 Found 140 hits with Last Name = 'darczak' and Initial = 'd'
Found 140 hits with Last Name = 'darczak' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50110068
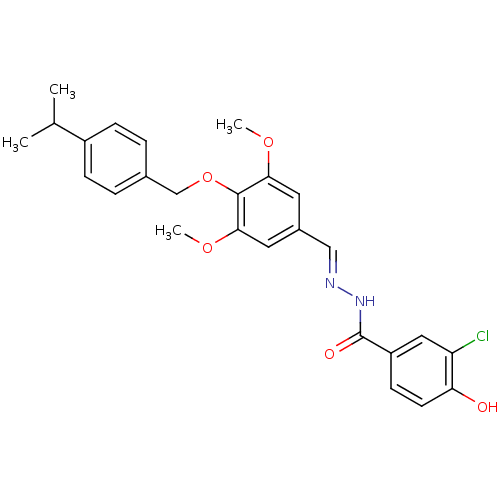
(3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)cc(OC)c1OCc1ccc(cc1)C(C)C Show InChI InChI=1S/C26H27ClN2O5/c1-16(2)19-7-5-17(6-8-19)15-34-25-23(32-3)11-18(12-24(25)33-4)14-28-29-26(31)20-9-10-22(30)21(27)13-20/h5-14,16,30H,15H2,1-4H3,(H,29,31)/b28-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50122102
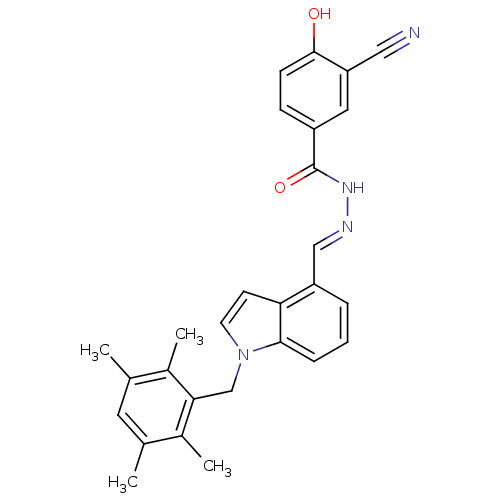
(3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...)Show SMILES Cc1cc(C)c(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(c4)C#N)cccc23)c1C Show InChI InChI=1S/C28H26N4O2/c1-17-12-18(2)20(4)25(19(17)3)16-32-11-10-24-22(6-5-7-26(24)32)15-30-31-28(34)21-8-9-27(33)23(13-21)14-29/h5-13,15,33H,16H2,1-4H3,(H,31,34)/b30-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374178

(CHEMBL411120)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1cccc2n(Cc3cccc(c3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H17ClF3N3O2/c25-20-12-16(7-8-22(20)32)23(33)30-29-13-17-4-2-6-21-19(17)9-10-31(21)14-15-3-1-5-18(11-15)24(26,27)28/h1-13,32H,14H2,(H,30,33)/b29-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374184
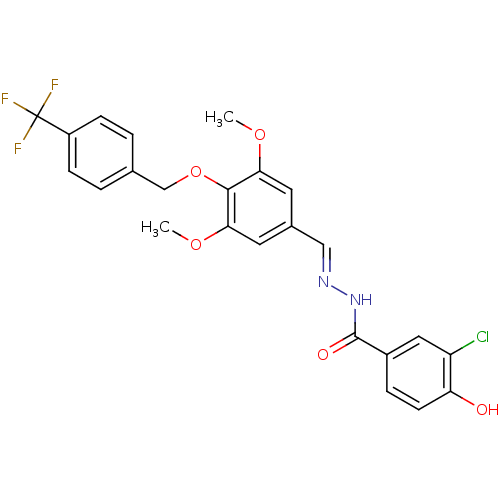
(CHEMBL270677)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)cc(OC)c1OCc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C24H20ClF3N2O5/c1-33-20-9-15(12-29-30-23(32)16-5-8-19(31)18(25)11-16)10-21(34-2)22(20)35-13-14-3-6-17(7-4-14)24(26,27)28/h3-12,31H,13H2,1-2H3,(H,30,32)/b29-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374179
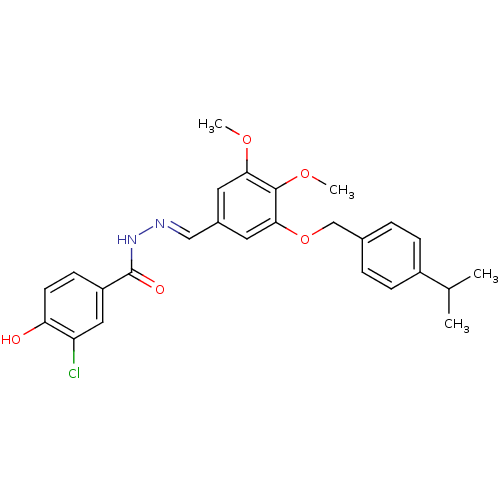
(CHEMBL409325)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)cc(OCc2ccc(cc2)C(C)C)c1OC Show InChI InChI=1S/C26H27ClN2O5/c1-16(2)19-7-5-17(6-8-19)15-34-24-12-18(11-23(32-3)25(24)33-4)14-28-29-26(31)20-9-10-22(30)21(27)13-20/h5-14,16,30H,15H2,1-4H3,(H,29,31)/b28-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374188

(CHEMBL270226)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)cc(OCc2c(C)c(C)cc(C)c2C)c1OC Show InChI InChI=1S/C27H29ClN2O5/c1-15-9-16(2)18(4)21(17(15)3)14-35-25-11-19(10-24(33-5)26(25)34-6)13-29-30-27(32)20-7-8-23(31)22(28)12-20/h7-13,31H,14H2,1-6H3,(H,30,32)/b29-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374187

(CHEMBL256379)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)cc(OC)c1OCc1ccccc1 Show InChI InChI=1S/C23H21ClN2O5/c1-29-20-10-16(13-25-26-23(28)17-8-9-19(27)18(24)12-17)11-21(30-2)22(20)31-14-15-6-4-3-5-7-15/h3-13,27H,14H2,1-2H3,(H,26,28)/b25-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374177

(CHEMBL409729)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)cc(OC)c1OCc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H20ClF3N2O5/c1-33-20-9-15(12-29-30-23(32)16-6-7-19(31)18(25)11-16)10-21(34-2)22(20)35-13-14-4-3-5-17(8-14)24(26,27)28/h3-12,31H,13H2,1-2H3,(H,30,32)/b29-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374180

(CHEMBL404071)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1cccc(COc2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C22H16ClF3N2O3/c23-19-11-16(4-9-20(19)29)21(30)28-27-12-14-2-1-3-15(10-14)13-31-18-7-5-17(6-8-18)22(24,25)26/h1-12,29H,13H2,(H,28,30)/b27-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374186
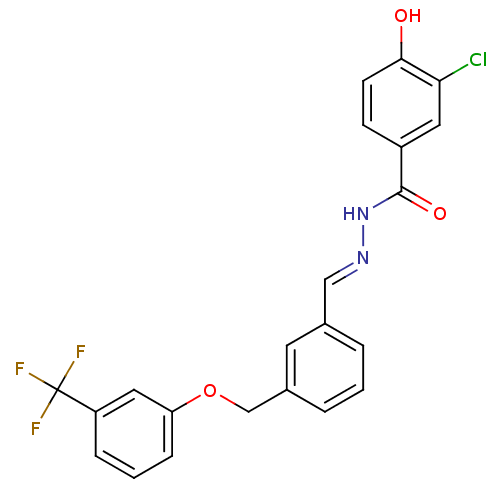
(CHEMBL272122)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1cccc(COc2cccc(c2)C(F)(F)F)c1 Show InChI InChI=1S/C22H16ClF3N2O3/c23-19-10-16(7-8-20(19)29)21(30)28-27-12-14-3-1-4-15(9-14)13-31-18-6-2-5-17(11-18)22(24,25)26/h1-12,29H,13H2,(H,28,30)/b27-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374183

(CHEMBL269990)Show SMILES COc1cc(\C=N\NC(=O)c2ccc(O)c(Cl)c2)ccc1OCc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H18ClF3N2O4/c1-32-21-10-14(12-28-29-22(31)16-6-7-19(30)18(24)11-16)5-8-20(21)33-13-15-3-2-4-17(9-15)23(25,26)27/h2-12,30H,13H2,1H3,(H,29,31)/b28-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374182

(CHEMBL272118)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C21H17ClN2O3/c22-19-12-17(8-11-20(19)25)21(26)24-23-13-15-6-9-18(10-7-15)27-14-16-4-2-1-3-5-16/h1-13,25H,14H2,(H,24,26)/b23-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374181

(CHEMBL256837)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1ccc(OCc2cccc(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H16ClF3N2O3/c23-19-11-16(6-9-20(19)29)21(30)28-27-12-14-4-7-18(8-5-14)31-13-15-2-1-3-17(10-15)22(24,25)26/h1-12,29H,13H2,(H,28,30)/b27-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50374185

(CHEMBL255824)Show SMILES Oc1ccc(cc1Cl)C(=O)N\N=C\c1cccc(OCc2cccc(c2)C(F)(F)F)c1 Show InChI InChI=1S/C22H16ClF3N2O3/c23-19-11-16(7-8-20(19)29)21(30)28-27-12-14-3-2-6-18(10-14)31-13-15-4-1-5-17(9-15)22(24,25)26/h1-12,29H,13H2,(H,28,30)/b27-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 554 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Pituitary adenylate cyclase-activating polypeptide type I receptor
(Homo sapiens (Human)) | BDBM50110055

(3-Chloro-4-hydroxy-benzoic acid [1-(4-isopropyl-be...)Show SMILES CC(C)c1ccc(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(Cl)c4)cccc23)cc1 Show InChI InChI=1S/C26H24ClN3O2/c1-17(2)19-8-6-18(7-9-19)16-30-13-12-22-21(4-3-5-24(22)30)15-28-29-26(32)20-10-11-25(31)23(27)14-20/h3-15,17,31H,16H2,1-2H3,(H,29,32)/b28-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 604 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells |
Bioorg Med Chem Lett 18: 2162-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.052
BindingDB Entry DOI: 10.7270/Q22J6CQ0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50386813
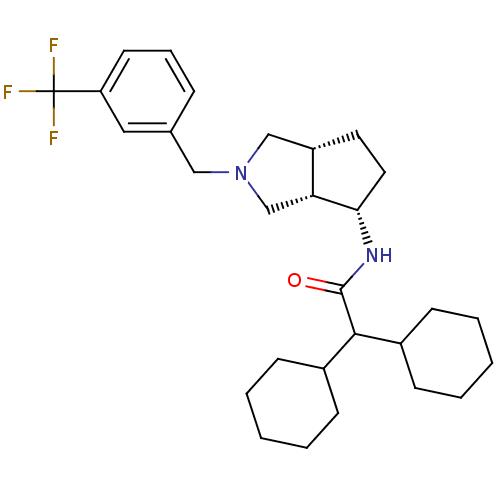
(CHEMBL2047726)Show SMILES FC(F)(F)c1cccc(CN2C[C@@H]3CC[C@H](NC(=O)C(C4CCCCC4)C4CCCCC4)[C@@H]3C2)c1 |r| Show InChI InChI=1S/C29H41F3N2O/c30-29(31,32)24-13-7-8-20(16-24)17-34-18-23-14-15-26(25(23)19-34)33-28(35)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h7-8,13,16,21-23,25-27H,1-6,9-12,14-15,17-19H2,(H,33,35)/t23-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat Cav2.2 expressed in HEK293 cells under inactivating depolarizing conditions at holding potential of -115mV and stepped to 0 mV once... |
Bioorg Med Chem 20: 4128-39 (2012)
Article DOI: 10.1016/j.bmc.2012.04.057
BindingDB Entry DOI: 10.7270/Q2TQ62JK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50386813

(CHEMBL2047726)Show SMILES FC(F)(F)c1cccc(CN2C[C@@H]3CC[C@H](NC(=O)C(C4CCCCC4)C4CCCCC4)[C@@H]3C2)c1 |r| Show InChI InChI=1S/C29H41F3N2O/c30-29(31,32)24-13-7-8-20(16-24)17-34-18-23-14-15-26(25(23)19-34)33-28(35)27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h7-8,13,16,21-23,25-27H,1-6,9-12,14-15,17-19H2,(H,33,35)/t23-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat Cav2.2 expressed in HEK293 cells under inactivating depolarizing conditions at holding potential of -115mV and stepped to 0 mV once... |
Bioorg Med Chem 20: 4128-39 (2012)
Article DOI: 10.1016/j.bmc.2012.04.057
BindingDB Entry DOI: 10.7270/Q2TQ62JK |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50438747
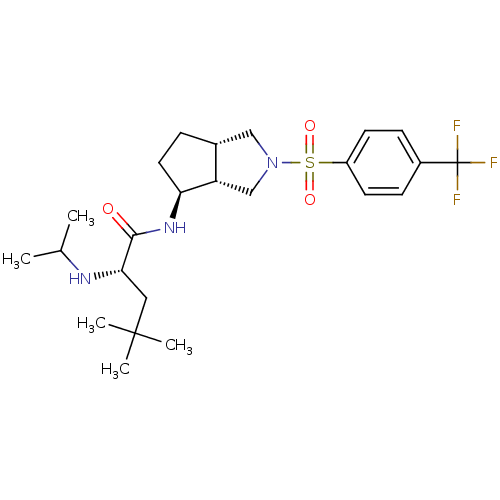
(CHEMBL2414787)Show SMILES CC(C)N[C@@H](CC(C)(C)C)C(=O)N[C@H]1CC[C@@H]2CN(C[C@H]12)S(=O)(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C24H36F3N3O3S/c1-15(2)28-21(12-23(3,4)5)22(31)29-20-11-6-16-13-30(14-19(16)20)34(32,33)18-9-7-17(8-10-18)24(25,26)27/h7-10,15-16,19-21,28H,6,11-14H2,1-5H3,(H,29,31)/t16-,19+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com
Curated by ChEMBL
| Assay Description
Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under hyperpolarized condition by whole cell patch-clamp manual electrophysiology a... |
Bioorg Med Chem Lett 23: 4857-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.074
BindingDB Entry DOI: 10.7270/Q2280915 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175237
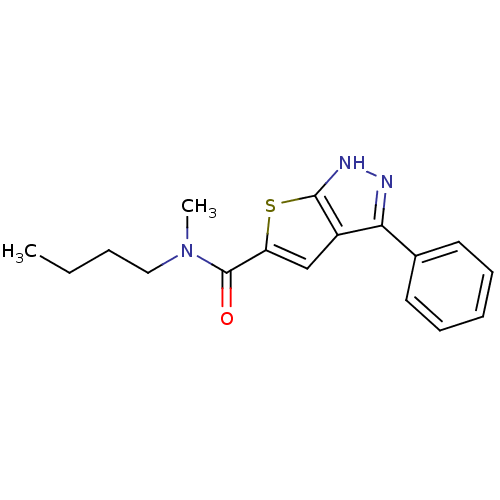
(CHEMBL199528 | N-butyl-N-methyl-3-phenyl-1H-thieno...)Show InChI InChI=1S/C17H19N3OS/c1-3-4-10-20(2)17(21)14-11-13-15(18-19-16(13)22-14)12-8-6-5-7-9-12/h5-9,11H,3-4,10H2,1-2H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175239
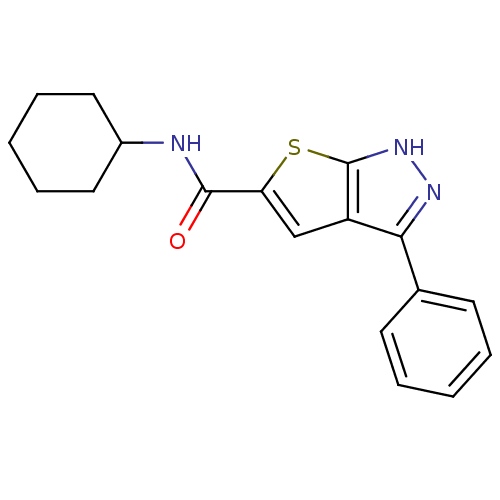
(CHEMBL199755 | N-cyclohexyl-3-phenyl-1H-thieno[2,3...)Show InChI InChI=1S/C18H19N3OS/c22-17(19-13-9-5-2-6-10-13)15-11-14-16(20-21-18(14)23-15)12-7-3-1-4-8-12/h1,3-4,7-8,11,13H,2,5-6,9-10H2,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175247

(CHEMBL200499 | N-benzyl-3-phenyl-1H-thieno[2,3-c]p...)Show InChI InChI=1S/C19H15N3OS/c23-18(20-12-13-7-3-1-4-8-13)16-11-15-17(21-22-19(15)24-16)14-9-5-2-6-10-14/h1-11H,12H2,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175241

(CHEMBL199383 | N-(4-methoxybenzyl)-3-phenyl-1H-thi...)Show SMILES COc1ccc(CNC(=O)c2cc3c(n[nH]c3s2)-c2ccccc2)cc1 Show InChI InChI=1S/C20H17N3O2S/c1-25-15-9-7-13(8-10-15)12-21-19(24)17-11-16-18(22-23-20(16)26-17)14-5-3-2-4-6-14/h2-11H,12H2,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50438746
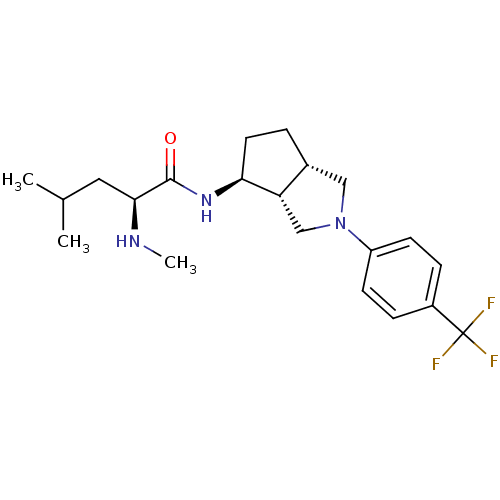
(CHEMBL2414771)Show SMILES CN[C@@H](CC(C)C)C(=O)N[C@H]1CC[C@@H]2CN(C[C@H]12)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C21H30F3N3O/c1-13(2)10-19(25-3)20(28)26-18-9-4-14-11-27(12-17(14)18)16-7-5-15(6-8-16)21(22,23)24/h5-8,13-14,17-19,25H,4,9-12H2,1-3H3,(H,26,28)/t14-,17+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com
Curated by ChEMBL
| Assay Description
Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under depolarized condition by whole cell patch-clamp manual electrophysiology assa... |
Bioorg Med Chem Lett 23: 4857-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.074
BindingDB Entry DOI: 10.7270/Q2280915 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175234
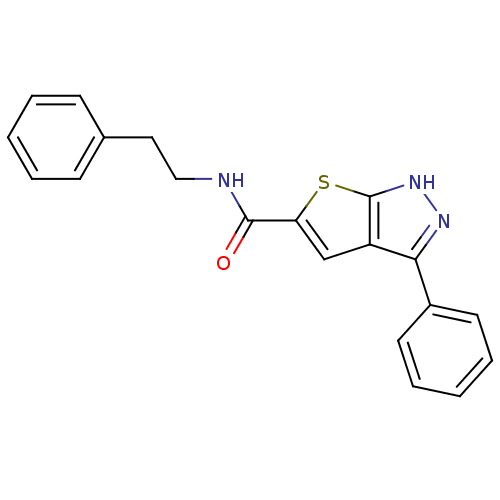
(CHEMBL197923 | N-phenethyl-3-phenyl-1H-thieno[2,3-...)Show InChI InChI=1S/C20H17N3OS/c24-19(21-12-11-14-7-3-1-4-8-14)17-13-16-18(22-23-20(16)25-17)15-9-5-2-6-10-15/h1-10,13H,11-12H2,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175232

((4-methylpiperazin-1-yl)(3-phenyl-1H-thieno[2,3-c]...)Show InChI InChI=1S/C17H18N4OS/c1-20-7-9-21(10-8-20)17(22)14-11-13-15(18-19-16(13)23-14)12-5-3-2-4-6-12/h2-6,11H,7-10H2,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50438746

(CHEMBL2414771)Show SMILES CN[C@@H](CC(C)C)C(=O)N[C@H]1CC[C@@H]2CN(C[C@H]12)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C21H30F3N3O/c1-13(2)10-19(25-3)20(28)26-18-9-4-14-11-27(12-17(14)18)16-7-5-15(6-8-16)21(22,23)24/h5-8,13-14,17-19,25H,4,9-12H2,1-3H3,(H,26,28)/t14-,17+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com
Curated by ChEMBL
| Assay Description
Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under hyperpolarized condition by whole cell patch-clamp manual electrophysiology a... |
Bioorg Med Chem Lett 23: 4857-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.074
BindingDB Entry DOI: 10.7270/Q2280915 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175246
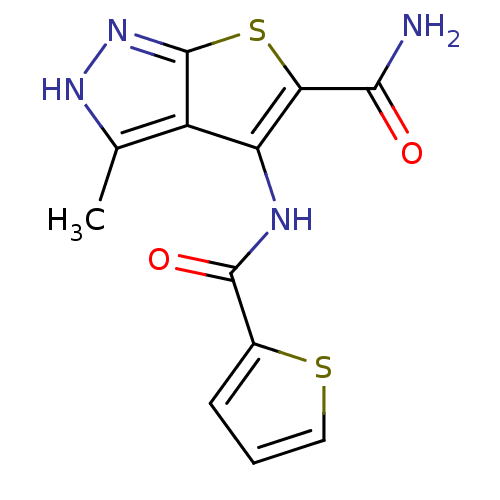
(3-methyl-4-(thiophene-2-carboxamido)-1H-thieno[2,3...)Show InChI InChI=1S/C12H10N4O2S2/c1-5-7-8(14-11(18)6-3-2-4-19-6)9(10(13)17)20-12(7)16-15-5/h2-4H,1H3,(H2,13,17)(H,14,18)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50175250

(CHEMBL370199 | methyl 2-(5-carbamoyl-3-methyl-1H-t...)Show InChI InChI=1S/C10H10N4O4S/c1-3-4-5(12-8(16)10(17)18-2)6(7(11)15)19-9(4)14-13-3/h1-2H3,(H2,11,15)(H,12,16)(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plk1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175235

(4-(2-chlorobenzamido)-3-methyl-1H-thieno[2,3-c]pyr...)Show InChI InChI=1S/C14H11ClN4O2S/c1-6-9-10(11(12(16)20)22-14(9)19-18-6)17-13(21)7-4-2-3-5-8(7)15/h2-5H,1H3,(H2,16,20)(H,17,21)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50175245
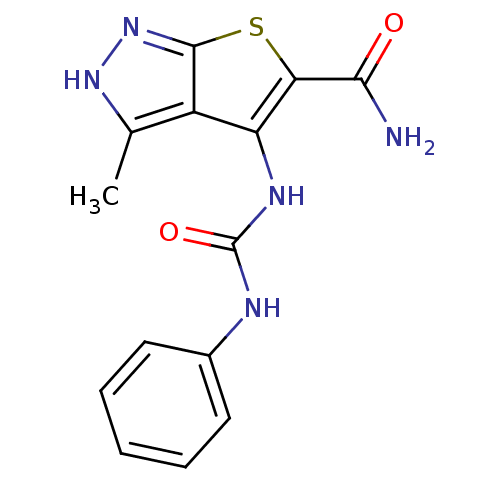
(1-(5-carbamoyl-3-methyl-1H-thieno[2,3-c]pyrazol-4-...)Show InChI InChI=1S/C14H13N5O2S/c1-7-9-10(11(12(15)20)22-13(9)19-18-7)17-14(21)16-8-5-3-2-4-6-8/h2-6H,1H3,(H2,15,20)(H,18,19)(H2,16,17,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Akt1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50438747

(CHEMBL2414787)Show SMILES CC(C)N[C@@H](CC(C)(C)C)C(=O)N[C@H]1CC[C@@H]2CN(C[C@H]12)S(=O)(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C24H36F3N3O3S/c1-15(2)28-21(12-23(3,4)5)22(31)29-20-11-6-16-13-30(14-19(16)20)34(32,33)18-9-7-17(8-10-18)24(25,26)27/h7-10,15-16,19-21,28H,6,11-14H2,1-5H3,(H,29,31)/t16-,19+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064, United States. xenia.b.searle@abbvie.com
Curated by ChEMBL
| Assay Description
Inhibition of rat N-type Cav2.2 channel expressed in HEK293 cells under depolarized condition by whole cell patch-clamp manual electrophysiology assa... |
Bioorg Med Chem Lett 23: 4857-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.074
BindingDB Entry DOI: 10.7270/Q2280915 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175252
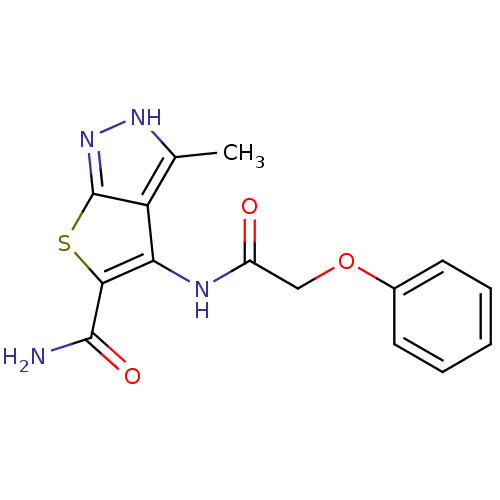
(3-methyl-4-(2-phenoxyacetamido)-1H-thieno[2,3-c]py...)Show InChI InChI=1S/C15H14N4O3S/c1-8-11-12(13(14(16)21)23-15(11)19-18-8)17-10(20)7-22-9-5-3-2-4-6-9/h2-6H,7H2,1H3,(H2,16,21)(H,17,20)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50175239

(CHEMBL199755 | N-cyclohexyl-3-phenyl-1H-thieno[2,3...)Show InChI InChI=1S/C18H19N3OS/c22-17(19-13-9-5-2-6-10-13)15-11-14-16(20-21-18(14)23-15)12-7-3-1-4-8-12/h1,3-4,7-8,11,13H,2,5-6,9-10H2,(H,19,22)(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDK2/Cyclin A |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50175239

(CHEMBL199755 | N-cyclohexyl-3-phenyl-1H-thieno[2,3...)Show InChI InChI=1S/C18H19N3OS/c22-17(19-13-9-5-2-6-10-13)15-11-14-16(20-21-18(14)23-15)12-7-3-1-4-8-12/h1,3-4,7-8,11,13H,2,5-6,9-10H2,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plk1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175243

(CHEMBL373001 | N-benzyl-3-methyl-1H-thieno[2,3-c]p...)Show InChI InChI=1S/C14H13N3OS/c1-9-11-7-12(19-14(11)17-16-9)13(18)15-8-10-5-3-2-4-6-10/h2-7H,8H2,1H3,(H,15,18)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50175245

(1-(5-carbamoyl-3-methyl-1H-thieno[2,3-c]pyrazol-4-...)Show InChI InChI=1S/C14H13N5O2S/c1-7-9-10(11(12(15)20)22-13(9)19-18-7)17-14(21)16-8-5-3-2-4-6-8/h2-6H,1H3,(H2,15,20)(H,18,19)(H2,16,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Pak4 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50175247

(CHEMBL200499 | N-benzyl-3-phenyl-1H-thieno[2,3-c]p...)Show InChI InChI=1S/C19H15N3OS/c23-18(20-12-13-7-3-1-4-8-13)16-11-15-17(21-22-19(15)24-16)14-9-5-2-6-10-14/h1-11H,12H2,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Akt1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50175251

(CHEMBL200796 | methyl 3-(phenylamino)-1H-thieno[2,...)Show InChI InChI=1S/C13H11N3O2S/c1-18-13(17)10-7-9-11(15-16-12(9)19-10)14-8-5-3-2-4-6-8/h2-7H,1H3,(H2,14,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Akt1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50175241

(CHEMBL199383 | N-(4-methoxybenzyl)-3-phenyl-1H-thi...)Show SMILES COc1ccc(CNC(=O)c2cc3c(n[nH]c3s2)-c2ccccc2)cc1 Show InChI InChI=1S/C20H17N3O2S/c1-25-15-9-7-13(8-10-15)12-21-19(24)17-11-16-18(22-23-20(16)26-17)14-5-3-2-4-6-14/h2-11H,12H2,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against MK2 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50175243

(CHEMBL373001 | N-benzyl-3-methyl-1H-thieno[2,3-c]p...)Show InChI InChI=1S/C14H13N3OS/c1-9-11-7-12(19-14(11)17-16-9)13(18)15-8-10-5-3-2-4-6-10/h2-7H,8H2,1H3,(H,15,18)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plk1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175249
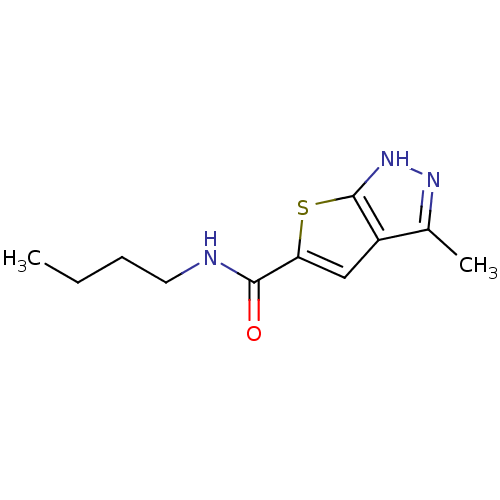
(CHEMBL371242 | N-butyl-3-methyl-1H-thieno[2,3-c]py...)Show InChI InChI=1S/C11H15N3OS/c1-3-4-5-12-10(15)9-6-8-7(2)13-14-11(8)16-9/h6H,3-5H2,1-2H3,(H,12,15)(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175250

(CHEMBL370199 | methyl 2-(5-carbamoyl-3-methyl-1H-t...)Show InChI InChI=1S/C10H10N4O4S/c1-3-4-5(12-8(16)10(17)18-2)6(7(11)15)19-9(4)14-13-3/h1-2H3,(H2,11,15)(H,12,16)(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50175234

(CHEMBL197923 | N-phenethyl-3-phenyl-1H-thieno[2,3-...)Show InChI InChI=1S/C20H17N3OS/c24-19(21-12-11-14-7-3-1-4-8-14)17-13-16-18(22-23-20(16)25-17)15-9-5-2-6-10-15/h1-10,13H,11-12H2,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Plk1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50175247

(CHEMBL200499 | N-benzyl-3-phenyl-1H-thieno[2,3-c]p...)Show InChI InChI=1S/C19H15N3OS/c23-18(20-12-13-7-3-1-4-8-13)16-11-15-17(21-22-19(15)24-16)14-9-5-2-6-10-14/h1-11H,12H2,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against MK2 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50175246

(3-methyl-4-(thiophene-2-carboxamido)-1H-thieno[2,3...)Show InChI InChI=1S/C12H10N4O2S2/c1-5-7-8(14-11(18)6-3-2-4-19-6)9(10(13)17)20-12(7)16-15-5/h2-4H,1H3,(H2,13,17)(H,14,18)(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Akt1 |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175238

(3-methyl-1H-thieno[2,3-c]pyrazole-5-carboxamide | ...)Show InChI InChI=1S/C7H7N3OS/c1-3-4-2-5(6(8)11)12-7(4)10-9-3/h2H,1H3,(H2,8,11)(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50175236
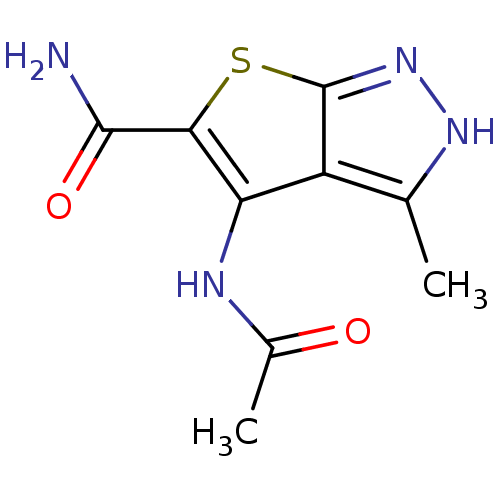
(4-acetamido-3-methyl-1H-thieno[2,3-c]pyrazole-5-ca...)Show InChI InChI=1S/C9H10N4O2S/c1-3-5-6(11-4(2)14)7(8(10)15)16-9(5)13-12-3/h1-2H3,(H2,10,15)(H,11,14)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50175237

(CHEMBL199528 | N-butyl-N-methyl-3-phenyl-1H-thieno...)Show InChI InChI=1S/C17H19N3OS/c1-3-4-10-20(2)17(21)14-11-13-15(18-19-16(13)22-14)12-8-6-5-7-9-12/h5-9,11H,3-4,10H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDK2/Cyclin A |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50175232

((4-methylpiperazin-1-yl)(3-phenyl-1H-thieno[2,3-c]...)Show InChI InChI=1S/C17H18N4OS/c1-20-7-9-21(10-8-20)17(22)14-11-13-15(18-19-16(13)23-14)12-5-3-2-4-6-12/h2-6,11H,7-10H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDK2/Cyclin A |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50175234

(CHEMBL197923 | N-phenethyl-3-phenyl-1H-thieno[2,3-...)Show InChI InChI=1S/C20H17N3OS/c24-19(21-12-11-14-7-3-1-4-8-14)17-13-16-18(22-23-20(16)25-17)15-9-5-2-6-10-15/h1-10,13H,11-12H2,(H,21,24)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDK2/Cyclin A |
Bioorg Med Chem Lett 16: 96-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.042
BindingDB Entry DOI: 10.7270/Q2JH3KRS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data