-5-44(6-2)29-14-13-25-21-31(38(47)51-33(25)24-29)36(45)39-15-16-48-17-18-49-19-20-50-32-12-8-11-30-34(41-43(4)35(30)32)37(46)40-26-22-27-9-7-10-28(23-26)42(27)3/h8,11-14,21,24,26-28H,5-7,9-10,15-20,22-23H2,1-4H3,(H,39,45)(H,40,46)/t27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50363289

(CHEMBL1945833)Show SMILES CN1[C@H]2CCC[C@H]1CC(C2)NC(=O)c1nn(CCOCCOCCNC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c2ccccc12 |r,wU:2.1,6.6,TLB:0:1:3.4.5:7.8.9,(26.42,5.72,;25.59,4.42,;25.3,2.95,;25.32,1.12,;23.81,.85,;24.6,1.89,;24.56,3.65,;22.86,3.71,;22.57,2.24,;23.53,2.99,;22.1,.78,;20.59,.46,;19.56,1.6,;20.11,-1.01,;21.03,-2.26,;20.11,-3.52,;20.88,-4.85,;22.42,-4.85,;23.2,-3.52,;24.74,-3.52,;25.51,-4.85,;27.05,-4.85,;27.82,-3.52,;29.36,-3.52,;30.13,-4.86,;31.67,-4.85,;32.4,-3.49,;32.49,-6.16,;31.77,-7.52,;30.23,-7.57,;29.5,-8.92,;30.32,-10.23,;31.86,-10.18,;32.58,-8.82,;32.68,-11.48,;34.22,-11.42,;33.14,-12.94,;29.6,-11.59,;30.42,-12.89,;31.95,-12.84,;32.76,-14.16,;32.02,-15.52,;32.83,-16.83,;30.49,-15.55,;29.68,-14.25,;28.14,-14.3,;27.33,-12.99,;25.81,-13.04,;25.01,-11.73,;23.47,-11.77,;25.73,-10.39,;27.26,-10.35,;28.06,-11.65,;18.64,-3.04,;17.3,-3.81,;15.97,-3.04,;15.97,-1.49,;17.3,-.72,;18.64,-1.49,)| Show InChI InChI=1S/C44H46N6O8S/c1-49-28-5-4-6-29(49)22-27(21-28)46-42(53)41-33-7-2-3-8-37(33)50(48-41)16-18-57-20-19-56-17-15-45-44(59)47-26-9-12-32(36(23-26)43(54)55)40-34-13-10-30(51)24-38(34)58-39-25-31(52)11-14-35(39)40/h2-3,7-14,23-25,27-29,51H,4-6,15-22H2,1H3,(H,46,53)(H,54,55)(H2,45,47,59)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5HT3A expressed in HEK293 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 22: 1151-5 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.097
BindingDB Entry DOI: 10.7270/Q237795K |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006848

(CHEMBL88091 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cyc...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCNCC1 Show InChI InChI=1S/C33H53N7O6S2/c1-22(2)17-29(41)30(42)26(18-23-9-5-3-6-10-23)37-31(43)27(20-25-21-47-33(34)36-25)38-32(44)28(19-24-11-7-4-8-12-24)39-48(45,46)40-15-13-35-14-16-40/h4,7-8,11-12,21-23,26-30,35,39,41-42H,3,5-6,9-10,13-20H2,1-2H3,(H2,34,36)(H,37,43)(H,38,44)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006850

(CHEMBL313063 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cscn1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H51N5O7S2/c1-23(2)17-30(39)31(40)27(18-24-9-5-3-6-10-24)35-32(41)28(20-26-21-46-22-34-26)36-33(42)29(19-25-11-7-4-8-12-25)37-47(43,44)38-13-15-45-16-14-38/h4,7-8,11-12,21-24,27-31,37,39-40H,3,5-6,9-10,13-20H2,1-2H3,(H,35,41)(H,36,42)/t27-,28-,29-,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006858
(CHEMBL315380 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S/c1-23(2)17-30(40)31(41)27(18-24-9-5-3-6-10-24)36-32(42)28(20-26-21-34-22-35-26)37-33(43)29(19-25-11-7-4-8-12-25)38-47(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-24,27-31,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H,34,35)(H,36,42)(H,37,43)/t27-,28-,29-,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006854

(CHEMBL88069 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cyc...)Show SMILES CC[C@H](O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C32H50N6O7S2/c1-2-25(39)20-29(40)26(17-22-9-5-3-6-10-22)35-30(41)27(19-24-21-46-32(33)34-24)36-31(42)28(18-23-11-7-4-8-12-23)37-47(43,44)38-13-15-45-16-14-38/h4,7-8,11-12,21-22,25-29,37,39-40H,2-3,5-6,9-10,13-20H2,1H3,(H2,33,34)(H,35,41)(H,36,42)/t25-,26-,27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006853
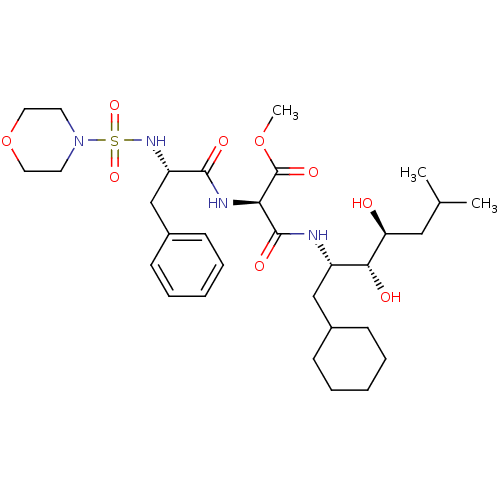
(CHEMBL315211 | N-(1-Cyclohexylmethyl-2,3-dihydroxy...)Show SMILES COC(=O)[C@H](NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C31H50N4O9S/c1-21(2)18-26(36)28(37)24(19-22-10-6-4-7-11-22)32-30(39)27(31(40)43-3)33-29(38)25(20-23-12-8-5-9-13-23)34-45(41,42)35-14-16-44-17-15-35/h5,8-9,12-13,21-22,24-28,34,36-37H,4,6-7,10-11,14-20H2,1-3H3,(H,32,39)(H,33,38)/t24-,25-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006845
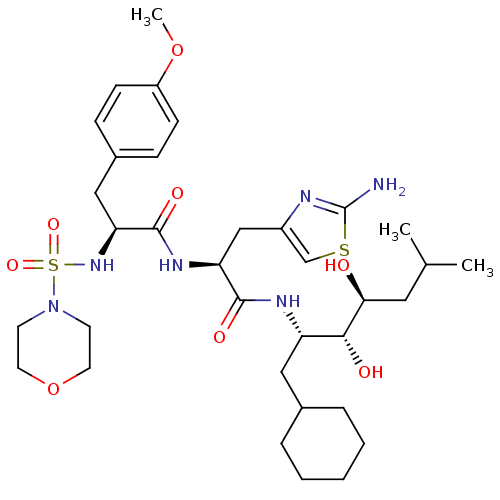
(CHEMBL316208 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...)Show SMILES COc1ccc(C[C@H](NS(=O)(=O)N2CCOCC2)C(=O)N[C@@H](Cc2csc(N)n2)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)[C@@H](O)CC(C)C)cc1 Show InChI InChI=1S/C34H54N6O8S2/c1-22(2)17-30(41)31(42)27(18-23-7-5-4-6-8-23)37-32(43)28(20-25-21-49-34(35)36-25)38-33(44)29(19-24-9-11-26(47-3)12-10-24)39-50(45,46)40-13-15-48-16-14-40/h9-12,21-23,27-31,39,41-42H,4-8,13-20H2,1-3H3,(H2,35,36)(H,37,43)(H,38,44)/t27-,28-,29-,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815

(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006844
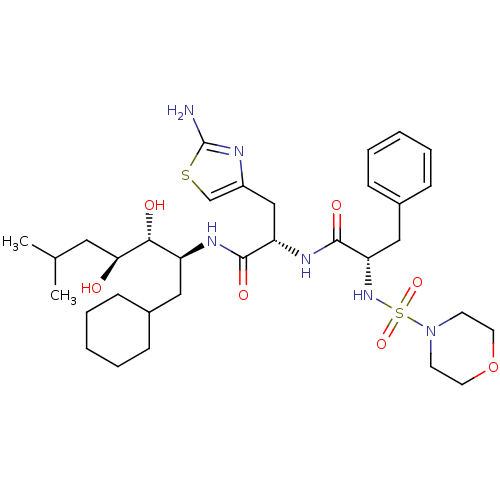
((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S2/c1-22(2)17-29(40)30(41)26(18-23-9-5-3-6-10-23)36-31(42)27(20-25-21-47-33(34)35-25)37-32(43)28(19-24-11-7-4-8-12-24)38-48(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-23,26-30,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H2,34,35)(H,36,42)(H,37,43)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006844

((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S2/c1-22(2)17-29(40)30(41)26(18-23-9-5-3-6-10-23)36-31(42)27(20-25-21-47-33(34)35-25)37-32(43)28(19-24-11-7-4-8-12-24)38-48(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-23,26-30,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H2,34,35)(H,36,42)(H,37,43)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of plasma renin activity in monkey |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230906
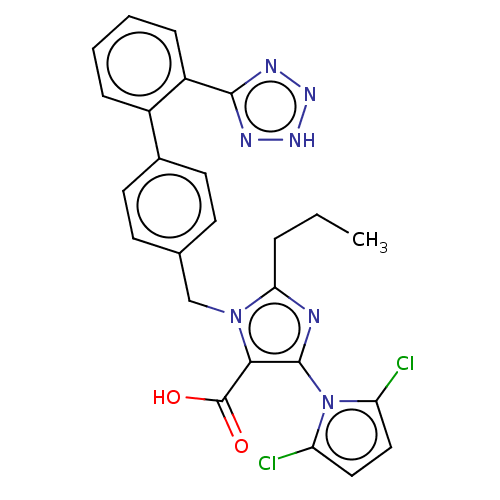
(CHEMBL308261)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(Cl)ccc1Cl |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;19.81,-14.18,;21.14,-13.41,;20.29,-15.64,;19.05,-16.57,;17.81,-15.66,;16.33,-16.06,)| Show InChI InChI=1S/C25H21Cl2N7O2/c1-2-5-21-28-24(34-19(26)12-13-20(34)27)22(25(35)36)33(21)14-15-8-10-16(11-9-15)17-6-3-4-7-18(17)23-29-31-32-30-23/h3-4,6-13H,2,5,14H2,1H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006851

(CHEMBL263531 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C36H54N4O7S/c1-26(2)22-33(41)34(42)30(23-27-12-6-3-7-13-27)37-35(43)31(24-28-14-8-4-9-15-28)38-36(44)32(25-29-16-10-5-11-17-29)39-48(45,46)40-18-20-47-21-19-40/h4-5,8-11,14-17,26-27,30-34,39,41-42H,3,6-7,12-13,18-25H2,1-2H3,(H,37,43)(H,38,44)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006856
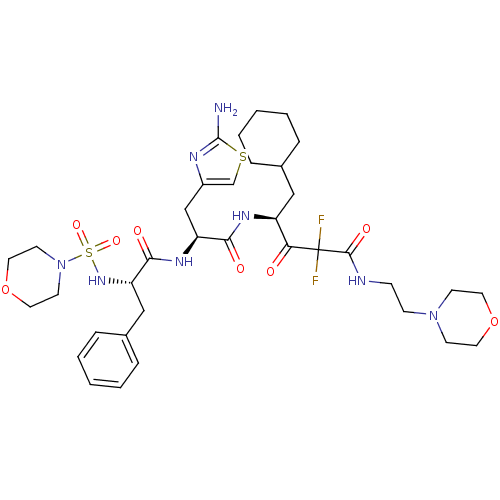
(4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...)Show SMILES Nc1nc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NS(=O)(=O)N2CCOCC2)C(=O)N[C@@H](CC2CCCCC2)C(=O)C(F)(F)C(=O)NCCN2CCOCC2)cs1 Show InChI InChI=1S/C36H52F2N8O8S2/c37-36(38,34(50)40-11-12-45-13-17-53-18-14-45)31(47)28(21-25-7-3-1-4-8-25)42-32(48)29(23-27-24-55-35(39)41-27)43-33(49)30(22-26-9-5-2-6-10-26)44-56(51,52)46-15-19-54-20-16-46/h2,5-6,9-10,24-25,28-30,44H,1,3-4,7-8,11-23H2,(H2,39,41)(H,40,50)(H,42,48)(H,43,49)/t28-,29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50009873
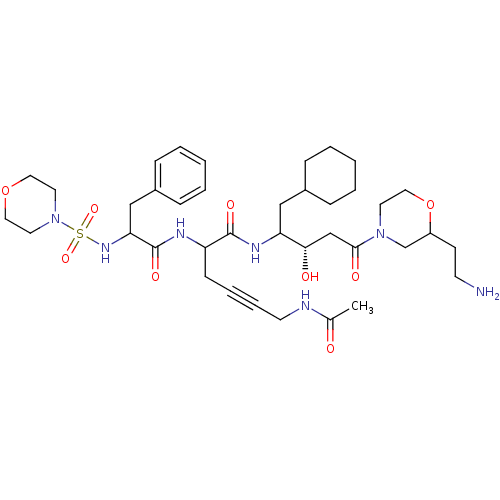
(6-Acetylamino-2-[2-(morpholine-4-sulfonylamino)-3-...)Show SMILES CC(=O)NCC#CCC(NC(=O)C(Cc1ccccc1)NS(=O)(=O)N1CCOCC1)C(=O)NC(CC1CCCCC1)[C@@H](O)CC(=O)N1CCOC(CCN)C1 Show InChI InChI=1S/C38H59N7O9S/c1-28(46)40-17-9-8-14-32(41-38(50)34(25-30-12-6-3-7-13-30)43-55(51,52)45-19-21-53-22-20-45)37(49)42-33(24-29-10-4-2-5-11-29)35(47)26-36(48)44-18-23-54-31(27-44)15-16-39/h3,6-7,12-13,29,31-35,43,47H,2,4-5,10-11,14-27,39H2,1H3,(H,40,46)(H,41,50)(H,42,49)/t31?,32?,33?,34?,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against monkey plasma renin. |
J Med Chem 34: 1258-71 (1991)
BindingDB Entry DOI: 10.7270/Q21C1VT6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006846
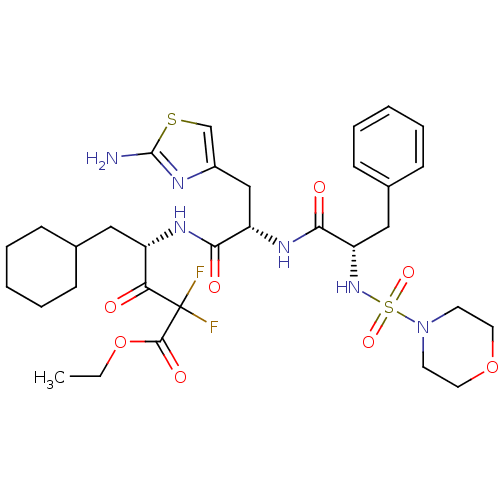
(4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...)Show SMILES CCOC(=O)C(F)(F)C(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C32H44F2N6O8S2/c1-2-48-30(44)32(33,34)27(41)24(17-21-9-5-3-6-10-21)37-28(42)25(19-23-20-49-31(35)36-23)38-29(43)26(18-22-11-7-4-8-12-22)39-50(45,46)40-13-15-47-16-14-40/h4,7-8,11-12,20-21,24-26,39H,2-3,5-6,9-10,13-19H2,1H3,(H2,35,36)(H,37,42)(H,38,43)/t24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006844

((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S2/c1-22(2)17-29(40)30(41)26(18-23-9-5-3-6-10-23)36-31(42)27(20-25-21-47-33(34)35-25)37-32(43)28(19-24-11-7-4-8-12-24)38-48(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-23,26-30,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H2,34,35)(H,36,42)(H,37,43)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of plasma renin activity in human |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230919
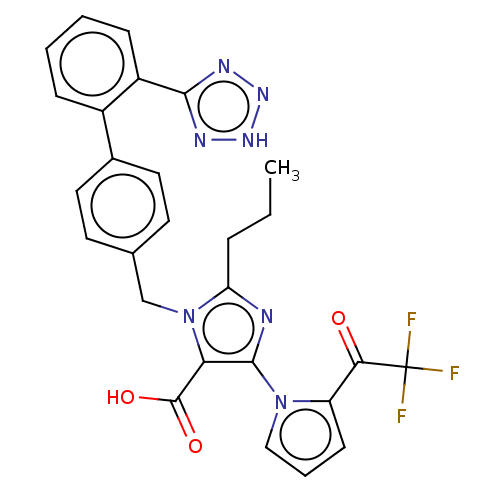
(CHEMBL307844 | CI-996)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006843

(CHEMBL314066 | N-[(2-Amino-thiazol-4-yl)-(1-cycloh...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)C(NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1)c1csc(N)n1 Show InChI InChI=1S/C32H50N6O7S2/c1-21(2)17-27(39)29(40)24(18-22-9-5-3-6-10-22)34-31(42)28(26-20-46-32(33)35-26)36-30(41)25(19-23-11-7-4-8-12-23)37-47(43,44)38-13-15-45-16-14-38/h4,7-8,11-12,20-22,24-25,27-29,37,39-40H,3,5-6,9-10,13-19H2,1-2H3,(H2,33,35)(H,34,42)(H,36,41)/t24-,25-,27-,28?,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.581 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006849
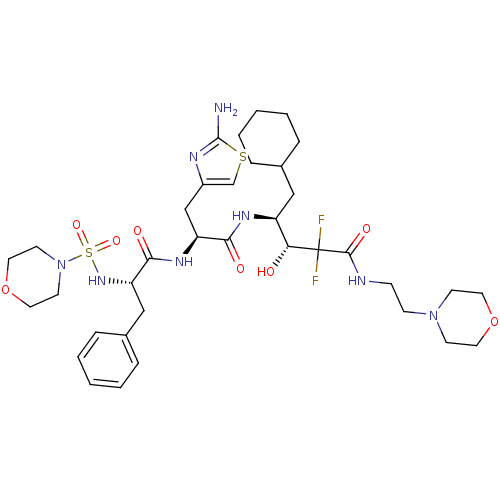
(4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...)Show SMILES Nc1nc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NS(=O)(=O)N2CCOCC2)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(F)(F)C(=O)NCCN2CCOCC2)cs1 Show InChI InChI=1S/C36H54F2N8O8S2/c37-36(38,34(50)40-11-12-45-13-17-53-18-14-45)31(47)28(21-25-7-3-1-4-8-25)42-32(48)29(23-27-24-55-35(39)41-27)43-33(49)30(22-26-9-5-2-6-10-26)44-56(51,52)46-15-19-54-20-16-46/h2,5-6,9-10,24-25,28-31,44,47H,1,3-4,7-8,11-23H2,(H2,39,41)(H,40,50)(H,42,48)(H,43,49)/t28-,29-,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006202

(3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COc1ccc(C[C@H](NC(=O)CC(C)(C)N)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)[C@@H](O)CC(C)C)cc1 Show InChI InChI=1S/C35H56N6O6/c1-22(2)15-30(42)32(44)27(16-23-9-7-6-8-10-23)40-34(46)29(18-25-20-37-21-38-25)41-33(45)28(39-31(43)19-35(3,4)36)17-24-11-13-26(47-5)14-12-24/h11-14,20-23,27-30,32,42,44H,6-10,15-19,36H2,1-5H3,(H,37,38)(H,39,43)(H,40,46)(H,41,45)/t27-,28-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230891

(CHEMBL76166)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C=O Show InChI InChI=1S/C26H23N7O3/c1-2-6-22-27-25(32-14-5-7-19(32)16-34)23(26(35)36)33(22)15-17-10-12-18(13-11-17)20-8-3-4-9-21(20)24-28-30-31-29-24/h3-5,7-14,16H,2,6,15H2,1H3,(H,35,36)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230883
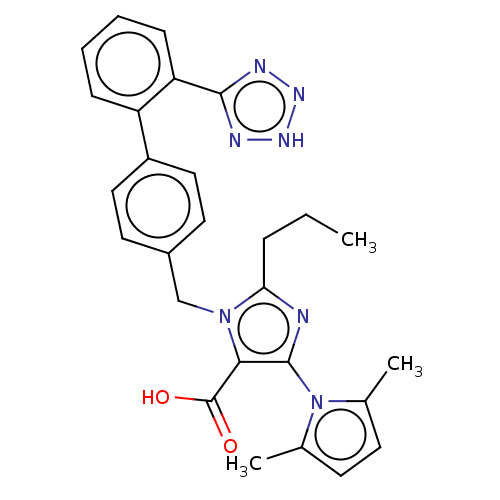
(CHEMBL309089)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;17.81,-15.66,;16.36,-16.14,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,)| Show InChI InChI=1S/C27H27N7O2/c1-4-7-23-28-26(34-17(2)10-11-18(34)3)24(27(35)36)33(23)16-19-12-14-20(15-13-19)21-8-5-6-9-22(21)25-29-31-32-30-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17941
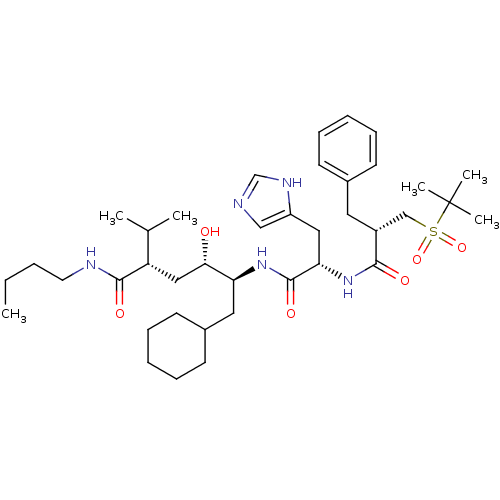
((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230915
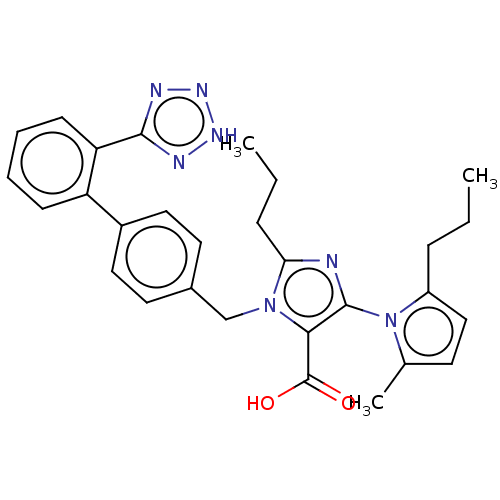
(CHEMBL76169)Show SMILES CCCc1ccc(C)n1-c1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c1C(O)=O |(13.76,-15.58,;15.23,-15.1,;16.36,-16.14,;17.81,-15.66,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,;18.28,-14.2,;17.36,-12.96,;15.83,-12.96,;15.36,-11.5,;13.89,-11.03,;12.74,-12.07,;11.28,-11.6,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,)| Show InChI InChI=1S/C29H31N7O2/c1-4-8-22-17-12-19(3)36(22)28-26(29(37)38)35(25(30-28)9-5-2)18-20-13-15-21(16-14-20)23-10-6-7-11-24(23)27-31-33-34-32-27/h6-7,10-17H,4-5,8-9,18H2,1-3H3,(H,37,38)(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006860

(CHEMBL85672 | {1-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NP(=O)(OC(C)C)OC(C)C Show InChI InChI=1S/C35H58N5O7PS/c1-22(2)17-31(41)32(42)28(18-25-13-9-7-10-14-25)38-33(43)29(20-27-21-49-35(36)37-27)39-34(44)30(19-26-15-11-8-12-16-26)40-48(45,46-23(3)4)47-24(5)6/h8,11-12,15-16,21-25,28-32,41-42H,7,9-10,13-14,17-20H2,1-6H3,(H2,36,37)(H,38,43)(H,39,44)(H,40,45)/t28-,29-,30-,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of monkey renin. |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230887
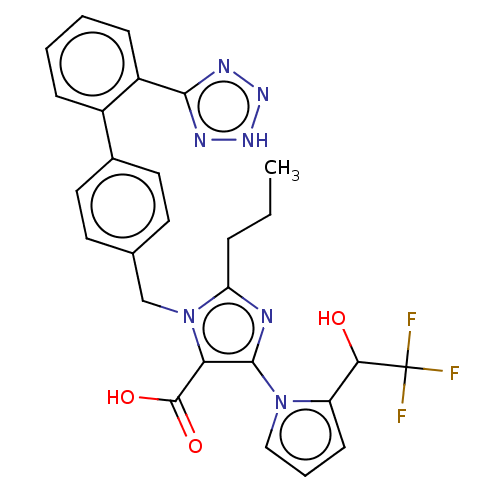
(CHEMBL306259)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(O)C(F)(F)F Show InChI InChI=1S/C27H24F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14,23,38H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215294

(2-(N-(6-chloro-7-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CC(O)=O)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H12ClN3O6S/c1-5-3-6-9(15-12(20)11(19)14-6)10(8(5)13)16(4-7(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
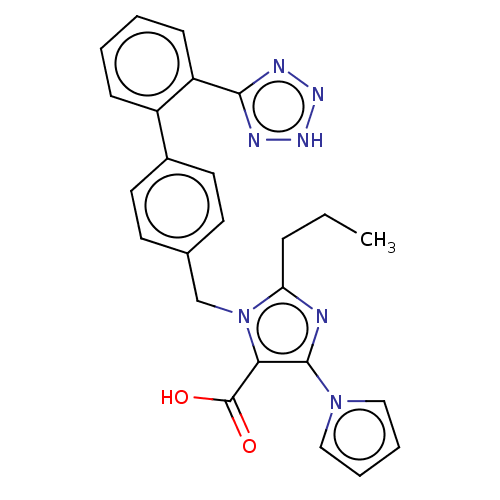
(RAT) | BDBM50230912
(CHEMBL72922)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C25H23N7O2/c1-2-7-21-26-24(31-14-5-6-15-31)22(25(33)34)32(21)16-17-10-12-18(13-11-17)19-8-3-4-9-20(19)23-27-29-30-28-23/h3-6,8-15H,2,7,16H2,1H3,(H,33,34)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230886
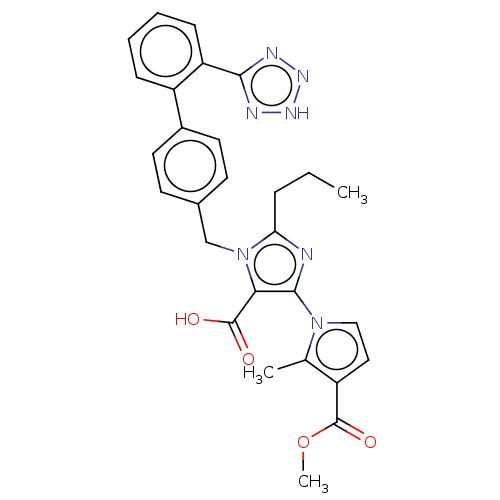
(CHEMBL307455)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1ccc(C(=O)OC)c1C Show InChI InChI=1S/C28H27N7O4/c1-4-7-23-29-26(34-15-14-20(17(34)2)28(38)39-3)24(27(36)37)35(23)16-18-10-12-19(13-11-18)21-8-5-6-9-22(21)25-30-32-33-31-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,36,37)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230910

(CHEMBL306066)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C26H25N7O2/c1-2-3-10-22-27-25(32-15-6-7-16-32)23(26(34)35)33(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)24-28-30-31-29-24/h4-9,11-16H,2-3,10,17H2,1H3,(H,34,35)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230882
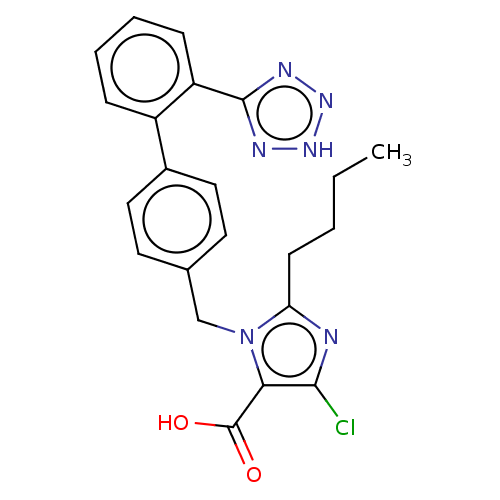
(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230882

(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against AT1 receptor binding affinity in rat liver |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data