 Found 190 hits with Last Name = 'deck' and Initial = 'lm'
Found 190 hits with Last Name = 'deck' and Initial = 'lm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81608

(Isocoumarin, 9b)Show SMILES NC(=N)SCCCOc1oc(=O)c2cc(NC(=O)c3ccccc3)ccc2c1Cl Show InChI InChI=1S/C20H18ClN3O4S/c21-16-14-8-7-13(24-17(25)12-5-2-1-3-6-12)11-15(14)18(26)28-19(16)27-9-4-10-29-20(22)23/h1-3,5-8,11H,4,9-10H2,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81603
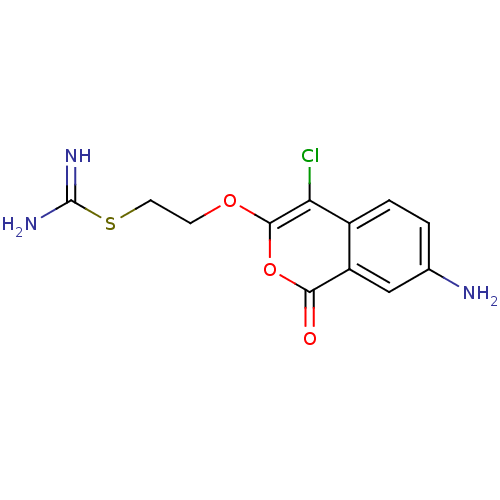
(Isocoumarin, 7a)Show InChI InChI=1S/C12H12ClN3O3S/c13-9-7-2-1-6(14)5-8(7)10(17)19-11(9)18-3-4-20-12(15)16/h1-2,5H,3-4,14H2,(H3,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081700
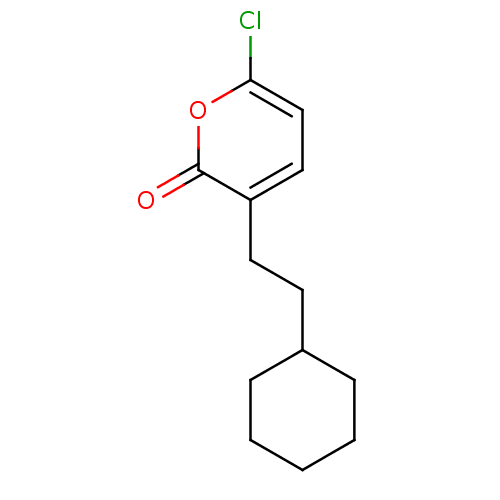
(6-Chloro-3-(2-cyclohexyl-ethyl)-pyran-2-one | CHEM...)Show InChI InChI=1S/C13H17ClO2/c14-12-9-8-11(13(15)16-12)7-6-10-4-2-1-3-5-10/h8-10H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81599
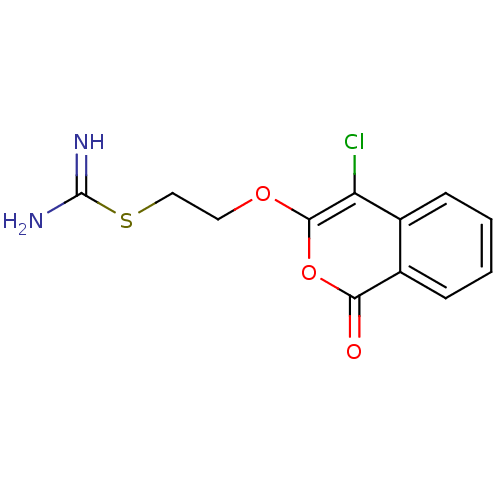
(Isocoumarin, 5c)Show InChI InChI=1S/C12H11ClN2O3S/c13-9-7-3-1-2-4-8(7)10(16)18-11(9)17-5-6-19-12(14)15/h1-4H,5-6H2,(H3,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81606
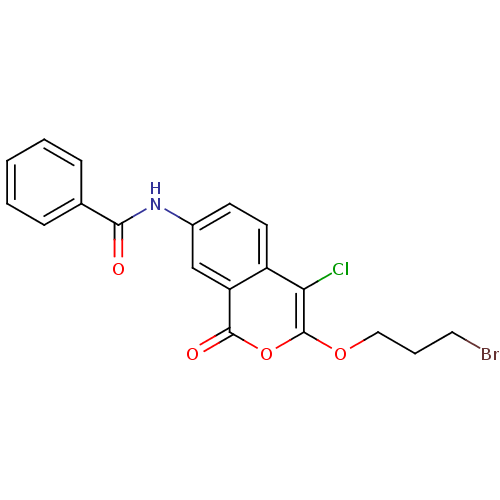
(Isocoumarin, 8b)Show SMILES Clc1c(OCCCBr)oc(=O)c2cc(NC(=O)c3ccccc3)ccc12 Show InChI InChI=1S/C19H15BrClNO4/c20-9-4-10-25-19-16(21)14-8-7-13(11-15(14)18(24)26-19)22-17(23)12-5-2-1-3-6-12/h1-3,5-8,11H,4,9-10H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81604
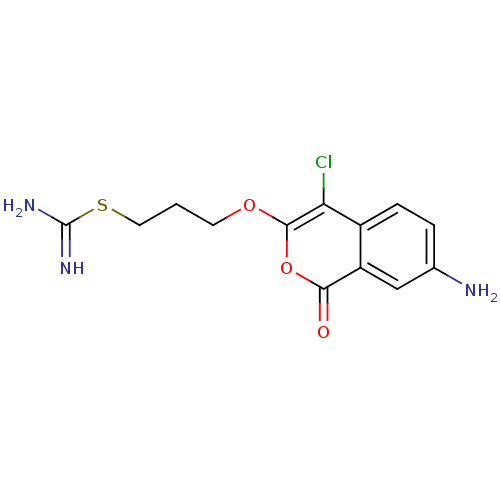
(Isocoumarin, 7b)Show InChI InChI=1S/C13H14ClN3O3S/c14-10-8-3-2-7(15)6-9(8)11(18)20-12(10)19-4-1-5-21-13(16)17/h2-3,6H,1,4-5,15H2,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081696

(6-Chloro-3-cyclopentyl-pyran-2-one | CHEMBL133091)Show InChI InChI=1S/C10H11ClO2/c11-9-6-5-8(10(12)13-9)7-3-1-2-4-7/h5-7H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81600
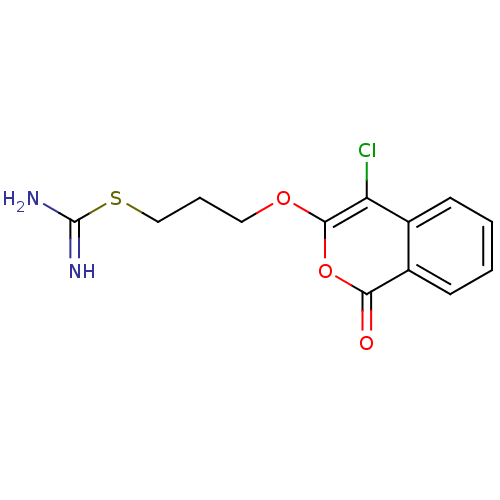
(Isocoumarin, 5d)Show InChI InChI=1S/C13H13ClN2O3S/c14-10-8-4-1-2-5-9(8)11(17)19-12(10)18-6-3-7-20-13(15)16/h1-2,4-5H,3,6-7H2,(H3,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081698
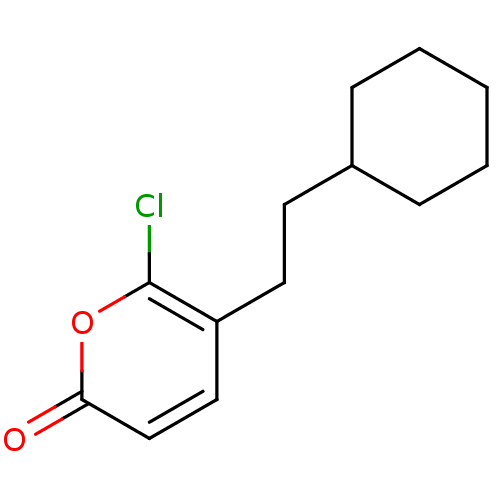
(6-Chloro-5-(2-cyclohexyl-ethyl)-pyran-2-one | CHEM...)Show InChI InChI=1S/C13H17ClO2/c14-13-11(8-9-12(15)16-13)7-6-10-4-2-1-3-5-10/h8-10H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081697

(6-Chloro-3-(3-cyclohexyl-propyl)-pyran-2-one | CHE...)Show InChI InChI=1S/C14H19ClO2/c15-13-10-9-12(14(16)17-13)8-4-7-11-5-2-1-3-6-11/h9-11H,1-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81607
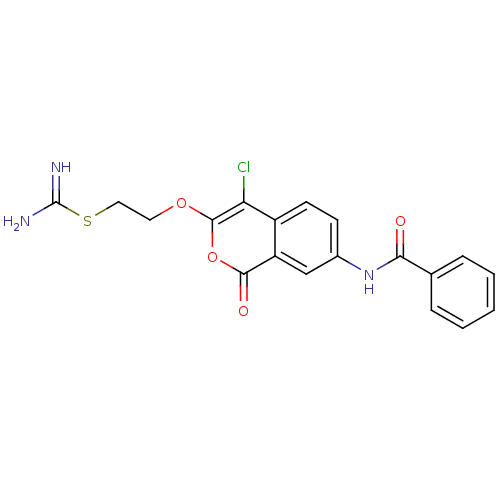
(Isocoumarin, 9a)Show SMILES NC(=N)SCCOc1oc(=O)c2cc(NC(=O)c3ccccc3)ccc2c1Cl Show InChI InChI=1S/C19H16ClN3O4S/c20-15-13-7-6-12(23-16(24)11-4-2-1-3-5-11)10-14(13)17(25)27-18(15)26-8-9-28-19(21)22/h1-7,10H,8-9H2,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066979
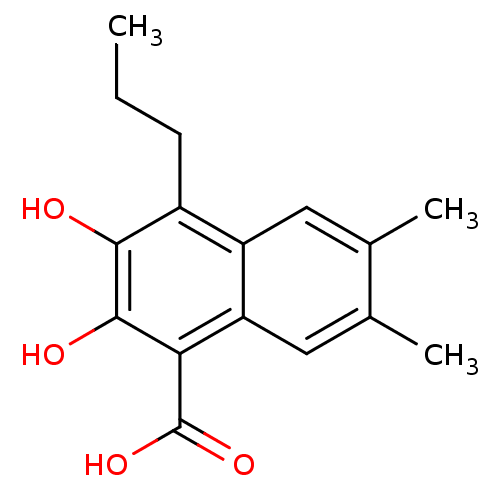
(2,3-Dihydroxy-6,7-dimethyl-4-propyl-naphthalene-1-...)Show InChI InChI=1S/C16H18O4/c1-4-5-10-11-6-8(2)9(3)7-12(11)13(16(19)20)15(18)14(10)17/h6-7,17-18H,4-5H2,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066979

(2,3-Dihydroxy-6,7-dimethyl-4-propyl-naphthalene-1-...)Show InChI InChI=1S/C16H18O4/c1-4-5-10-11-6-8(2)9(3)7-12(11)13(16(19)20)15(18)14(10)17/h6-7,17-18H,4-5H2,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81601
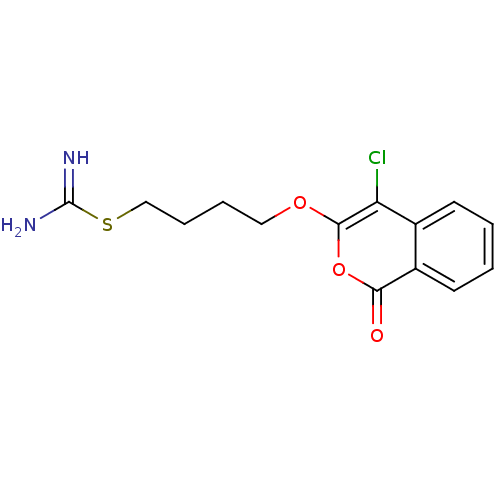
(Isocoumarin, 5e)Show InChI InChI=1S/C14H15ClN2O3S/c15-11-9-5-1-2-6-10(9)12(18)20-13(11)19-7-3-4-8-21-14(16)17/h1-2,5-6H,3-4,7-8H2,(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010449
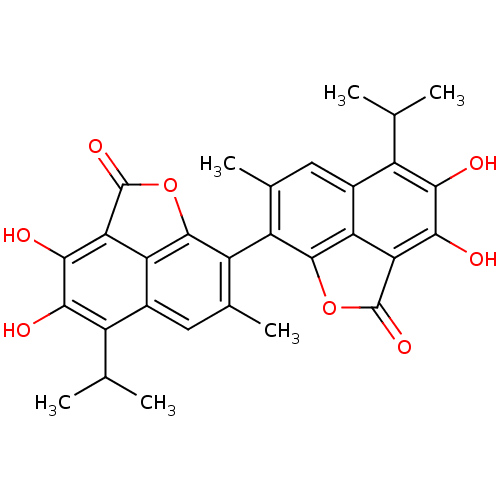
(3,4,3',4'-Tetrahydroxy-5,5'-diisopropyl-7,7'-dimet...)Show SMILES CC(C)c1c(O)c(O)c2C(=O)Oc3c(c(C)cc1c23)-c1c2OC(=O)c3c(O)c(O)c(C(C)C)c(cc1C)c23 |(17.54,-15.98,;16.19,-15.21,;14.86,-15.98,;16.19,-13.65,;17.54,-12.91,;18.85,-13.69,;17.54,-11.35,;18.85,-10.58,;16.19,-10.58,;16.18,-9.03,;17.26,-7.93,;13.55,-9.03,;13.55,-10.58,;12.21,-11.35,;12.21,-12.91,;12.24,-14.01,;13.55,-13.65,;14.86,-12.91,;14.86,-11.35,;9.55,-11.35,;8.21,-10.58,;8.21,-9.03,;5.59,-9.03,;4.5,-7.93,;5.59,-10.58,;4.26,-11.35,;2.92,-10.58,;4.26,-12.91,;2.92,-13.69,;5.59,-13.65,;5.59,-15.21,;6.92,-15.98,;4.26,-15.98,;6.92,-12.91,;8.21,-13.65,;9.55,-12.91,;9.55,-14.01,;6.92,-11.35,)| Show InChI InChI=1S/C30H26O8/c1-9(2)15-13-7-11(5)17(27-19(13)21(29(35)37-27)25(33)23(15)31)18-12(6)8-14-16(10(3)4)24(32)26(34)22-20(14)28(18)38-30(22)36/h7-10,31-34H,1-6H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066973

(2,3-Dihydroxy-4-isopropyl-6-methyl-7-(4-trifluorom...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccc(cc3)C(F)(F)F)c(C)cc12 Show InChI InChI=1S/C23H21F3O4/c1-11(2)18-16-8-12(3)14(9-13-4-6-15(7-5-13)23(24,25)26)10-17(16)19(22(29)30)21(28)20(18)27/h4-8,10-11,27-28H,9H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066975

(7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-12(2)18-16-9-13(3)15(10-14-7-5-4-6-8-14)11-17(16)19(22(25)26)21(24)20(18)23/h4-9,11-12,23-24H,10H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010436

(6,7,6',7'-Tetrahydroxy-5,5'-diisopropyl-1,1'-dimet...)Show SMILES COc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1OC |(9.55,-8.26,;8.21,-9.03,;8.21,-10.58,;9.55,-11.35,;9.55,-12.91,;9.55,-14.01,;8.21,-13.65,;6.92,-12.91,;5.59,-13.65,;5.59,-15.21,;6.92,-15.98,;4.26,-15.98,;4.26,-12.91,;2.92,-13.69,;4.26,-11.35,;2.92,-10.58,;5.59,-10.58,;5.59,-9.03,;4.5,-7.93,;6.92,-11.35,;12.21,-11.35,;12.21,-12.91,;12.24,-14.01,;13.55,-13.65,;14.86,-12.91,;16.19,-13.65,;16.19,-15.21,;17.54,-15.98,;14.86,-15.98,;17.54,-12.91,;18.85,-13.69,;17.54,-11.35,;18.85,-10.58,;16.19,-10.58,;16.18,-9.03,;17.26,-7.93,;14.86,-11.35,;13.55,-10.58,;13.55,-9.03,;12.21,-8.26,)| Show InChI InChI=1S/C32H34O8/c1-13(2)21-17-9-15(5)23(31(39-7)25(17)19(11-33)27(35)29(21)37)24-16(6)10-18-22(14(3)4)30(38)28(36)20(12-34)26(18)32(24)40-8/h9-14,35-38H,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066976

(7-Benzyl-2,3-dihydroxy-4,6-dimethyl-naphthalene-1-...)Show InChI InChI=1S/C20H18O4/c1-11-8-15-12(2)18(21)19(22)17(20(23)24)16(15)10-14(11)9-13-6-4-3-5-7-13/h3-8,10,21-22H,9H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM23223
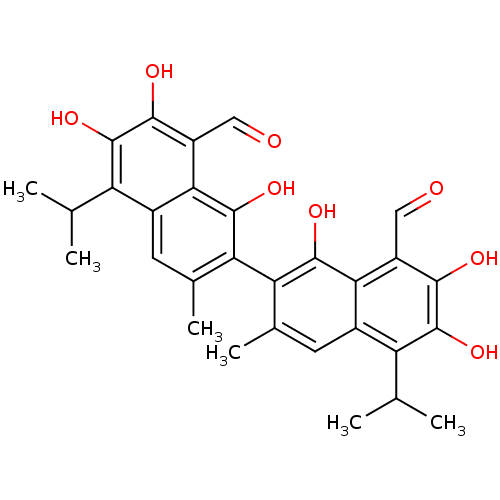
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against Aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081695
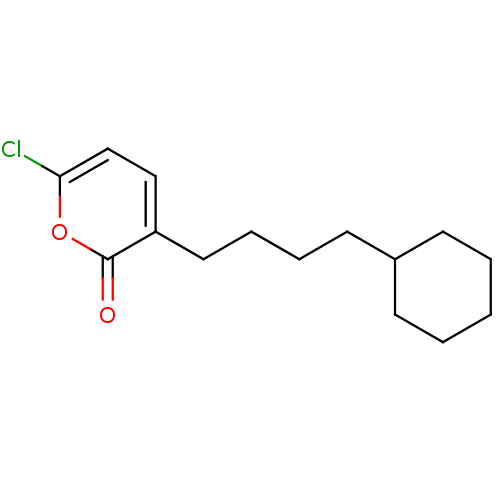
(6-Chloro-3-(4-cyclohexyl-butyl)-pyran-2-one | CHEM...)Show InChI InChI=1S/C15H21ClO2/c16-14-11-10-13(15(17)18-14)9-5-4-8-12-6-2-1-3-7-12/h10-12H,1-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066975

(7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-12(2)18-16-9-13(3)15(10-14-7-5-4-6-8-14)11-17(16)19(22(25)26)21(24)20(18)23/h4-9,11-12,23-24H,10H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066977
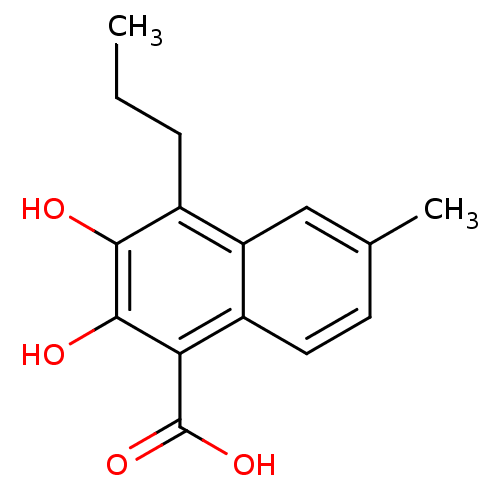
(2,3-Dihydroxy-6-methyl-4-propyl-naphthalene-1-carb...)Show InChI InChI=1S/C15H16O4/c1-3-4-10-11-7-8(2)5-6-9(11)12(15(18)19)14(17)13(10)16/h5-7,16-17H,3-4H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066981
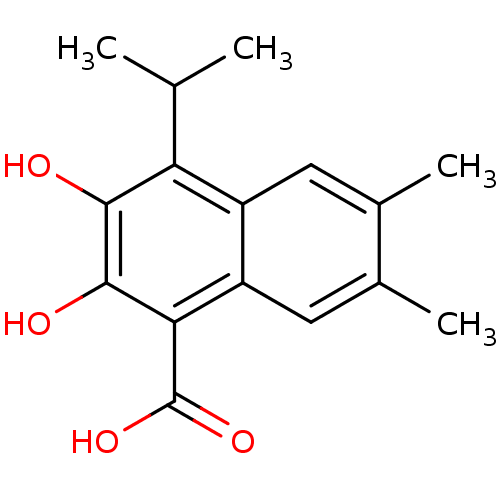
(2,3-Dihydroxy-4-isopropyl-6,7-dimethyl-naphthalene...)Show InChI InChI=1S/C16H18O4/c1-7(2)12-10-5-8(3)9(4)6-11(10)13(16(19)20)15(18)14(12)17/h5-7,17-18H,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-H) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010451

(But-2-enoic acid 1'-but-2-enoyloxy-8,8'-dicyano-6,...)Show SMILES C\C=C\C(=O)Oc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(=O)\C=C\C |(9.21,2.87,;9.21,1.32,;7.88,.55,;7.88,-.99,;9.21,-1.76,;6.55,-1.76,;6.55,-3.3,;7.88,-4.08,;7.88,-5.63,;7.88,-6.74,;6.55,-6.38,;5.25,-5.63,;3.91,-6.38,;3.91,-7.93,;5.25,-8.71,;2.58,-8.71,;2.58,-5.63,;1.25,-6.41,;2.58,-4.08,;1.25,-3.3,;3.91,-3.3,;3.91,-1.76,;3.9,-.22,;5.25,-4.08,;10.55,-4.08,;10.55,-5.63,;10.57,-6.74,;11.88,-6.38,;13.19,-5.63,;14.52,-6.38,;14.52,-7.93,;15.86,-8.71,;13.19,-8.71,;15.86,-5.63,;17.19,-6.41,;15.86,-4.08,;17.19,-3.3,;14.52,-3.3,;14.5,-1.76,;14.5,-.22,;13.19,-4.08,;11.88,-3.3,;11.88,-1.76,;13.21,-.99,;11.86,-.21,;13.21,.55,;14.54,1.32,;14.54,2.87,)| Show InChI InChI=1S/C38H36N2O8/c1-9-11-25(41)47-37-29(19(7)13-21-27(17(3)4)35(45)33(43)23(15-39)31(21)37)30-20(8)14-22-28(18(5)6)36(46)34(44)24(16-40)32(22)38(30)48-26(42)12-10-2/h9-14,17-18,43-46H,1-8H3/b11-9+,12-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against Aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81605
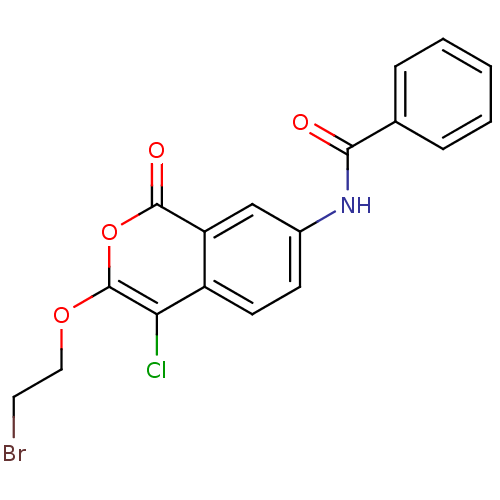
(Isocoumarin, 8a)Show SMILES Clc1c(OCCBr)oc(=O)c2cc(NC(=O)c3ccccc3)ccc12 Show InChI InChI=1S/C18H13BrClNO4/c19-8-9-24-18-15(20)13-7-6-12(10-14(13)17(23)25-18)21-16(22)11-4-2-1-3-5-11/h1-7,10H,8-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010446
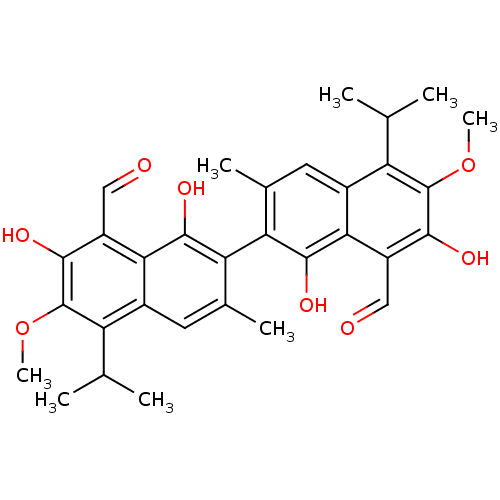
(1,7,1',7'-Tetrahydroxy-5,5'-diisopropyl-6,6'-dimet...)Show SMILES COc1c(O)c(C=O)c2c(O)c(c(C)cc2c1C(C)C)-c1c(C)cc2c(C(C)C)c(OC)c(O)c(C=O)c2c1O |(5.52,-15.79,;4.18,-15.03,;2.85,-15.81,;2.87,-17.36,;4.21,-18.11,;1.54,-18.13,;1.55,-19.67,;.22,-20.45,;.21,-17.37,;-1.11,-18.15,;-1.1,-19.69,;-2.45,-17.39,;-2.46,-15.86,;-3.81,-15.1,;-1.14,-15.08,;.21,-15.84,;1.52,-15.06,;1.51,-13.52,;2.84,-12.74,;.17,-12.76,;-3.78,-18.16,;-3.77,-19.71,;-2.43,-20.48,;-5.1,-20.49,;-6.44,-19.72,;-7.77,-20.5,;-7.77,-22.04,;-9.1,-22.81,;-6.43,-22.81,;-9.1,-19.73,;-10.44,-20.5,;-11.96,-19.65,;-9.1,-18.18,;-10.44,-17.41,;-7.77,-17.41,;-7.78,-15.87,;-6.45,-15.1,;-6.44,-18.18,;-5.11,-17.4,;-5.12,-15.86,)| Show InChI InChI=1S/C32H34O8/c1-13(2)21-17-9-15(5)23(29(37)25(17)19(11-33)27(35)31(21)39-7)24-16(6)10-18-22(14(3)4)32(40-8)28(36)20(12-34)26(18)30(24)38/h9-14,35-38H,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against Aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81594

(Isocoumarin, 4a)Show InChI InChI=1S/C11H7BrClNO5/c12-3-4-18-11-9(13)7-2-1-6(14(16)17)5-8(7)10(15)19-11/h1-2,5H,3-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066981

(2,3-Dihydroxy-4-isopropyl-6,7-dimethyl-naphthalene...)Show InChI InChI=1S/C16H18O4/c1-7(2)12-10-5-8(3)9(4)6-11(10)13(16(19)20)15(18)14(12)17/h5-7,17-18H,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50031638

(2,3-Dihydroxy-4-isopropyl-6-methyl-naphthalene-1-c...)Show InChI InChI=1S/C15H16O4/c1-7(2)11-10-6-8(3)4-5-9(10)12(15(18)19)14(17)13(11)16/h4-7,16-17H,1-3H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081694

(6-Chloro-3-cyclohexylmethyl-pyran-2-one | CHEMBL44...)Show InChI InChI=1S/C12H15ClO2/c13-11-7-6-10(12(14)15-11)8-9-4-2-1-3-5-9/h6-7,9H,1-5,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
Bile salt-activated lipase
(Homo sapiens (Human)) | BDBM50081699

(6-Chloro-3-cyclohexyl-pyran-2-one | CHEMBL134790)Show InChI InChI=1S/C11H13ClO2/c12-10-7-6-9(11(13)14-10)8-4-2-1-3-5-8/h6-8H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
Curated by ChEMBL
| Assay Description
Inhibition of the Pancreatic Cholesterol Esterase from porcine |
J Med Chem 42: 4250-6 (1999)
BindingDB Entry DOI: 10.7270/Q2KP81C5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81595
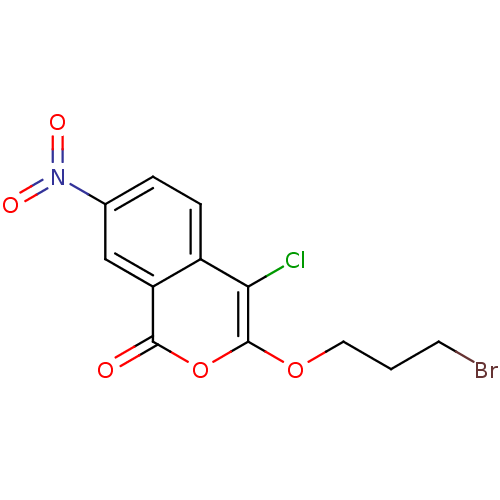
(Isocoumarin, 4b)Show InChI InChI=1S/C12H9BrClNO5/c13-4-1-5-19-12-10(14)8-3-2-7(15(17)18)6-9(8)11(16)20-12/h2-3,6H,1,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010437
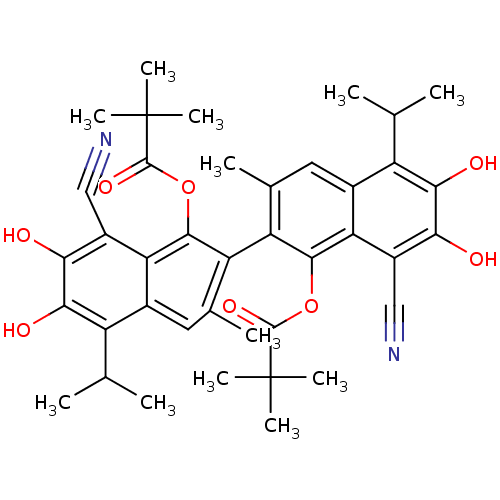
(2,2-Dimethyl-propionic acid 8,8'-dicyano-1'-(2,2-d...)Show SMILES CC(C)c1c(O)c(O)c(C#N)c2c(OC(=O)C(C)(C)C)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(=O)C(C)(C)C |(11.54,-10.43,;12.87,-9.66,;14.22,-10.43,;12.87,-8.1,;14.22,-7.35,;15.53,-8.14,;14.22,-5.8,;15.53,-5.03,;12.87,-5.03,;12.87,-3.48,;12.84,-1.94,;11.54,-5.8,;10.23,-5.03,;10.23,-3.48,;11.57,-2.71,;10.23,-1.94,;11.58,-1.17,;12.91,-.41,;10.25,-.4,;11.18,.32,;8.89,-5.8,;8.89,-7.35,;8.92,-8.46,;10.23,-8.1,;11.54,-7.35,;6.23,-5.8,;6.23,-7.35,;6.23,-8.46,;4.89,-8.1,;3.61,-7.35,;2.28,-8.1,;2.28,-9.66,;.94,-10.43,;3.61,-10.43,;.94,-7.35,;-.39,-8.14,;.94,-5.8,;-.39,-5.03,;2.28,-5.03,;2.28,-3.48,;2.25,-1.94,;3.61,-5.8,;4.89,-5.03,;4.89,-3.48,;6.22,-2.7,;7.56,-3.46,;6.2,-1.15,;4.87,-.4,;5.8,.34,;7.54,-.37,)| Show InChI InChI=1S/C40H44N2O8/c1-17(2)25-21-13-19(5)27(35(49-37(47)39(7,8)9)29(21)23(15-41)31(43)33(25)45)28-20(6)14-22-26(18(3)4)34(46)32(44)24(16-42)30(22)36(28)50-38(48)40(10,11)12/h13-14,17-18,43-46H,1-12H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against Aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010450
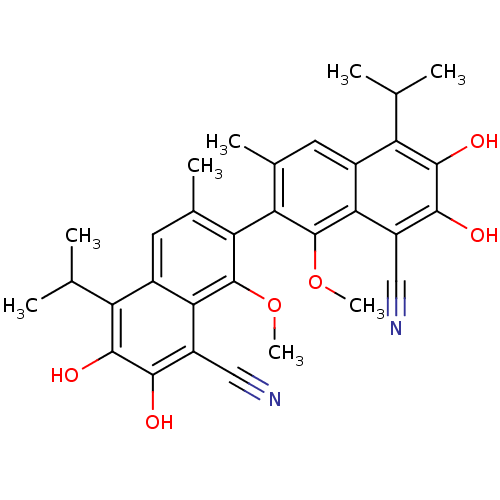
(6,7,6',7'-Tetrahydroxy-5,5'-diisopropyl-1,1'-dimet...)Show SMILES COc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC |(9.55,-8.26,;8.21,-9.03,;8.21,-10.58,;9.55,-11.35,;9.55,-12.91,;9.55,-14.01,;8.21,-13.65,;6.92,-12.91,;5.59,-13.65,;5.59,-15.21,;6.92,-15.98,;4.26,-15.98,;4.26,-12.91,;2.92,-13.69,;4.26,-11.35,;2.92,-10.58,;5.59,-10.58,;5.59,-9.03,;5.57,-7.49,;6.92,-11.35,;12.21,-11.35,;12.21,-12.91,;12.24,-14.01,;13.55,-13.65,;14.86,-12.91,;16.19,-13.65,;16.19,-15.21,;17.54,-15.98,;14.86,-15.98,;17.54,-12.91,;18.85,-13.69,;17.54,-11.35,;18.85,-10.58,;16.19,-10.58,;16.18,-9.03,;16.16,-7.49,;14.86,-11.35,;13.55,-10.58,;13.55,-9.03,;12.21,-8.26,)| Show InChI InChI=1S/C32H32N2O6/c1-13(2)21-17-9-15(5)23(31(39-7)25(17)19(11-33)27(35)29(21)37)24-16(6)10-18-22(14(3)4)30(38)28(36)20(12-34)26(18)32(24)40-8/h9-10,13-14,35-38H,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against Aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50031638

(2,3-Dihydroxy-4-isopropyl-6-methyl-naphthalene-1-c...)Show InChI InChI=1S/C15H16O4/c1-7(2)11-10-6-8(3)4-5-9(10)12(15(18)19)14(17)13(11)16/h4-7,16-17H,1-3H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010440
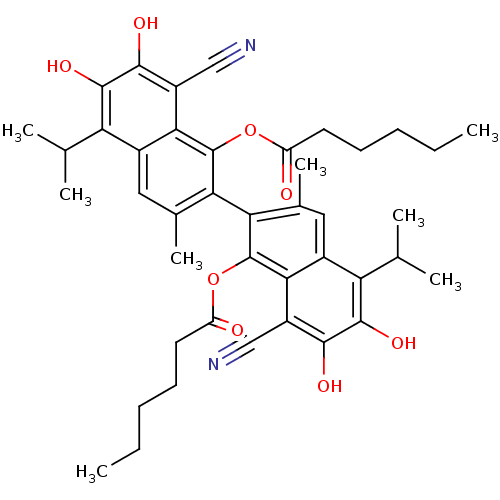
(CHEMBL326383 | Hexanoic acid 8,8'-dicyano-1'-hexan...)Show SMILES CCCCCC(=O)Oc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(=O)CCCCC |(9.32,2.54,;9.32,1,;7.99,.21,;8.01,-1.33,;9.34,-2.08,;9.36,-3.62,;10.69,-4.39,;8.03,-4.41,;8.03,-5.95,;9.36,-6.72,;9.36,-8.27,;9.36,-9.38,;8.03,-9.01,;6.75,-8.27,;5.42,-9.01,;5.42,-10.57,;6.75,-11.34,;4.09,-11.34,;4.09,-8.27,;2.76,-9.05,;4.09,-6.72,;2.75,-5.95,;5.42,-5.95,;5.42,-4.41,;5.4,-2.87,;6.75,-6.72,;12.02,-6.72,;12.02,-8.27,;12.05,-9.38,;13.35,-9.01,;14.66,-8.27,;15.99,-9.01,;15.99,-10.57,;17.34,-11.34,;14.66,-11.34,;17.34,-8.27,;18.67,-9.05,;17.34,-6.72,;18.67,-5.93,;15.99,-5.95,;15.99,-4.41,;15.97,-2.87,;14.66,-6.72,;13.35,-5.95,;13.35,-4.41,;12.02,-3.62,;13.52,-3.23,;12.04,-2.08,;13.38,-1.33,;13.38,.21,;12.05,1,;12.05,2.54,)| Show InChI InChI=1S/C42H48N2O8/c1-9-11-13-15-29(45)51-41-33(23(7)17-25-31(21(3)4)39(49)37(47)27(19-43)35(25)41)34-24(8)18-26-32(22(5)6)40(50)38(48)28(20-44)36(26)42(34)52-30(46)16-14-12-10-2/h17-18,21-22,47-50H,9-16H2,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066980
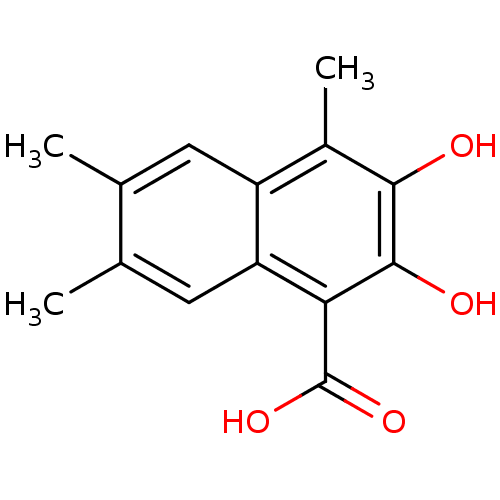
(2,3-Dihydroxy-4,6,7-trimethyl-naphthalene-1-carbox...)Show InChI InChI=1S/C14H14O4/c1-6-4-9-8(3)12(15)13(16)11(14(17)18)10(9)5-7(6)2/h4-5,15-16H,1-3H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-M) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81609
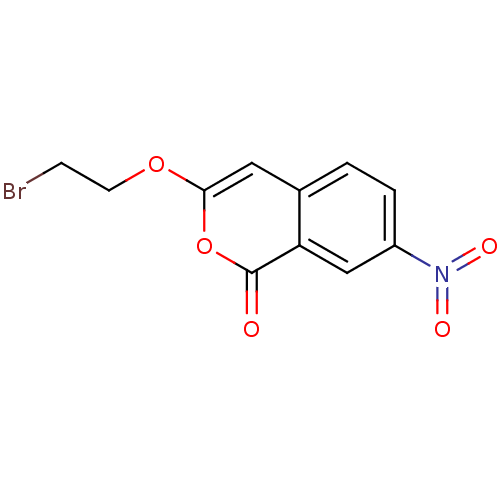
(Isocoumarin, 10a)Show InChI InChI=1S/C11H8BrNO5/c12-3-4-17-10-5-7-1-2-8(13(15)16)6-9(7)11(14)18-10/h1-2,5-6H,3-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81610
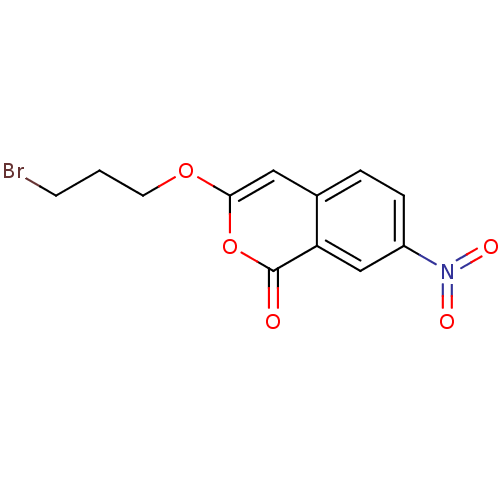
(Isocoumarin, 10b)Show InChI InChI=1S/C12H10BrNO5/c13-4-1-5-18-11-6-8-2-3-9(14(16)17)7-10(8)12(15)19-11/h2-3,6-7H,1,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico
| Assay Description
Inhibition of human urokinase-type plasminogen activator (uPA). |
BMC Chem Biol 6: 1 (2006)
Article DOI: 10.1186/1472-6769-6-1
BindingDB Entry DOI: 10.7270/Q20C4T8F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010447
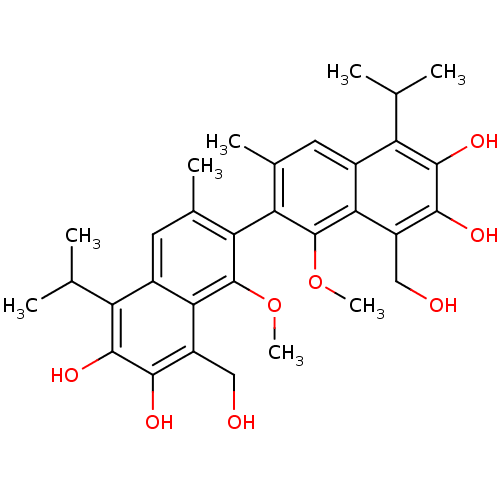
(8,8'-Bis-hydroxymethyl-5,5'-diisopropyl-1,1'-dimet...)Show SMILES COc1c(c(C)cc2c(C(C)C)c(O)c(O)c(CO)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(CO)c2c1OC |(9.55,-8.26,;8.21,-9.03,;8.21,-10.58,;9.55,-11.35,;9.55,-12.91,;9.55,-14.01,;8.21,-13.65,;6.92,-12.91,;5.59,-13.65,;5.59,-15.21,;6.92,-15.98,;4.26,-15.98,;4.26,-12.91,;2.92,-13.69,;4.26,-11.35,;2.92,-10.58,;5.59,-10.58,;5.59,-9.03,;6.93,-8.26,;6.92,-11.35,;12.21,-11.35,;12.21,-12.91,;12.24,-14.01,;13.55,-13.65,;14.86,-12.91,;16.19,-13.65,;16.19,-15.21,;17.54,-15.98,;14.86,-15.98,;17.54,-12.91,;18.85,-13.69,;17.54,-11.35,;18.85,-10.58,;16.19,-10.58,;16.18,-9.03,;14.83,-8.26,;14.86,-11.35,;13.55,-10.58,;13.55,-9.03,;12.21,-8.26,)| Show InChI InChI=1S/C32H38O8/c1-13(2)21-17-9-15(5)23(31(39-7)25(17)19(11-33)27(35)29(21)37)24-16(6)10-18-22(14(3)4)30(38)28(36)20(12-34)26(18)32(24)40-8/h9-10,13-14,33-38H,11-12H2,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against Aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010444
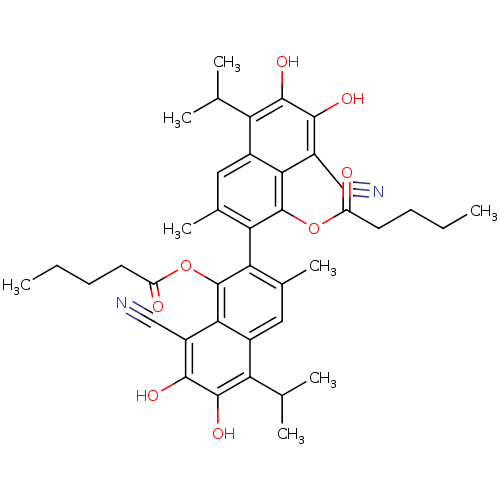
(CHEMBL52639 | Pentanoic acid 8,8'-dicyano-6,7,6',7...)Show SMILES CCCCC(=O)Oc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(=O)CCCC |(8.66,3.2,;7.33,2.42,;7.33,.88,;6,.11,;6,-1.43,;7.33,-2.2,;4.67,-2.2,;4.67,-3.74,;6,-4.51,;6,-6.07,;6,-7.17,;4.67,-6.82,;3.37,-6.07,;2.04,-6.82,;2.04,-8.36,;.71,-9.15,;3.37,-9.15,;.71,-6.07,;-.65,-6.84,;.71,-4.51,;-.65,-3.72,;2.04,-3.74,;2.04,-2.2,;2.04,-.66,;3.37,-4.51,;8.66,-4.51,;8.66,-6.07,;8.68,-7.17,;9.99,-6.82,;11.29,-6.07,;12.61,-6.82,;12.61,-8.36,;13.96,-9.15,;11.29,-9.15,;13.96,-6.07,;15.31,-6.84,;13.96,-4.51,;15.31,-3.72,;12.61,-3.74,;12.61,-2.2,;12.61,-.66,;11.29,-4.51,;9.99,-3.74,;9.99,-2.2,;9.2,-.86,;7.66,-.86,;9.97,.48,;9.19,1.81,;9.95,3.14,;9.18,4.47,)| Show InChI InChI=1S/C40H44N2O8/c1-9-11-13-27(43)49-39-31(21(7)15-23-29(19(3)4)37(47)35(45)25(17-41)33(23)39)32-22(8)16-24-30(20(5)6)38(48)36(46)26(18-42)34(24)40(32)50-28(44)14-12-10-2/h15-16,19-20,45-48H,9-14H2,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010435

(Butyric acid 1'-butyryloxy-8,8'-dicyano-6,7,6',7'-...)Show SMILES CCCC(=O)Oc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(=O)CCC |(9.95,3.14,;9.19,1.81,;9.97,.48,;9.2,-.86,;7.66,-.86,;9.99,-2.2,;9.99,-3.74,;8.66,-4.51,;8.66,-6.07,;8.68,-7.17,;9.99,-6.82,;11.29,-6.07,;12.61,-6.82,;12.61,-8.36,;13.96,-9.15,;11.29,-9.15,;13.96,-6.07,;15.31,-6.84,;13.96,-4.51,;15.31,-3.72,;12.61,-3.74,;12.61,-2.2,;12.61,-.66,;11.29,-4.51,;6,-4.51,;6,-6.07,;6,-7.17,;4.67,-6.82,;3.37,-6.07,;2.04,-6.82,;2.04,-8.36,;.71,-9.15,;3.37,-9.15,;.71,-6.07,;-.65,-6.84,;.71,-4.51,;-.65,-3.72,;2.04,-3.74,;2.04,-2.2,;2.04,-.66,;3.37,-4.51,;4.67,-3.74,;4.67,-2.2,;6,-1.43,;7.33,-2.2,;6,.11,;7.33,.88,;7.33,2.42,)| Show InChI InChI=1S/C38H40N2O8/c1-9-11-25(41)47-37-29(19(7)13-21-27(17(3)4)35(45)33(43)23(15-39)31(21)37)30-20(8)14-22-28(18(5)6)36(46)34(44)24(16-40)32(22)38(30)48-26(42)12-10-2/h13-14,17-18,43-46H,9-12H2,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066977

(2,3-Dihydroxy-6-methyl-4-propyl-naphthalene-1-carb...)Show InChI InChI=1S/C15H16O4/c1-3-4-10-11-7-8(2)5-6-9(11)12(15(18)19)14(17)13(10)16/h5-7,16-17H,3-4H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010439
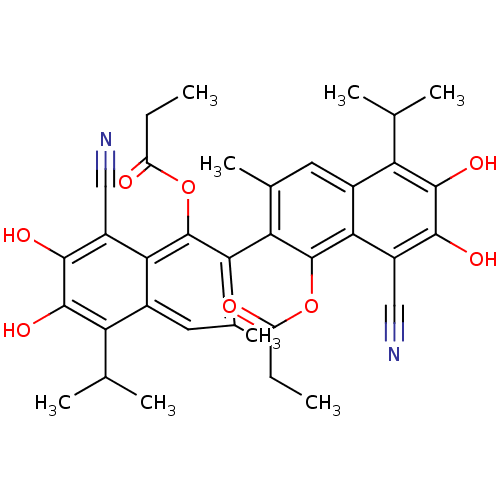
(CHEMBL297483 | Propionic acid 8,8'-dicyano-6,7,6',...)Show SMILES CCC(=O)Oc1c(c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(=O)CC |(7.33,.88,;6,.11,;6,-1.43,;7.33,-2.2,;4.67,-2.2,;4.67,-3.74,;6,-4.51,;6,-6.07,;6,-7.17,;4.67,-6.82,;3.37,-6.07,;2.04,-6.82,;2.04,-8.36,;.71,-9.15,;3.37,-9.15,;.71,-6.07,;-.65,-6.84,;.71,-4.51,;-.65,-3.72,;2.04,-3.74,;2.04,-2.2,;2.04,-.66,;3.37,-4.51,;8.66,-4.51,;8.66,-6.07,;8.68,-7.17,;9.99,-6.82,;11.29,-6.07,;12.61,-6.82,;12.61,-8.36,;13.96,-9.15,;11.29,-9.15,;13.96,-6.07,;15.31,-6.84,;13.96,-4.51,;15.31,-3.72,;12.61,-3.74,;12.61,-2.2,;12.61,-.66,;11.29,-4.51,;9.99,-3.74,;9.99,-2.2,;9.2,-.86,;7.66,-.86,;9.97,.48,;9.19,1.81,)| Show InChI InChI=1S/C36H36N2O8/c1-9-23(39)45-35-27(17(7)11-19-25(15(3)4)33(43)31(41)21(13-37)29(19)35)28-18(8)12-20-26(16(5)6)34(44)32(42)22(14-38)30(20)36(28)46-24(40)10-2/h11-12,15-16,41-44H,9-10H2,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50066975

(7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-12(2)18-16-9-13(3)15(10-14-7-5-4-6-8-14)11-17(16)19(22(25)26)21(24)20(18)23/h4-9,11-12,23-24H,10H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against Human Lactate Dehydrogenase (LDH-H) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50010438

(Acetic acid 1'-acetoxy-8,8'-dicyano-6,7,6',7'-tetr...)Show SMILES CC(C)c1c(O)c(O)c(C#N)c2c(OC(C)=O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(C)=O |(.71,-9.15,;2.04,-8.36,;3.37,-9.15,;2.04,-6.82,;.71,-6.07,;-.65,-6.84,;.71,-4.51,;-.65,-3.72,;2.04,-3.74,;2.04,-2.2,;2.04,-.66,;3.37,-4.51,;4.67,-3.74,;4.67,-2.2,;6,-1.42,;7.33,-2.18,;5.98,.12,;6,-4.51,;6,-6.07,;6,-7.17,;4.67,-6.82,;3.37,-6.07,;8.66,-4.51,;8.66,-6.07,;8.68,-7.17,;9.99,-6.82,;11.29,-6.07,;12.61,-6.82,;12.61,-8.36,;13.96,-9.15,;11.29,-9.15,;13.96,-6.07,;15.31,-6.84,;13.96,-4.51,;15.31,-3.72,;12.61,-3.74,;12.61,-2.2,;12.61,-.66,;11.29,-4.51,;9.99,-3.74,;9.99,-2.2,;8.89,-1.1,;7.4,-1.49,;9.29,.39,)| Show InChI InChI=1S/C34H32N2O8/c1-13(2)23-19-9-15(5)25(33(43-17(7)37)27(19)21(11-35)29(39)31(23)41)26-16(6)10-20-24(14(3)4)32(42)30(40)22(12-36)28(20)34(26)44-18(8)38/h9-10,13-14,39-42H,1-8H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
The compound was tested for inhibitory activity against aldose reductase from human placenta |
J Med Chem 34: 3301-5 (1991)
BindingDB Entry DOI: 10.7270/Q2VM4B7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data