 Found 382 hits with Last Name = 'croom' and Initial = 'd'
Found 382 hits with Last Name = 'croom' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826
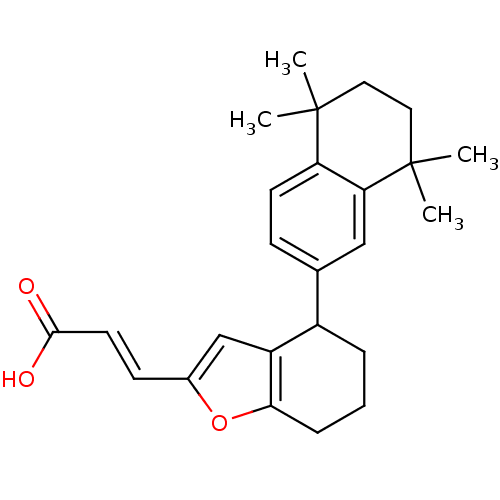
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85153
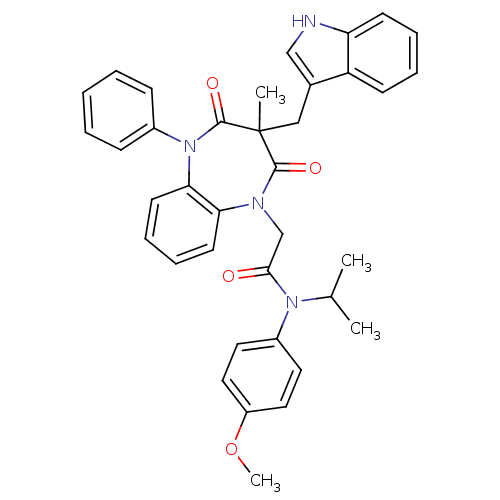
(CCK-A Agonist 20)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C37H36N4O4/c1-25(2)40(28-18-20-29(45-4)21-19-28)34(42)24-39-32-16-10-11-17-33(32)41(27-12-6-5-7-13-27)36(44)37(3,35(39)43)22-26-23-38-31-15-9-8-14-30(26)31/h5-21,23,25,38H,22,24H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85145
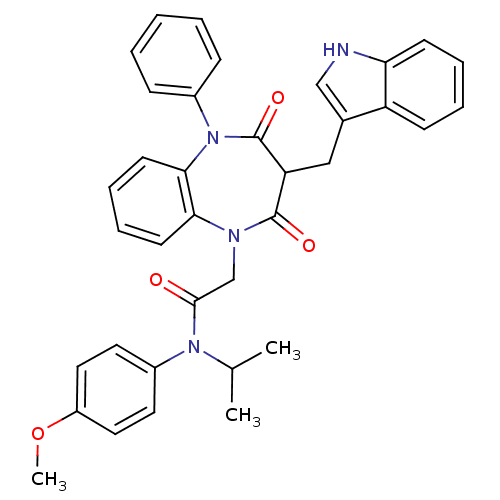
(CCK-A Agonist 15)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C36H34N4O4/c1-24(2)39(27-17-19-28(44-3)20-18-27)34(41)23-38-32-15-9-10-16-33(32)40(26-11-5-4-6-12-26)36(43)30(35(38)42)21-25-22-37-31-14-8-7-13-29(25)31/h4-20,22,24,30,37H,21,23H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173960
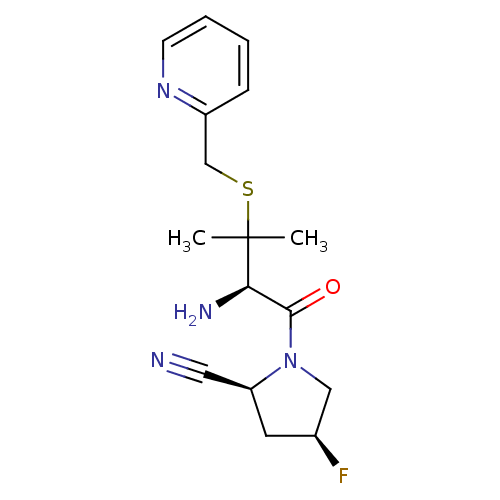
((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-2-ylmet...)Show SMILES CC(C)(SCc1ccccn1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C16H21FN4OS/c1-16(2,23-10-12-5-3-4-6-20-12)14(19)15(22)21-9-11(17)7-13(21)8-18/h3-6,11,13-14H,7,9-10,19H2,1-2H3/t11-,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85152
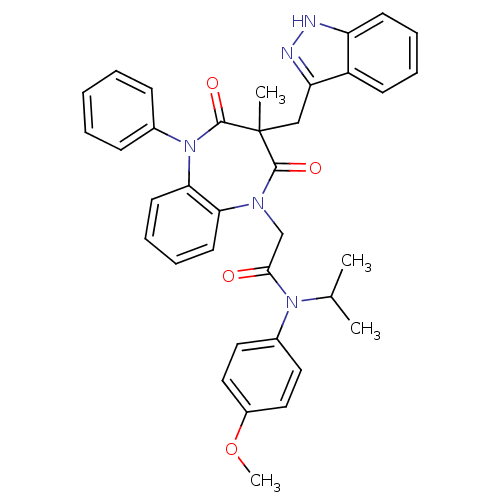
(CCK-A Agonist 23)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C36H35N5O4/c1-24(2)40(26-18-20-27(45-4)21-19-26)33(42)23-39-31-16-10-11-17-32(31)41(25-12-6-5-7-13-25)35(44)36(3,34(39)43)22-30-28-14-8-9-15-29(28)37-38-30/h5-21,24H,22-23H2,1-4H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85148

(CCK-A Agonist 21)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2c[nH]c3ccccc23)(OC)C1=O Show InChI InChI=1S/C37H36N4O5/c1-25(2)40(28-18-20-29(45-3)21-19-28)34(42)24-39-32-16-10-11-17-33(32)41(27-12-6-5-7-13-27)36(44)37(46-4,35(39)43)22-26-23-38-31-15-9-8-14-30(26)31/h5-21,23,25,38H,22,24H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85141
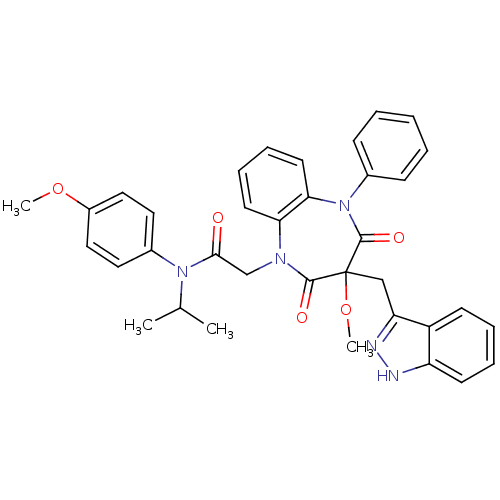
(CCK-A Agonist 24)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)(OC)C1=O Show InChI InChI=1S/C36H35N5O5/c1-24(2)40(26-18-20-27(45-3)21-19-26)33(42)23-39-31-16-10-11-17-32(31)41(25-12-6-5-7-13-25)35(44)36(46-4,34(39)43)22-30-28-14-8-9-15-29(28)37-38-30/h5-21,24H,22-23H2,1-4H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85161
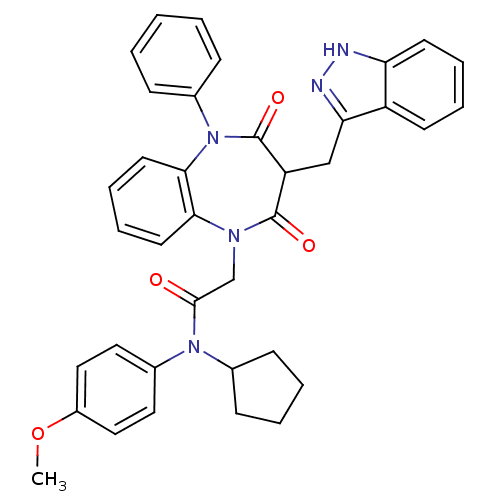
(CCK-A Agonist 43)Show SMILES COc1ccc(cc1)N(C1CCCC1)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C37H35N5O4/c1-46-28-21-19-27(20-22-28)41(25-13-5-6-14-25)35(43)24-40-33-17-9-10-18-34(33)42(26-11-3-2-4-12-26)37(45)30(36(40)44)23-32-29-15-7-8-16-31(29)38-39-32/h2-4,7-12,15-22,25,30H,5-6,13-14,23-24H2,1H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85155
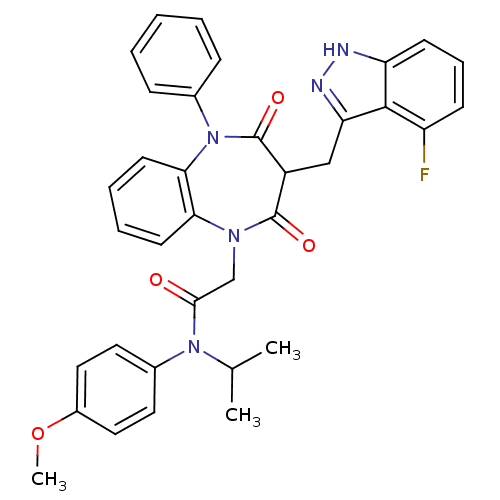
(CCK-A Agonist 31)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3cccc(F)c23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(24-16-18-25(45-3)19-17-24)32(42)21-39-30-14-7-8-15-31(30)41(23-10-5-4-6-11-23)35(44)26(34(39)43)20-29-33-27(36)12-9-13-28(33)37-38-29/h4-19,22,26H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85147
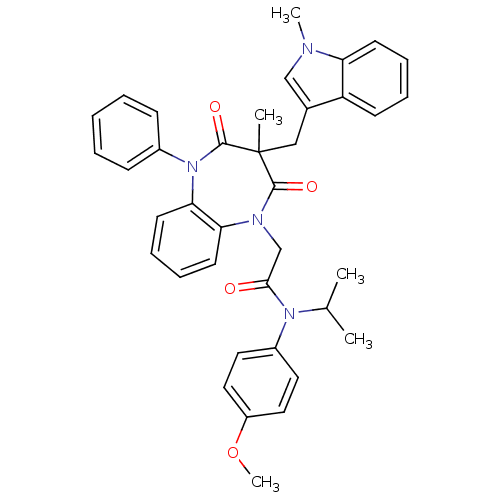
(CCK-A Agonist 22)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2cn(C)c3ccccc23)C1=O Show InChI InChI=1S/C38H38N4O4/c1-26(2)41(29-19-21-30(46-5)22-20-29)35(43)25-40-33-17-11-12-18-34(33)42(28-13-7-6-8-14-28)37(45)38(3,36(40)44)23-27-24-39(4)32-16-10-9-15-31(27)32/h6-22,24,26H,23,25H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173976
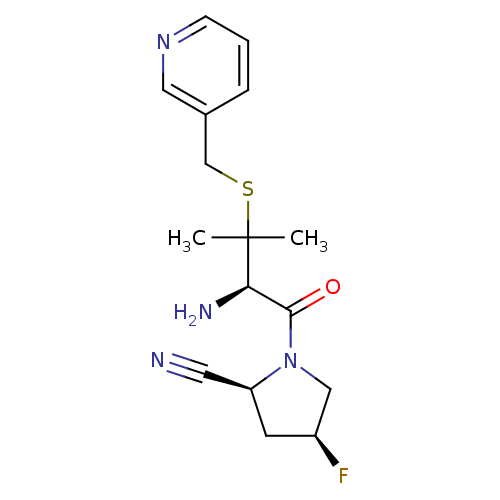
((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-3-ylmet...)Show SMILES CC(C)(SCc1cccnc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C16H21FN4OS/c1-16(2,23-10-11-4-3-5-20-8-11)14(19)15(22)21-9-12(17)6-13(21)7-18/h3-5,8,12-14H,6,9-10,19H2,1-2H3/t12-,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143827

((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...)Show SMILES COc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O4/c1-25(2)11-12-26(3,4)21-15-23(29-5)19(14-20(21)25)17-7-6-8-22-18(17)13-16(30-22)9-10-24(27)28/h9-10,13-15,17H,6-8,11-12H2,1-5H3,(H,27,28)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143825
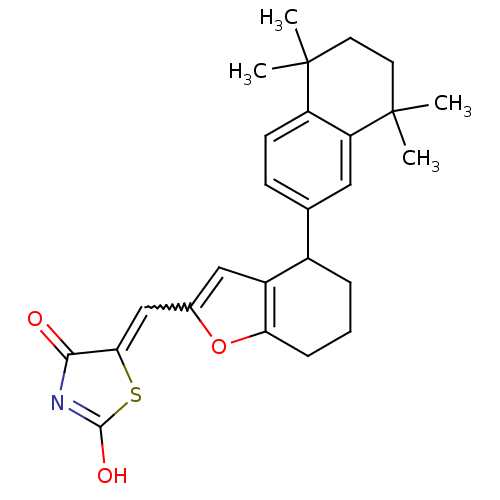
(5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(C=C3SC(O)=NC3=O)cc12 |w:21.22,c:27| Show InChI InChI=1S/C26H29NO3S/c1-25(2)10-11-26(3,4)20-12-15(8-9-19(20)25)17-6-5-7-21-18(17)13-16(30-21)14-22-23(28)27-24(29)31-22/h8-9,12-14,17H,5-7,10-11H2,1-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85143
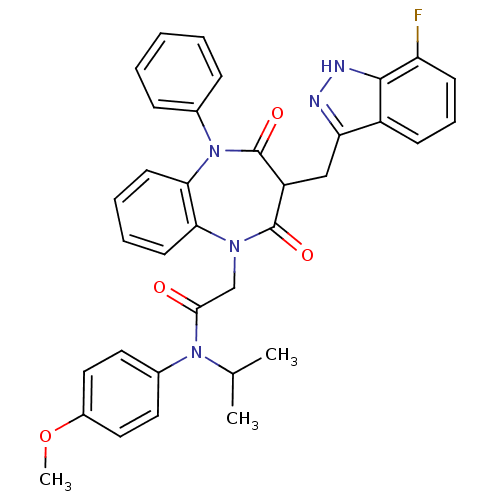
(CCK-A Agonist 34)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3c(F)cccc23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(24-16-18-25(45-3)19-17-24)32(42)21-39-30-14-7-8-15-31(30)41(23-10-5-4-6-11-23)35(44)27(34(39)43)20-29-26-12-9-13-28(36)33(26)38-37-29/h4-19,22,27H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173983
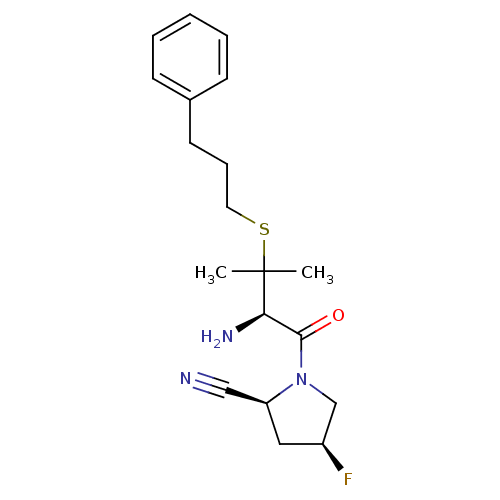
((2S,4S)-1-((R)-2-amino-3-methyl-3-(3-phenylpropylt...)Show SMILES CC(C)(SCCCc1ccccc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C19H26FN3OS/c1-19(2,25-10-6-9-14-7-4-3-5-8-14)17(22)18(24)23-13-15(20)11-16(23)12-21/h3-5,7-8,15-17H,6,9-11,13,22H2,1-2H3/t15-,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85162
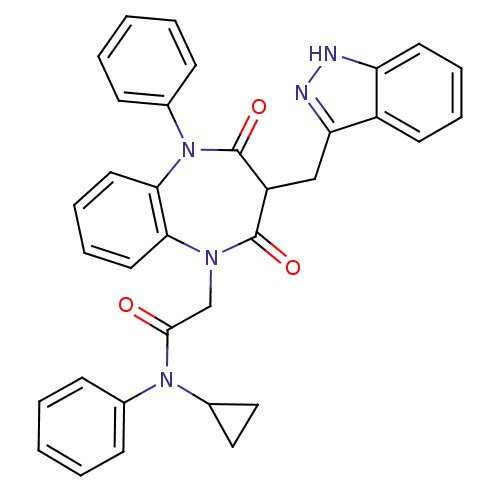
(CCK-A Agonist 44)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O)N(C1CC1)c1ccccc1 Show InChI InChI=1S/C34H29N5O3/c40-32(38(25-19-20-25)23-11-3-1-4-12-23)22-37-30-17-9-10-18-31(30)39(24-13-5-2-6-14-24)34(42)27(33(37)41)21-29-26-15-7-8-16-28(26)35-36-29/h1-18,25,27H,19-22H2,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173981
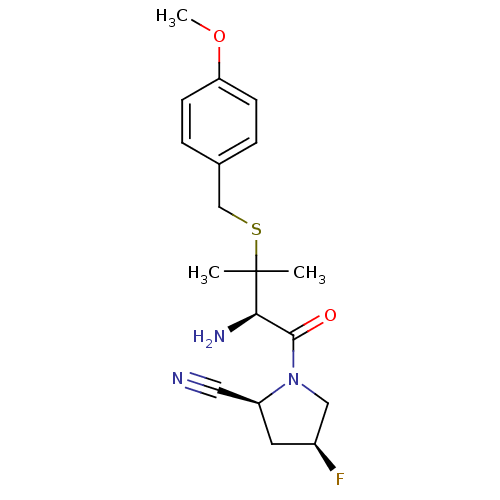
((2S,4S)-1-((R)-3-(4-methoxybenzylthio)-2-amino-3-m...)Show SMILES COc1ccc(CSC(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 Show InChI InChI=1S/C18H24FN3O2S/c1-18(2,25-11-12-4-6-15(24-3)7-5-12)16(21)17(23)22-10-13(19)8-14(22)9-20/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85149
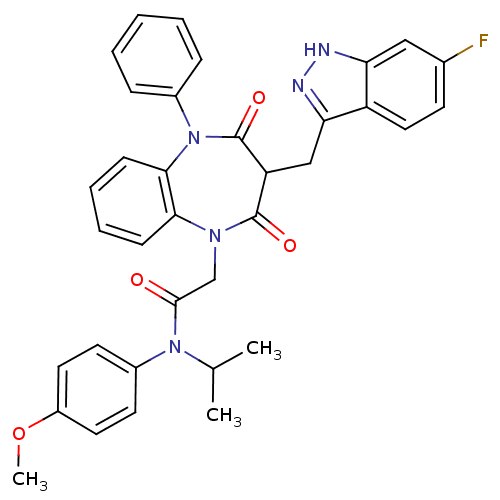
(CCK-A Agonist 33)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3cc(F)ccc23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(25-14-16-26(45-3)17-15-25)33(42)21-39-31-11-7-8-12-32(31)41(24-9-5-4-6-10-24)35(44)28(34(39)43)20-30-27-18-13-23(36)19-29(27)37-38-30/h4-19,22,28H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173958
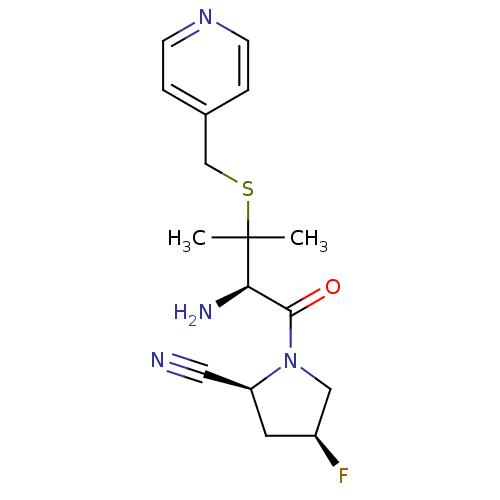
((2S,4S)-1-((R)-2-amino-3-methyl-3-(pyridin-4-ylmet...)Show SMILES CC(C)(SCc1ccncc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C16H21FN4OS/c1-16(2,23-10-11-3-5-20-6-4-11)14(19)15(22)21-9-12(17)7-13(21)8-18/h3-6,12-14H,7,9-10,19H2,1-2H3/t12-,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85160
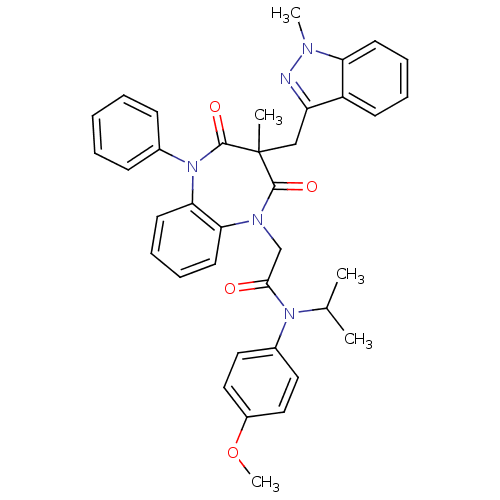
(CCK-A Agonist 27)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(C)(Cc2nn(C)c3ccccc23)C1=O Show InChI InChI=1S/C37H37N5O4/c1-25(2)41(27-19-21-28(46-5)22-20-27)34(43)24-40-32-17-11-12-18-33(32)42(26-13-7-6-8-14-26)36(45)37(3,35(40)44)23-30-29-15-9-10-16-31(29)39(4)38-30/h6-22,25H,23-24H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173967
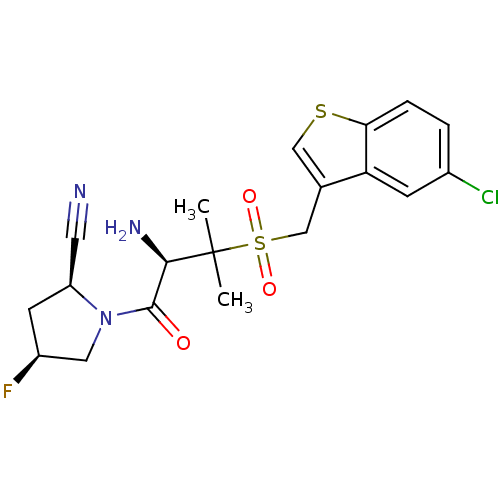
((2S,4S)-1-((R)-2-amino-3-((5-chlorobenzo[b]thiophe...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1csc2ccc(Cl)cc12 Show InChI InChI=1S/C19H21ClFN3O3S2/c1-19(2,17(23)18(25)24-8-13(21)6-14(24)7-22)29(26,27)10-11-9-28-16-4-3-12(20)5-15(11)16/h3-5,9,13-14,17H,6,8,10,23H2,1-2H3/t13-,14-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143833
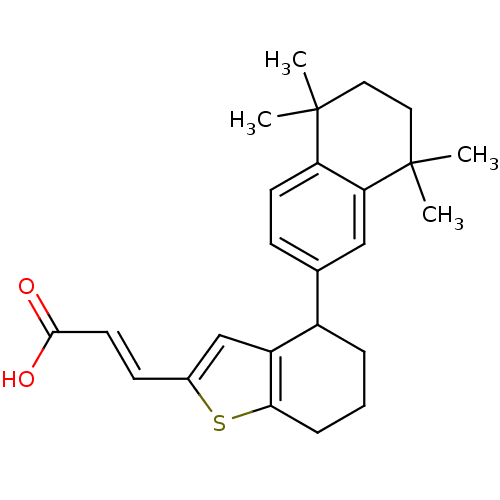
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2sc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O2S/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143832

((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES Cc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O3/c1-16-13-21-22(26(4,5)12-11-25(21,2)3)15-19(16)18-7-6-8-23-20(18)14-17(29-23)9-10-24(27)28/h9-10,13-15,18H,6-8,11-12H2,1-5H3,(H,27,28)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85150
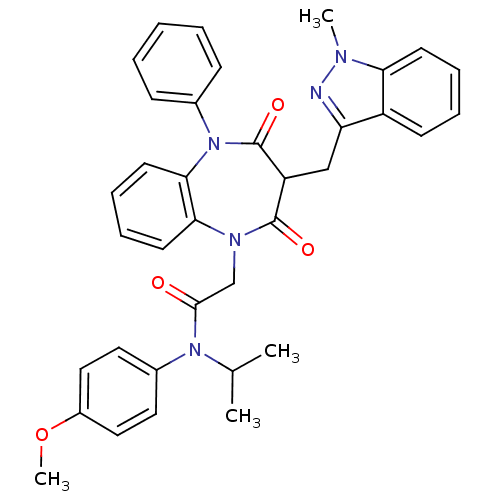
(CCK-A Agonist 25)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2nn(C)c3ccccc23)C1=O Show InChI InChI=1S/C36H35N5O4/c1-24(2)40(26-18-20-27(45-4)21-19-26)34(42)23-39-32-16-10-11-17-33(32)41(25-12-6-5-7-13-25)36(44)29(35(39)43)22-30-28-14-8-9-15-31(28)38(3)37-30/h5-21,24,29H,22-23H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85140
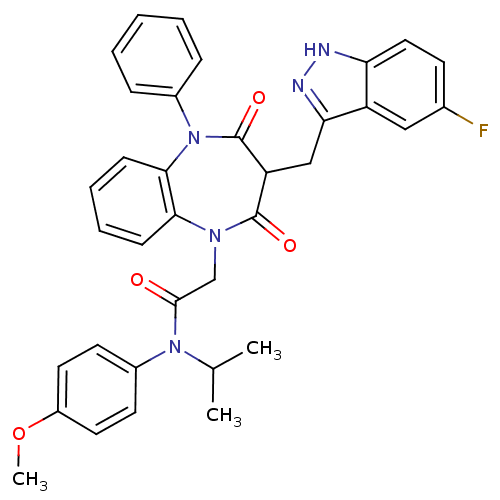
(CCK-A Agonist 32)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccc(F)cc23)C1=O Show InChI InChI=1S/C35H32FN5O4/c1-22(2)40(25-14-16-26(45-3)17-15-25)33(42)21-39-31-11-7-8-12-32(31)41(24-9-5-4-6-10-24)35(44)28(34(39)43)20-30-27-19-23(36)13-18-29(27)37-38-30/h4-19,22,28H,20-21H2,1-3H3,(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173977
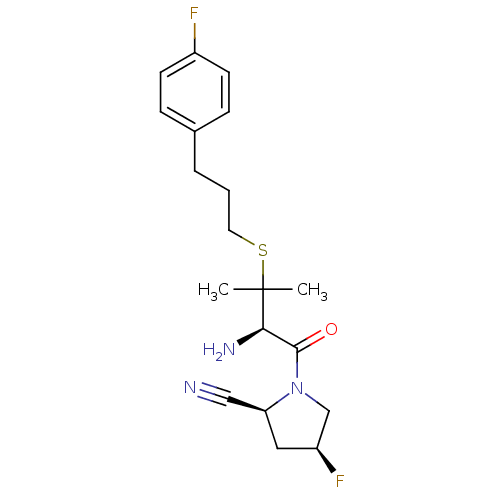
((2S,4S)-1-((R)-2-amino-3-(3-(4-fluorophenyl)propyl...)Show SMILES CC(C)(SCCCc1ccc(F)cc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C19H25F2N3OS/c1-19(2,26-9-3-4-13-5-7-14(20)8-6-13)17(23)18(25)24-12-15(21)10-16(24)11-22/h5-8,15-17H,3-4,9-10,12,23H2,1-2H3/t15-,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173971
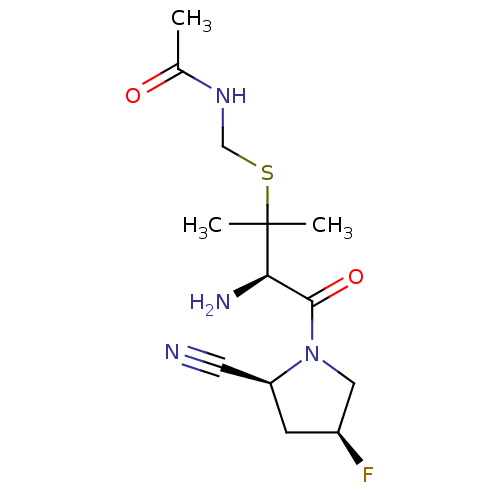
(CHEMBL427193 | N-(((R)-3-amino-4-((2S,4S)-2-cyano-...)Show SMILES CC(=O)NCSC(C)(C)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C13H21FN4O2S/c1-8(19)17-7-21-13(2,3)11(16)12(20)18-6-9(14)4-10(18)5-15/h9-11H,4,6-7,16H2,1-3H3,(H,17,19)/t9-,10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
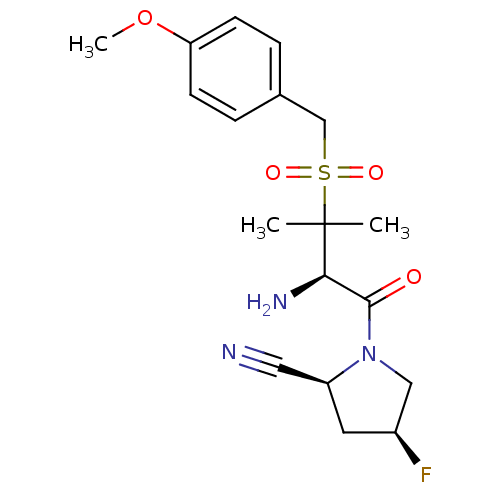
(Homo sapiens (Human)) | BDBM50173963
((2S,4S)-1-((R)-2-amino-3-(4-methoxybenzylsulfonyl)...)Show SMILES COc1ccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 |r| Show InChI InChI=1S/C18H24FN3O4S/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)27(24,25)11-12-4-6-15(26-3)7-5-12/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173979

((2S,4S)-1-((R)-2-amino-3-methyl-3-(phenethylthio)b...)Show SMILES CC(C)(SCCc1ccccc1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C18H24FN3OS/c1-18(2,24-9-8-13-6-4-3-5-7-13)16(21)17(23)22-12-14(19)10-15(22)11-20/h3-7,14-16H,8-10,12,21H2,1-2H3/t14-,15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
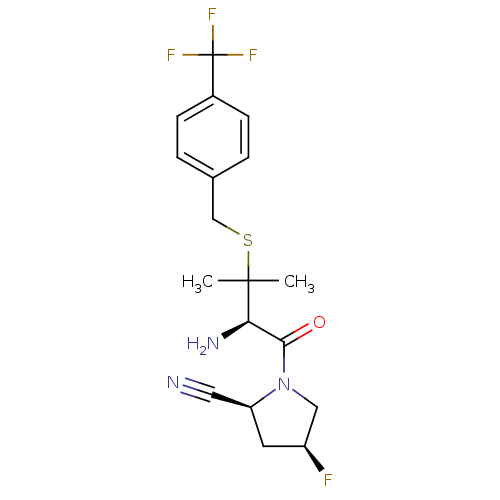
(Homo sapiens (Human)) | BDBM50173972
((2S,4S)-1-((R)-3-(4-(trifluoromethyl)benzylthio)-2...)Show SMILES CC(C)(SCc1ccc(cc1)C(F)(F)F)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C18H21F4N3OS/c1-17(2,15(24)16(26)25-9-13(19)7-14(25)8-23)27-10-11-3-5-12(6-4-11)18(20,21)22/h3-6,13-15H,7,9-10,24H2,1-2H3/t13-,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
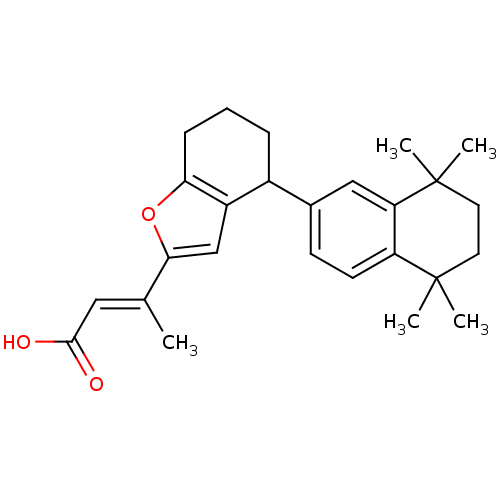
(Homo sapiens (Human)) | BDBM50409928
(CHEMBL2113737)Show SMILES C\C(=C/C(O)=O)c1cc2C(CCCc2o1)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H32O3/c1-16(13-24(27)28)23-15-19-18(7-6-8-22(19)29-23)17-9-10-20-21(14-17)26(4,5)12-11-25(20,2)3/h9-10,13-15,18H,6-8,11-12H2,1-5H3,(H,27,28)/b16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
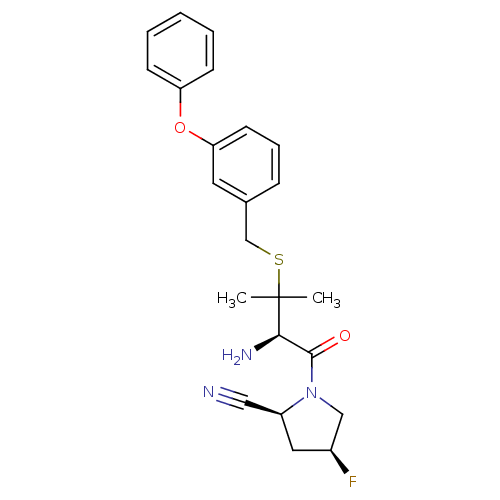
(Homo sapiens (Human)) | BDBM50173961
((2S,4S)-1-((R)-3-(3-phenoxybenzylthio)-2-amino-3-m...)Show SMILES CC(C)(SCc1cccc(Oc2ccccc2)c1)[C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N Show InChI InChI=1S/C23H26FN3O2S/c1-23(2,21(26)22(28)27-14-17(24)12-18(27)13-25)30-15-16-7-6-10-20(11-16)29-19-8-4-3-5-9-19/h3-11,17-18,21H,12,14-15,26H2,1-2H3/t17-,18-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173978
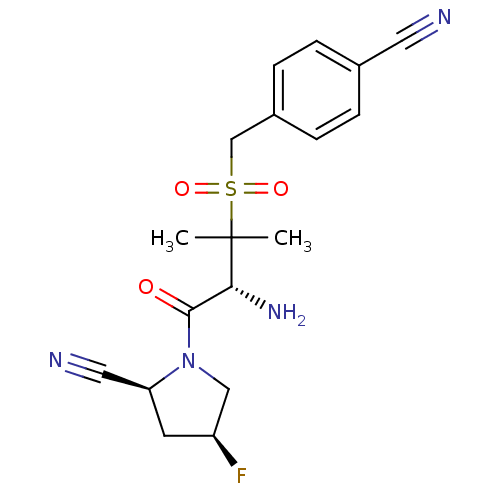
((2S,4S)-1-((R)-3-(4-cyanobenzylsulfonyl)-2-amino-3...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(cc1)C#N Show InChI InChI=1S/C18H21FN4O3S/c1-18(2,16(22)17(24)23-10-14(19)7-15(23)9-21)27(25,26)11-13-5-3-12(8-20)4-6-13/h3-6,14-16H,7,10-11,22H2,1-2H3/t14-,15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173965
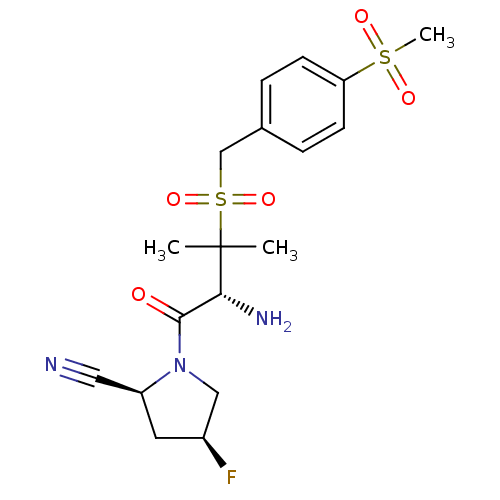
((2S,4S)-1-((R)-3-(4-(methylsulfonyl)benzylsulfonyl...)Show SMILES CC(C)([C@H](N)C(=O)N1C[C@@H](F)C[C@H]1C#N)S(=O)(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H24FN3O5S2/c1-18(2,16(21)17(23)22-10-13(19)8-14(22)9-20)29(26,27)11-12-4-6-15(7-5-12)28(3,24)25/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50409929
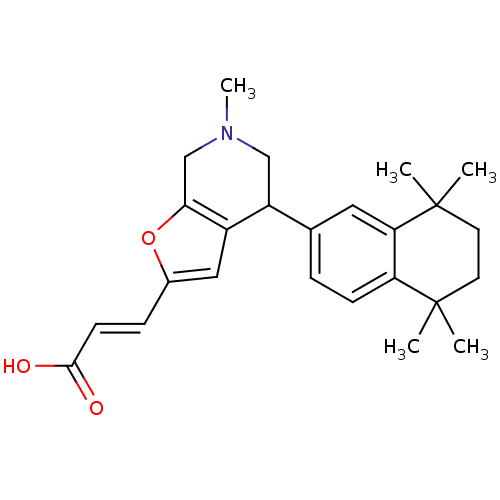
(CHEMBL2113736)Show SMILES CN1CC(c2cc(\C=C\C(O)=O)oc2C1)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H31NO3/c1-24(2)10-11-25(3,4)21-12-16(6-8-20(21)24)19-14-26(5)15-22-18(19)13-17(29-22)7-9-23(27)28/h6-9,12-13,19H,10-11,14-15H2,1-5H3,(H,27,28)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85139
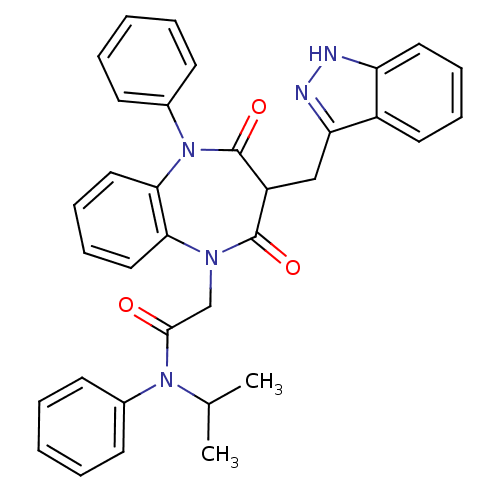
(CCK-A Agonist 37)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O)c1ccccc1 Show InChI InChI=1S/C34H31N5O3/c1-23(2)38(24-13-5-3-6-14-24)32(40)22-37-30-19-11-12-20-31(30)39(25-15-7-4-8-16-25)34(42)27(33(37)41)21-29-26-17-9-10-18-28(26)35-36-29/h3-20,23,27H,21-22H2,1-2H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85151
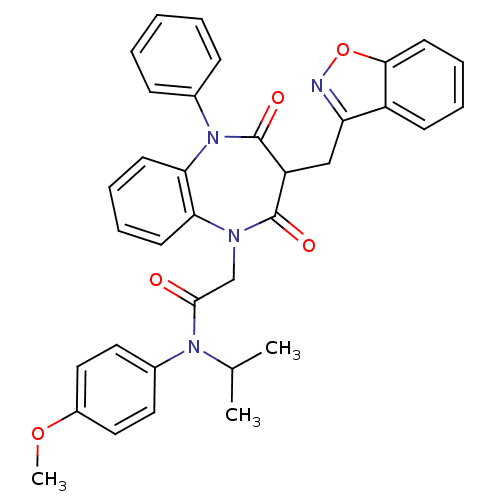
(CCK-A Agonist 18)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2noc3ccccc23)C1=O Show InChI InChI=1S/C35H32N4O5/c1-23(2)38(25-17-19-26(43-3)20-18-25)33(40)22-37-30-14-8-9-15-31(30)39(24-11-5-4-6-12-24)35(42)28(34(37)41)21-29-27-13-7-10-16-32(27)44-36-29/h4-20,23,28H,21-22H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50143824
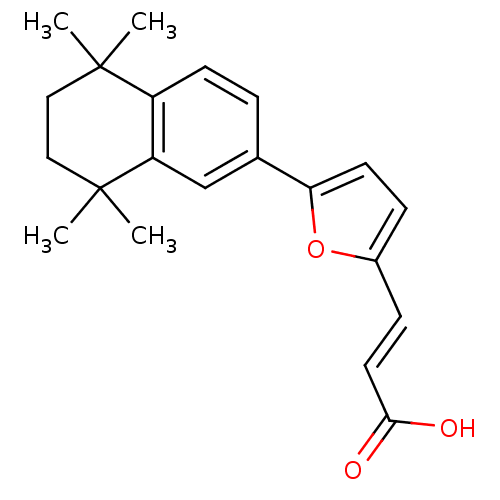
((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)-c1ccc(\C=C\C(O)=O)o1 Show InChI InChI=1S/C21H24O3/c1-20(2)11-12-21(3,4)17-13-14(5-8-16(17)20)18-9-6-15(24-18)7-10-19(22)23/h5-10,13H,11-12H2,1-4H3,(H,22,23)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143821
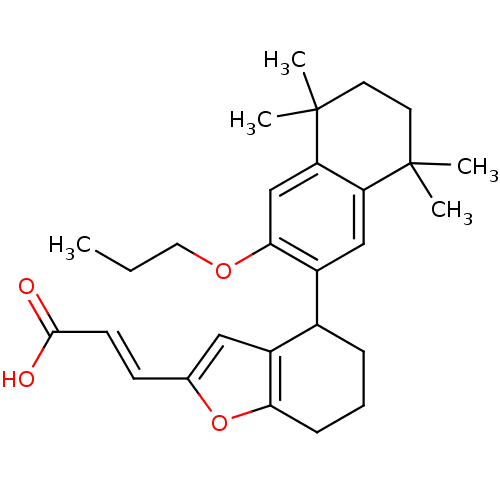
((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...)Show SMILES CCCOc1cc2c(cc1C1CCCc3oc(\C=C\C(O)=O)cc13)C(C)(C)CCC2(C)C Show InChI InChI=1S/C28H36O4/c1-6-14-31-25-17-23-22(27(2,3)12-13-28(23,4)5)16-21(25)19-8-7-9-24-20(19)15-18(32-24)10-11-26(29)30/h10-11,15-17,19H,6-9,12-14H2,1-5H3,(H,29,30)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173957

((2S,4S)-1-((R)-3-(4-methoxybenzylsulfinyl)-2-amino...)Show SMILES COc1ccc(CS(=O)C(C)(C)[C@H](N)C(=O)N2C[C@@H](F)C[C@H]2C#N)cc1 Show InChI InChI=1S/C18H24FN3O3S/c1-18(2,26(24)11-12-4-6-15(25-3)7-5-12)16(21)17(23)22-10-13(19)8-14(22)9-20/h4-7,13-14,16H,8,10-11,21H2,1-3H3/t13-,14-,16+,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85144

(CCK-A Agonist 28)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ncccc23)C1=O Show InChI InChI=1S/C34H32N6O4/c1-22(2)39(24-15-17-25(44-3)18-16-24)31(41)21-38-29-13-7-8-14-30(29)40(23-10-5-4-6-11-23)34(43)27(33(38)42)20-28-26-12-9-19-35-32(26)37-36-28/h4-19,22,27H,20-21H2,1-3H3,(H,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50173959
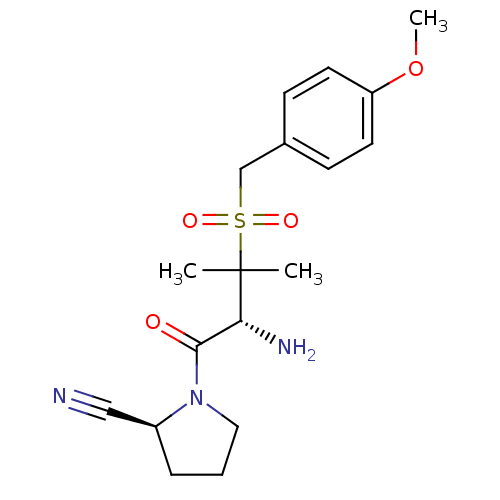
((S)-1-((R)-3-(4-methoxybenzylsulfonyl)-2-amino-3-m...)Show SMILES COc1ccc(CS(=O)(=O)C(C)(C)[C@H](N)C(=O)N2CCC[C@H]2C#N)cc1 Show InChI InChI=1S/C18H25N3O4S/c1-18(2,16(20)17(22)21-10-4-5-14(21)11-19)26(23,24)12-13-6-8-15(25-3)9-7-13/h6-9,14,16H,4-5,10,12,20H2,1-3H3/t14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human DPP4 |
Bioorg Med Chem Lett 15: 5257-61 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.050
BindingDB Entry DOI: 10.7270/Q21R6Q2C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85156

(CCK-A Agonist 41)Show SMILES COc1ccccc1N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2n[nH]c3ccccc23)C1=O Show InChI InChI=1S/C35H33N5O4/c1-23(2)39(31-19-11-12-20-32(31)44-3)33(41)22-38-29-17-9-10-18-30(29)40(24-13-5-4-6-14-24)35(43)26(34(38)42)21-28-25-15-7-8-16-27(25)36-37-28/h4-20,23,26H,21-22H2,1-3H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM85146

(CCK-A Agonist 30)Show SMILES COc1ccc(cc1)N(C(C)C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(Cc2[nH]nc3CCCCc23)C1=O Show InChI InChI=1S/C35H37N5O4/c1-23(2)39(25-17-19-26(44-3)20-18-25)33(41)22-38-31-15-9-10-16-32(31)40(24-11-5-4-6-12-24)35(43)28(34(38)42)21-30-27-13-7-8-14-29(27)36-37-30/h4-6,9-12,15-20,23,28H,7-8,13-14,21-22H2,1-3H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143835
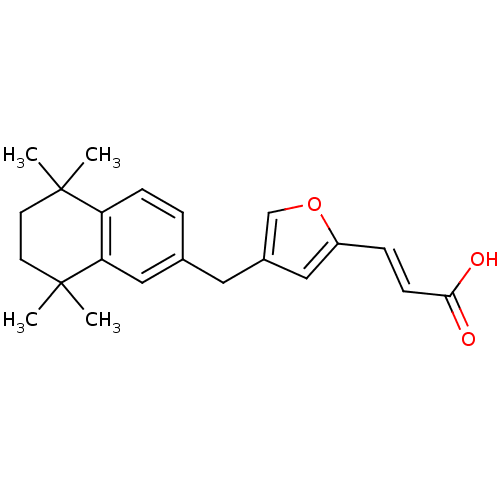
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(Cc3coc(\C=C\C(O)=O)c3)ccc12 Show InChI InChI=1S/C22H26O3/c1-21(2)9-10-22(3,4)19-13-15(5-7-18(19)21)11-16-12-17(25-14-16)6-8-20(23)24/h5-8,12-14H,9-11H2,1-4H3,(H,23,24)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data