 Found 73 hits with Last Name = 'demong' and Initial = 'de'
Found 73 hits with Last Name = 'demong' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742
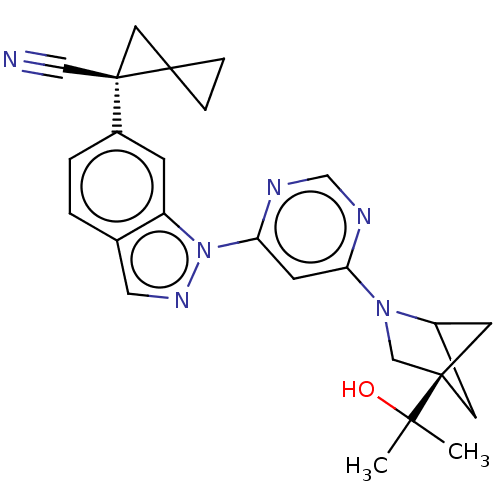
(CHEMBL5286106)Show SMILES CC(C)(O)[C@]12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,wD:4.3,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.67,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.85,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609728

(CHEMBL5267350)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1n[nH]c2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742

(CHEMBL5286106)Show SMILES CC(C)(O)[C@]12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,wD:4.3,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.67,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.85,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609741

(CHEMBL5284341)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;4.54,-2.51,;4.14,-4,;5.68,-3.91,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207
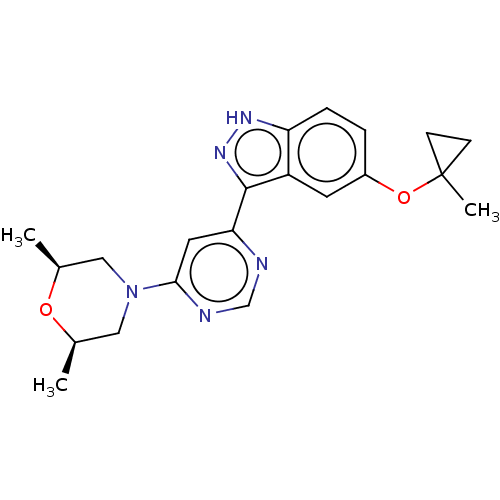
(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609741

(CHEMBL5284341)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;4.54,-2.51,;4.14,-4,;5.68,-3.91,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609742

(CHEMBL5286106)Show SMILES CC(C)(O)[C@]12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)[C@@]1(CC11CC1)C#N |r,wU:25.29,wD:4.3,(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.67,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.85,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207

(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496
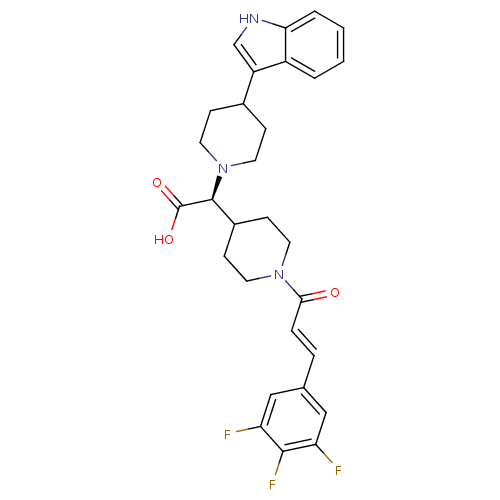
((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium flux |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609738
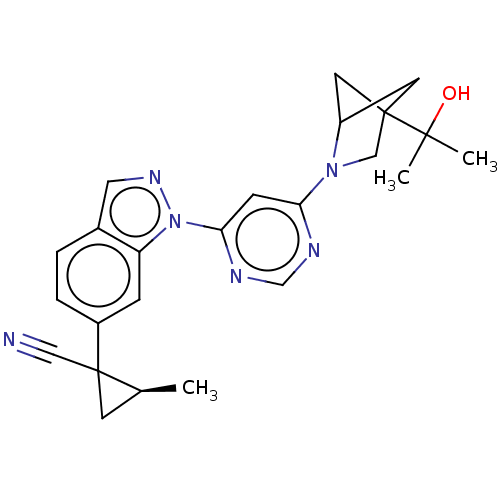
(CHEMBL5267690)Show SMILES C[C@H]1CC1(C#N)c1ccc2cnn(-c3cc(ncn3)N3CC4(CC3C4)C(C)(C)O)c2c1 |r,wU:1.0,(-3.54,-1.2,;-4.87,-.43,;-6.42,-.43,;-5.64,.91,;-6.98,1.68,;-8.31,2.45,;-4.31,1.68,;-4.31,3.23,;-2.98,4,;-1.65,3.23,;-.18,3.7,;.72,2.46,;-.18,1.21,;.22,-.28,;1.7,-.68,;2.1,-2.16,;1.01,-3.25,;-.47,-2.86,;-.88,-1.37,;3.59,-2.56,;4.78,-1.59,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;6.77,-1.06,;6.77,.49,;5.44,-.29,;8.31,-.98,;-1.65,1.69,;-2.97,.92,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609738

(CHEMBL5267690)Show SMILES C[C@H]1CC1(C#N)c1ccc2cnn(-c3cc(ncn3)N3CC4(CC3C4)C(C)(C)O)c2c1 |r,wU:1.0,(-3.54,-1.2,;-4.87,-.43,;-6.42,-.43,;-5.64,.91,;-6.98,1.68,;-8.31,2.45,;-4.31,1.68,;-4.31,3.23,;-2.98,4,;-1.65,3.23,;-.18,3.7,;.72,2.46,;-.18,1.21,;.22,-.28,;1.7,-.68,;2.1,-2.16,;1.01,-3.25,;-.47,-2.86,;-.88,-1.37,;3.59,-2.56,;4.78,-1.59,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;6.77,-1.06,;6.77,.49,;5.44,-.29,;8.31,-.98,;-1.65,1.69,;-2.97,.92,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224501
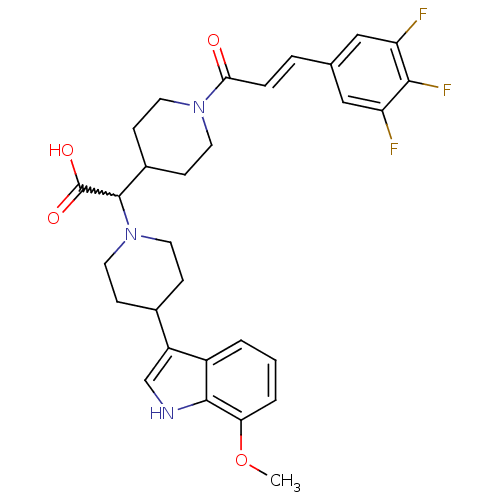
((E)-2-(4-(7-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1cccc2c(c[nH]c12)C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:17.41| Show InChI InChI=1S/C30H32F3N3O4/c1-40-25-4-2-3-21-22(17-34-28(21)25)19-7-13-36(14-8-19)29(30(38)39)20-9-11-35(12-10-20)26(37)6-5-18-15-23(31)27(33)24(32)16-18/h2-6,15-17,19-20,29,34H,7-14H2,1H3,(H,38,39)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609729

(CHEMBL5290520)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1ncn2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224502
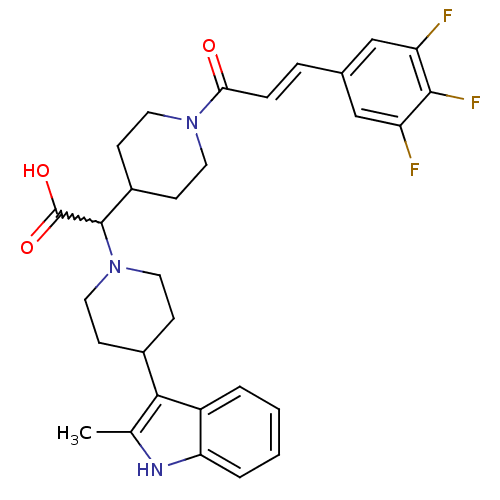
((E)-2-(4-(2-methyl-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:16.40| Show InChI InChI=1S/C30H32F3N3O3/c1-18-27(22-4-2-3-5-25(22)34-18)20-8-14-36(15-9-20)29(30(38)39)21-10-12-35(13-11-21)26(37)7-6-19-16-23(31)28(33)24(32)17-19/h2-7,16-17,20-21,29,34H,8-15H2,1H3,(H,38,39)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224500

((E)-2-(4-(5-amino-1H-indol-3-yl)piperidin-1-yl)-2-...)Show SMILES Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:14.36| Show InChI InChI=1S/C29H31F3N4O3/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)35-9-7-19(8-10-35)28(29(38)39)36-11-5-18(6-12-36)22-16-34-25-3-2-20(33)15-21(22)25/h1-4,13-16,18-19,28,34H,5-12,33H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609734
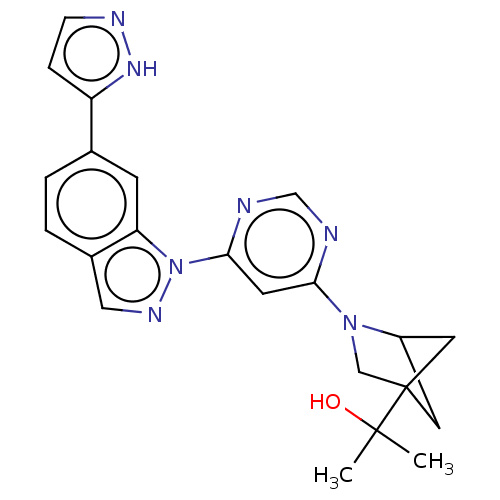
(CHEMBL5279615)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |(8.37,-.94,;7.28,-2.03,;6.88,-.54,;8.37,-3.12,;5.79,-2.43,;5.39,-3.92,;3.85,-4,;4.42,-2.54,;3.3,-2.56,;4.5,-1.59,;1.81,-2.16,;1.42,-.68,;-.07,-.28,;-1.16,-1.37,;-.76,-2.86,;.72,-3.25,;-.47,1.21,;.44,2.46,;-.47,3.7,;-1.93,3.23,;-3.26,4,;-4.6,3.23,;-4.6,1.68,;-3.26,.92,;-1.93,1.69,;-5.93,.91,;-7.34,1.54,;-8.37,.39,;-7.6,-.94,;-6.09,-.62,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224523
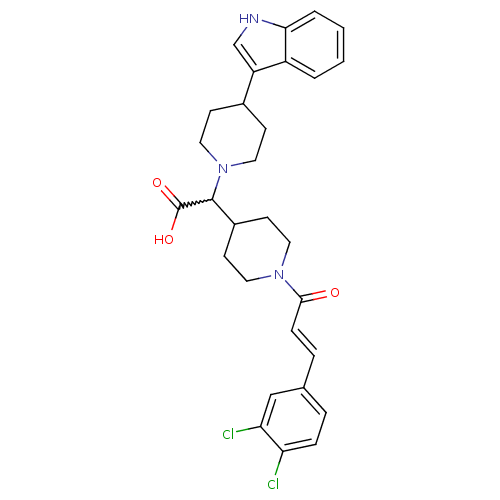
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31Cl2N3O3/c30-24-7-5-19(17-25(24)31)6-8-27(35)33-13-11-21(12-14-33)28(29(36)37)34-15-9-20(10-16-34)23-18-32-26-4-2-1-3-22(23)26/h1-8,17-18,20-21,28,32H,9-16H2,(H,36,37)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609728

(CHEMBL5267350)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1n[nH]c2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224511
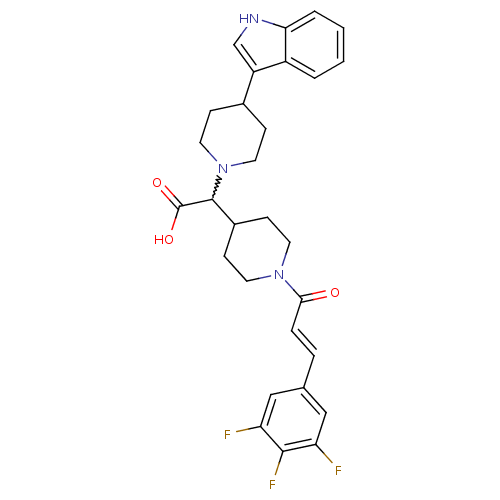
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609733
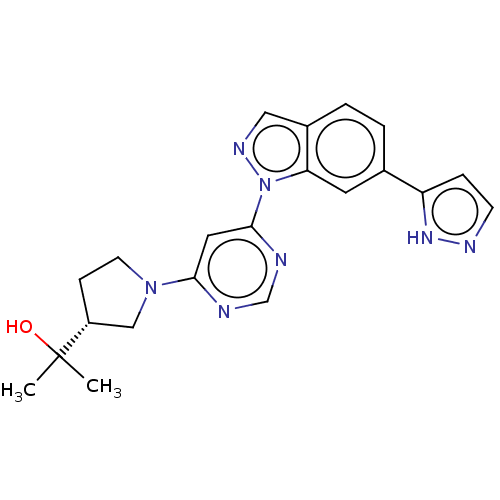
(CHEMBL5282583)Show SMILES CC(C)(O)[C@@H]1CCN(C1)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224524
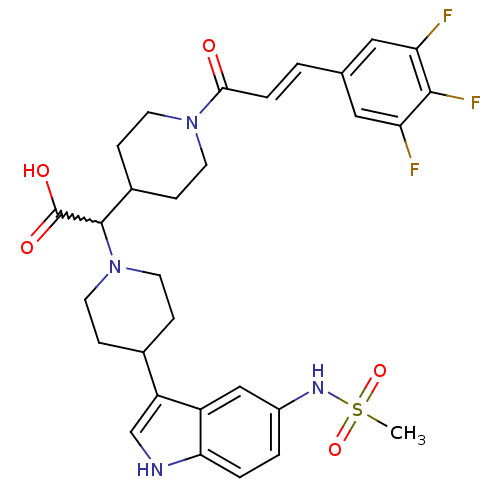
((E)-2-(4-(5-(methylsulfonamido)-1H-indol-3-yl)pipe...)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:18.40| Show InChI InChI=1S/C30H33F3N4O5S/c1-43(41,42)35-21-3-4-26-22(16-21)23(17-34-26)19-6-12-37(13-7-19)29(30(39)40)20-8-10-36(11-9-20)27(38)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34-35H,6-13H2,1H3,(H,39,40)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224519
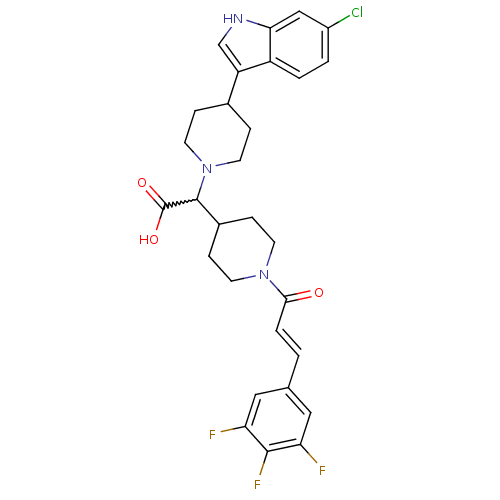
((E)-2-(4-(6-chloro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2cc(Cl)ccc12 |w:3.2| Show InChI InChI=1S/C29H29ClF3N3O3/c30-20-2-3-21-22(16-34-25(21)15-20)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224512
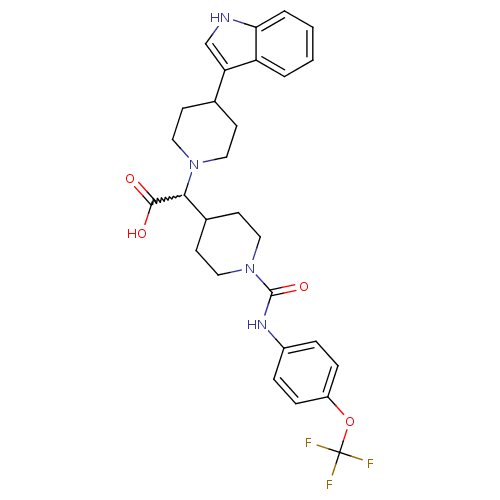
(2-(1-((4-(trifluoromethoxy)phenyl)carbamoyl)piperi...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(OC(F)(F)F)cc1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C28H31F3N4O4/c29-28(30,31)39-21-7-5-20(6-8-21)33-27(38)35-15-11-19(12-16-35)25(26(36)37)34-13-9-18(10-14-34)23-17-32-24-4-2-1-3-22(23)24/h1-8,17-19,25,32H,9-16H2,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609731
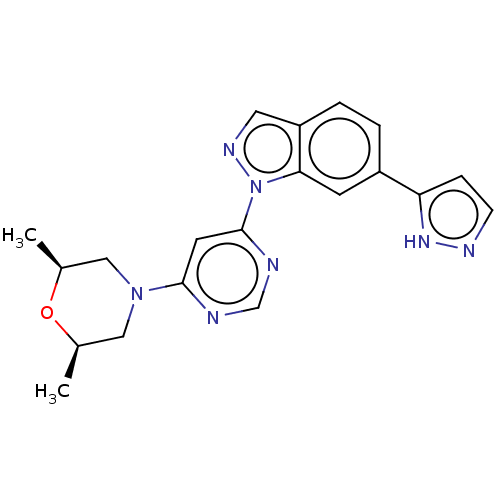
(CHEMBL5283154)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609737
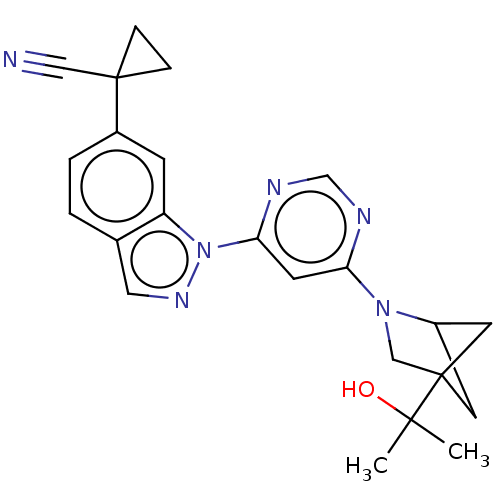
(CHEMBL5266092)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)C1(CC1)C#N |(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.22,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.88,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224508
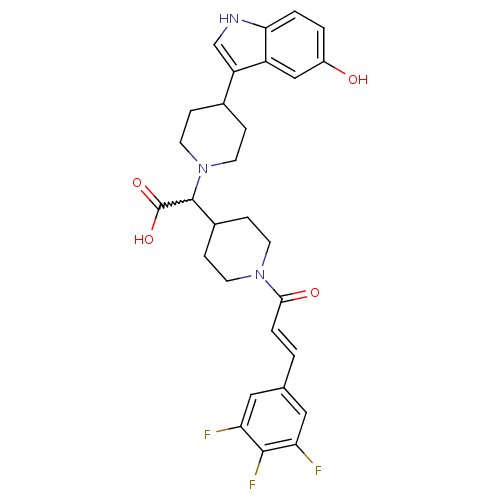
((E)-2-(4-(5-hydroxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(O)cc12 |w:3.2| Show InChI InChI=1S/C29H30F3N3O4/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)34-9-7-19(8-10-34)28(29(38)39)35-11-5-18(6-12-35)22-16-33-25-3-2-20(36)15-21(22)25/h1-4,13-16,18-19,28,33,36H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224510
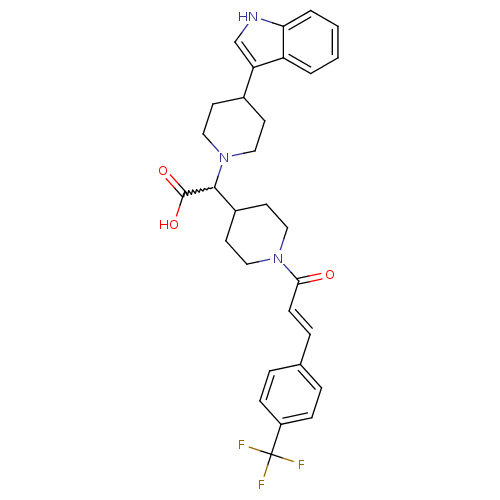
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(cc1)C(F)(F)F)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C30H32F3N3O3/c31-30(32,33)23-8-5-20(6-9-23)7-10-27(37)35-15-13-22(14-16-35)28(29(38)39)36-17-11-21(12-18-36)25-19-34-26-4-2-1-3-24(25)26/h1-10,19,21-22,28,34H,11-18H2,(H,38,39)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224521

((E)-2-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:15.37| Show InChI InChI=1S/C30H32F3N3O4/c1-40-21-3-4-26-22(16-21)23(17-34-26)19-6-12-36(13-7-19)29(30(38)39)20-8-10-35(11-9-20)27(37)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34H,6-13H2,1H3,(H,38,39)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609733

(CHEMBL5282583)Show SMILES CC(C)(O)[C@@H]1CCN(C1)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609734

(CHEMBL5279615)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |(8.37,-.94,;7.28,-2.03,;6.88,-.54,;8.37,-3.12,;5.79,-2.43,;5.39,-3.92,;3.85,-4,;4.42,-2.54,;3.3,-2.56,;4.5,-1.59,;1.81,-2.16,;1.42,-.68,;-.07,-.28,;-1.16,-1.37,;-.76,-2.86,;.72,-3.25,;-.47,1.21,;.44,2.46,;-.47,3.7,;-1.93,3.23,;-3.26,4,;-4.6,3.23,;-4.6,1.68,;-3.26,.92,;-1.93,1.69,;-5.93,.91,;-7.34,1.54,;-8.37,.39,;-7.6,-.94,;-6.09,-.62,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224499
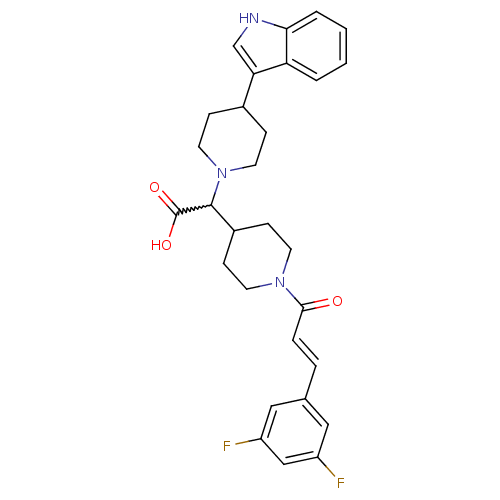
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31F2N3O3/c30-22-15-19(16-23(31)17-22)5-6-27(35)33-11-9-21(10-12-33)28(29(36)37)34-13-7-20(8-14-34)25-18-32-26-4-2-1-3-24(25)26/h1-6,15-18,20-21,28,32H,7-14H2,(H,36,37)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224506
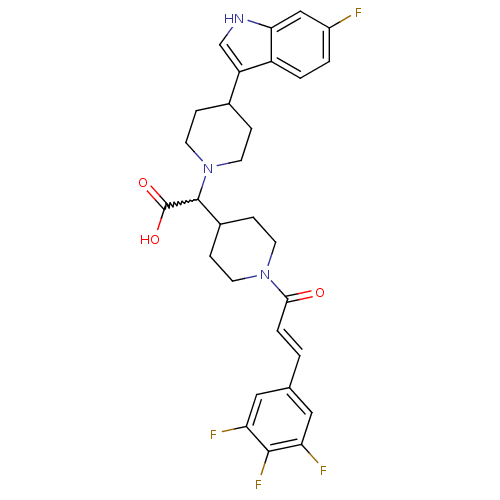
((E)-2-(4-(6-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2cc(F)ccc12 |w:3.2| Show InChI InChI=1S/C29H29F4N3O3/c30-20-2-3-21-22(16-34-25(21)15-20)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609732
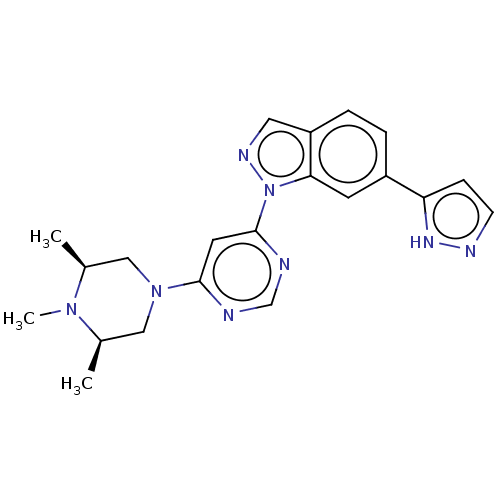
(CHEMBL5276834)Show SMILES C[C@H]1CN(C[C@@H](C)N1C)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609729

(CHEMBL5290520)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1ncn2ccc(cc12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609734

(CHEMBL5279615)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |(8.37,-.94,;7.28,-2.03,;6.88,-.54,;8.37,-3.12,;5.79,-2.43,;5.39,-3.92,;3.85,-4,;4.42,-2.54,;3.3,-2.56,;4.5,-1.59,;1.81,-2.16,;1.42,-.68,;-.07,-.28,;-1.16,-1.37,;-.76,-2.86,;.72,-3.25,;-.47,1.21,;.44,2.46,;-.47,3.7,;-1.93,3.23,;-3.26,4,;-4.6,3.23,;-4.6,1.68,;-3.26,.92,;-1.93,1.69,;-5.93,.91,;-7.34,1.54,;-8.37,.39,;-7.6,-.94,;-6.09,-.62,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609731

(CHEMBL5283154)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224516

(2-(1-((3,4-dichlorophenyl)carbamoyl)piperidin-4-yl...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C27H30Cl2N4O3/c28-22-6-5-19(15-23(22)29)31-27(36)33-13-9-18(10-14-33)25(26(34)35)32-11-7-17(8-12-32)21-16-30-24-4-2-1-3-20(21)24/h1-6,15-18,25,30H,7-14H2,(H,31,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609737

(CHEMBL5266092)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)C1(CC1)C#N |(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.22,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.88,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609739

(CHEMBL5266616)Show SMILES CC(C)(O)C12CC(C1)N(C2)c1cc(ncn1)-n1ncc2ccc(cc12)C1(CC1(C)C)C#N |(6.77,.49,;6.77,-1.06,;5.44,-.29,;8.31,-.98,;6.07,-2.43,;5.68,-3.91,;4.14,-4,;4.54,-2.51,;3.59,-2.56,;4.78,-1.59,;2.1,-2.16,;1.7,-.68,;.21,-.28,;-.88,-1.37,;-.47,-2.86,;1.01,-3.25,;-.18,1.21,;.72,2.46,;-.18,3.7,;-1.65,3.23,;-2.98,4,;-4.31,3.23,;-4.31,1.68,;-2.97,.92,;-1.65,1.69,;-5.64,.91,;-6.42,-.43,;-4.87,-.43,;-4.1,-1.76,;-3.33,-.43,;-6.98,1.68,;-8.31,2.45,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50236734

(CHEMBL4071886)Show SMILES CN(c1cccc(c1)S(C)(=O)=O)c1ccc2[nH]nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C20H18N4O2S/c1-24(15-4-3-5-17(12-15)27(2,25)26)16-6-7-19-18(13-16)20(23-22-19)14-8-10-21-11-9-14/h3-13H,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant LRRK2 expressed in baculovirus using fluorescein-ERM as substrate preincubated for 30 mins followed by subs... |
J Med Chem 60: 2983-2992 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00045
BindingDB Entry DOI: 10.7270/Q2H997GZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224498
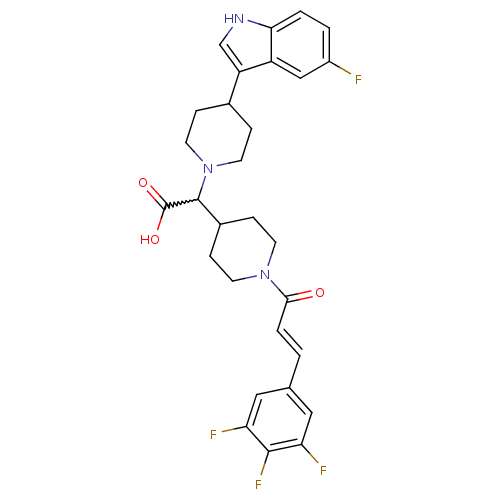
((E)-2-(4-(5-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(F)cc12 |w:3.2| Show InChI InChI=1S/C29H29F4N3O3/c30-20-2-3-25-21(15-20)22(16-34-25)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224515

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-cinna...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccccc1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C29H33N3O3/c33-27(11-10-21-6-2-1-3-7-21)31-16-14-23(15-17-31)28(29(34)35)32-18-12-22(13-19-32)25-20-30-26-9-5-4-8-24(25)26/h1-11,20,22-23,28,30H,12-19H2,(H,34,35)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609732

(CHEMBL5276834)Show SMILES C[C@H]1CN(C[C@@H](C)N1C)c1cc(ncn1)-n1ncc2ccc(cc12)-c1ccn[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM488343
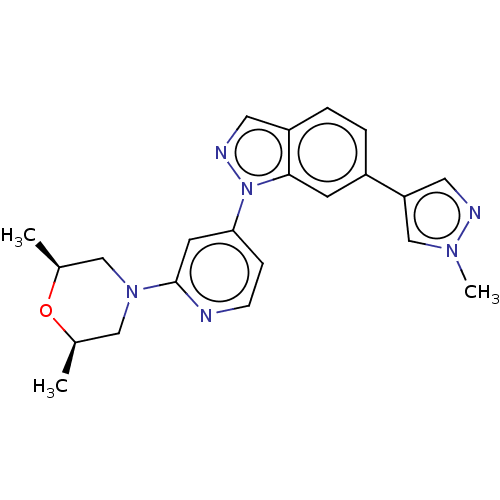
(US10954240, Example 47)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-n1ncc2ccc(cc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C22H24N6O/c1-15-12-27(13-16(2)29-15)22-9-20(6-7-23-22)28-21-8-17(4-5-18(21)10-25-28)19-11-24-26(3)14-19/h4-11,14-16H,12-13H2,1-3H3/t15-,16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224505
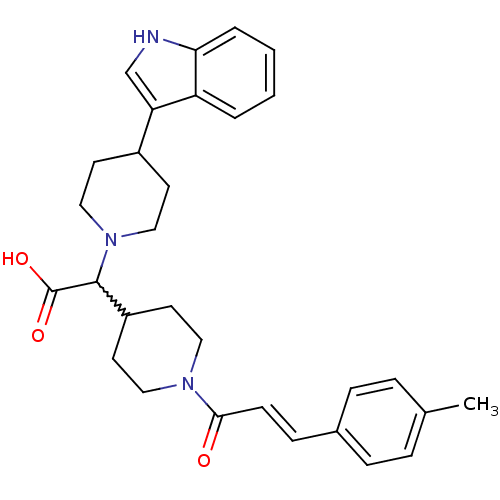
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-p-...)Show SMILES Cc1ccc(\C=C\C(=O)N2CCC(CC2)C(N2CCC(CC2)c2c[nH]c3ccccc23)C(O)=O)cc1 |w:15.15| Show InChI InChI=1S/C30H35N3O3/c1-21-6-8-22(9-7-21)10-11-28(34)32-16-14-24(15-17-32)29(30(35)36)33-18-12-23(13-19-33)26-20-31-27-5-3-2-4-25(26)27/h2-11,20,23-24,29,31H,12-19H2,1H3,(H,35,36)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM488343

(US10954240, Example 47)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-n1ncc2ccc(cc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C22H24N6O/c1-15-12-27(13-16(2)29-15)22-9-20(6-7-23-22)28-21-8-17(4-5-18(21)10-25-28)19-11-24-26(3)14-19/h4-11,14-16H,12-13H2,1-3H3/t15-,16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224517

(2-(1-((4-(trifluoromethyl)phenyl)carbamoyl)piperid...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(cc1)C(F)(F)F)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C28H31F3N4O3/c29-28(30,31)20-5-7-21(8-6-20)33-27(38)35-15-11-19(12-16-35)25(26(36)37)34-13-9-18(10-14-34)23-17-32-24-4-2-1-3-22(23)24/h1-8,17-19,25,32H,9-16H2,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50609730
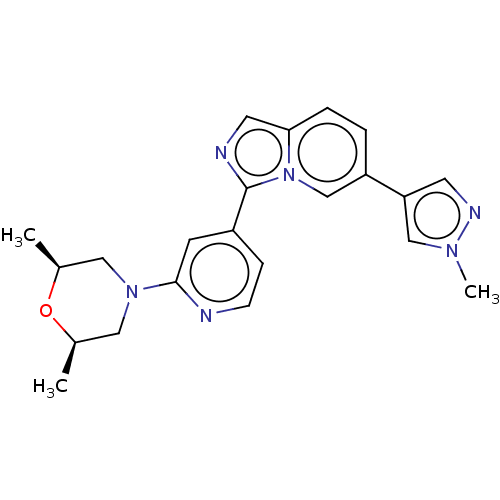
(CHEMBL5268380)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ccn1)-c1ncc2ccc(cn12)-c1cnn(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224514
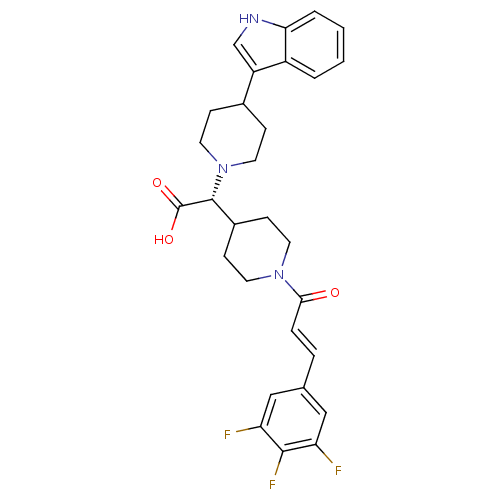
((R,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data