 Found 3077 hits with Last Name = 'demont' and Initial = 'e'
Found 3077 hits with Last Name = 'demont' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572134
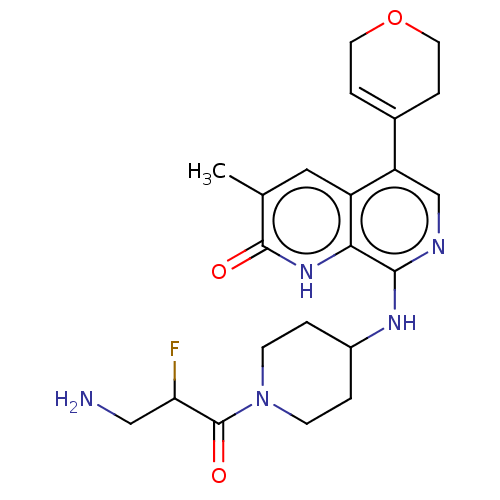
(CHEMBL4868363)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:28| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572127
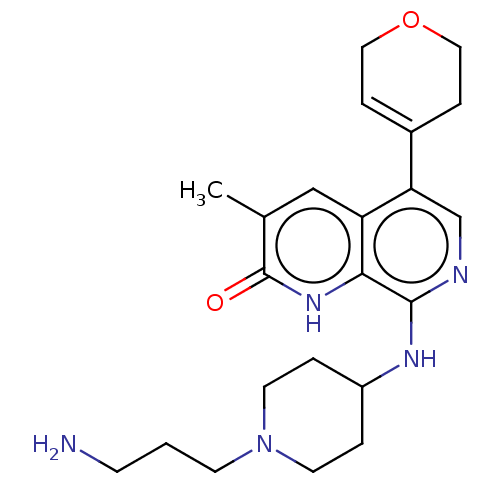
(CHEMBL4850335)Show SMILES Cc1cc2c(cnc(NC3CCN(CCCN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:26| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572130
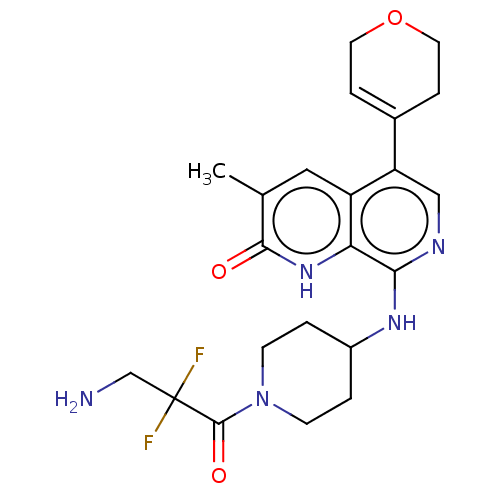
(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572131
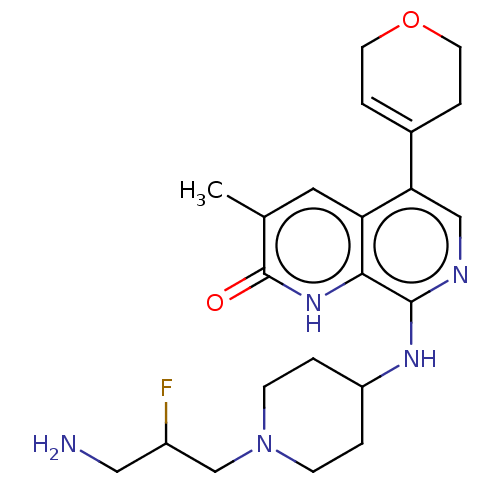
(CHEMBL4873236)Show SMILES Cc1cc2c(cnc(NC3CCN(CC(F)CN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:27| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572133
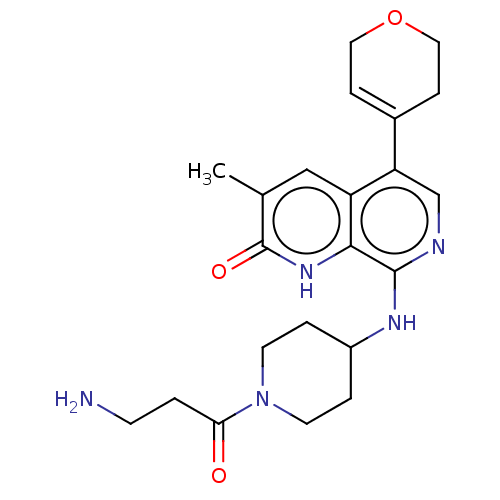
(CHEMBL4854161)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)CCN)c2[nH]c1=O)C1=CCOCC1 |t:27| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50098311
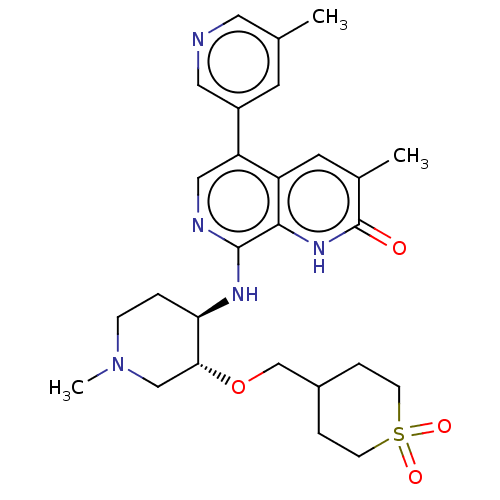
(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to ATAD2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to ATAD2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 9
(Homo sapiens (Human)) | BDBM50572130

(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD9 (R130 to V259 residues) expressed in bacterial expression system measured by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2B
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to ATAD2B (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TAF1 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572132
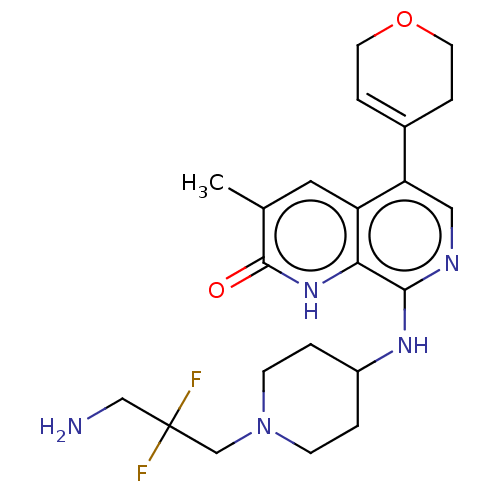
(CHEMBL4874167)Show SMILES Cc1cc2c(cnc(NC3CCN(CC(F)(F)CN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:28| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TAF1L (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572135
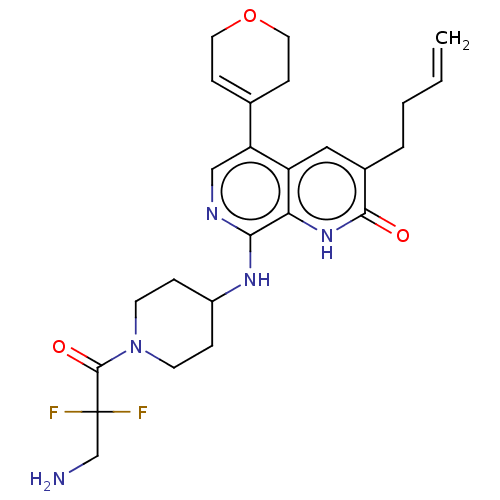
(CHEMBL4871251)Show SMILES NCC(F)(F)C(=O)N1CCC(CC1)Nc1ncc(C2=CCOCC2)c2cc(CCC=C)c(=O)[nH]c12 |t:19| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 1
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRPF2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572134

(CHEMBL4868363)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:28| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572133

(CHEMBL4854161)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)CCN)c2[nH]c1=O)C1=CCOCC1 |t:27| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572130

(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD2 (K333 to E460 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Chromatin remodeling regulator CECR2
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to CECR2 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain and PHD finger-containing protein 3
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRPF3 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2B
(Homo sapiens (Human)) | BDBM50572130

(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BAZ2B (S2054 to S2168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Chromatin remodeling regulator CECR2
(Homo sapiens (Human)) | BDBM50572130

(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length CECR2 (P423 to D543 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572127

(CHEMBL4850335)Show SMILES Cc1cc2c(cnc(NC3CCN(CCCN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:26| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572131

(CHEMBL4873236)Show SMILES Cc1cc2c(cnc(NC3CCN(CC(F)CN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:27| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572130

(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Peregrin
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRPF1 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Peregrin
(Homo sapiens (Human)) | BDBM50572130

(CHEMBL4864027)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:29| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRPF1 (E627 to G740 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572132

(CHEMBL4874167)Show SMILES Cc1cc2c(cnc(NC3CCN(CC(F)(F)CN)CC3)c2[nH]c1=O)C1=CCOCC1 |t:28| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50098311

(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRD4 BD1 (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50572135

(CHEMBL4871251)Show SMILES NCC(F)(F)C(=O)N1CCC(CC1)Nc1ncc(C2=CCOCC2)c2cc(CCC=C)c(=O)[nH]c12 |t:19| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length BRD4 BD1 (N44 to E168 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50148603
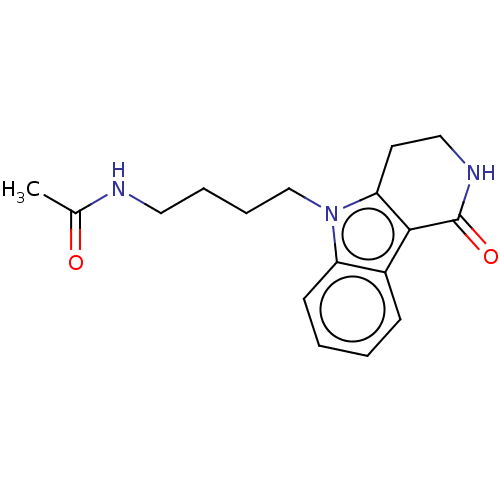
(CHEMBL3770724)Show InChI InChI=1S/C17H21N3O2/c1-12(21)18-9-4-5-11-20-14-7-3-2-6-13(14)16-15(20)8-10-19-17(16)22/h2-3,6-7H,4-5,8-11H2,1H3,(H,18,21)(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
Binding affinity to BRD2 BD1 (unknown origin) by fluorescence anisotropy competition binding assay |
J Med Chem 63: 9020-9044 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00566
BindingDB Entry DOI: 10.7270/Q2348Q0N |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50148603

(CHEMBL3770724)Show InChI InChI=1S/C17H21N3O2/c1-12(21)18-9-4-5-11-20-14-7-3-2-6-13(14)16-15(20)8-10-19-17(16)22/h2-3,6-7H,4-5,8-11H2,1H3,(H,18,21)(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
Binding affinity to BRD4 BD1 (unknown origin) by fluorescence anisotropy competition binding assay |
J Med Chem 63: 9020-9044 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00566
BindingDB Entry DOI: 10.7270/Q2348Q0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50148603

(CHEMBL3770724)Show InChI InChI=1S/C17H21N3O2/c1-12(21)18-9-4-5-11-20-14-7-3-2-6-13(14)16-15(20)8-10-19-17(16)22/h2-3,6-7H,4-5,8-11H2,1H3,(H,18,21)(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
Binding affinity to BRD3 BD1 (unknown origin) by fluorescence anisotropy competition binding assay |
J Med Chem 63: 9020-9044 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00566
BindingDB Entry DOI: 10.7270/Q2348Q0N |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50028142
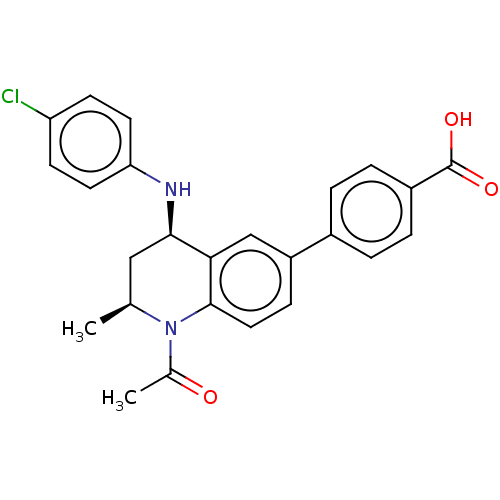
(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Binding affinity to full-length BRD4 short isoform (unknown origin) by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50028142

(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human BRD4 bromodomain 1 by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50028142

(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human BRD4 bromodomain 1/2 by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50028142

(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human BRD3 bromodomain 1 by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Bromodomain and PHD finger-containing protein 3
(Homo sapiens (Human)) | BDBM50028142

(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human BRPF3 by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50576438

(CHEMBL4869149)Show SMILES [H][C@@]12C[C@@H](O)C[C@]1([H])[C@H]2NC(=O)c1cc2c(OC[C@@]2(C)c2ccccc2)c(c1)C(=O)NC |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BRD4 BD2 (333 to 460 residues) expressed in bacterial expression system by bromoscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00344
BindingDB Entry DOI: 10.7270/Q2S46WSG |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 7
(Homo sapiens (Human)) | BDBM50028142

(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human BRD7 by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50576438

(CHEMBL4869149)Show SMILES [H][C@@]12C[C@@H](O)C[C@]1([H])[C@H]2NC(=O)c1cc2c(OC[C@@]2(C)c2ccccc2)c(c1)C(=O)NC |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BRD3 BD2 (306 to 416 residues) expressed in bacterial expression system by bromoscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00344
BindingDB Entry DOI: 10.7270/Q2S46WSG |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50576438

(CHEMBL4869149)Show SMILES [H][C@@]12C[C@@H](O)C[C@]1([H])[C@H]2NC(=O)c1cc2c(OC[C@@]2(C)c2ccccc2)c(c1)C(=O)NC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BRDT BD2 (250 to 382 residues) expressed in bacterial expression system by bromoscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00344
BindingDB Entry DOI: 10.7270/Q2S46WSG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26503
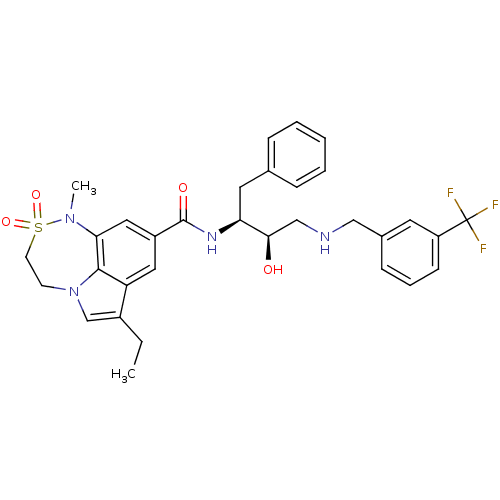
(3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H35F3N4O4S/c1-3-23-20-39-12-13-44(42,43)38(2)28-17-24(16-26(23)30(28)39)31(41)37-27(15-21-8-5-4-6-9-21)29(40)19-36-18-22-10-7-11-25(14-22)32(33,34)35/h4-11,14,16-17,20,27,29,36,40H,3,12-13,15,18-19H2,1-2H3,(H,37,41)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3674-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.149
BindingDB Entry DOI: 10.7270/Q25H7DKK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26788
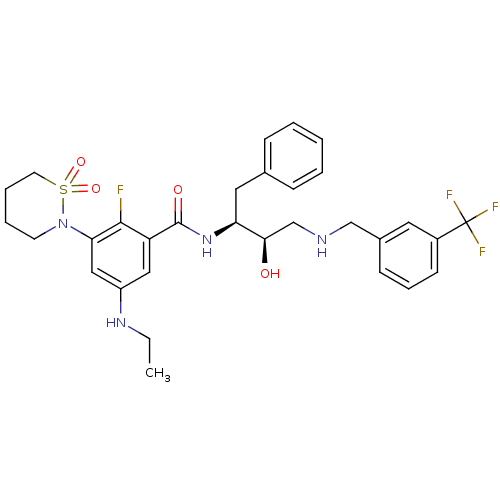
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H36F4N4O4S/c1-2-37-24-17-25(29(32)27(18-24)39-13-6-7-14-44(39,42)43)30(41)38-26(16-21-9-4-3-5-10-21)28(40)20-36-19-22-11-8-12-23(15-22)31(33,34)35/h3-5,8-12,15,17-18,26,28,36-37,40H,2,6-7,13-14,16,19-20H2,1H3,(H,38,41)/t26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.165
BindingDB Entry DOI: 10.7270/Q2F18X23 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26503

(3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H35F3N4O4S/c1-3-23-20-39-12-13-44(42,43)38(2)28-17-24(16-26(23)30(28)39)31(41)37-27(15-21-8-5-4-6-9-21)29(40)19-36-18-22-10-7-11-25(14-22)32(33,34)35/h4-11,14,16-17,20,27,29,36,40H,3,12-13,15,18-19H2,1-2H3,(H,37,41)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
J Med Chem 51: 3313-7 (2008)
Article DOI: 10.1021/jm800138h
BindingDB Entry DOI: 10.7270/Q2XS5SQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 3
(Homo sapiens (Human)) | BDBM50028142

(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human BRD3 bromodomain 2 by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29782
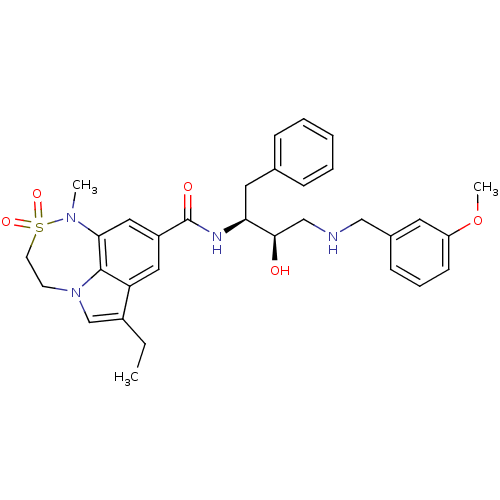
(7,6,5 tricyclic sulfonamide, 22)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC)c1 |r| Show InChI InChI=1S/C32H38N4O5S/c1-4-24-21-36-13-14-42(39,40)35(2)29-18-25(17-27(24)31(29)36)32(38)34-28(16-22-9-6-5-7-10-22)30(37)20-33-19-23-11-8-12-26(15-23)41-3/h5-12,15,17-18,21,28,30,33,37H,4,13-14,16,19-20H2,1-3H3,(H,34,38)/t28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3669-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.150
BindingDB Entry DOI: 10.7270/Q29885BR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26503

(3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H35F3N4O4S/c1-3-23-20-39-12-13-44(42,43)38(2)28-17-24(16-26(23)30(28)39)31(41)37-27(15-21-8-5-4-6-9-21)29(40)19-36-18-22-10-7-11-25(14-22)32(33,34)35/h4-11,14,16-17,20,27,29,36,40H,3,12-13,15,18-19H2,1-2H3,(H,37,41)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3669-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.150
BindingDB Entry DOI: 10.7270/Q29885BR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peregrin
(Homo sapiens (Human)) | BDBM50189403
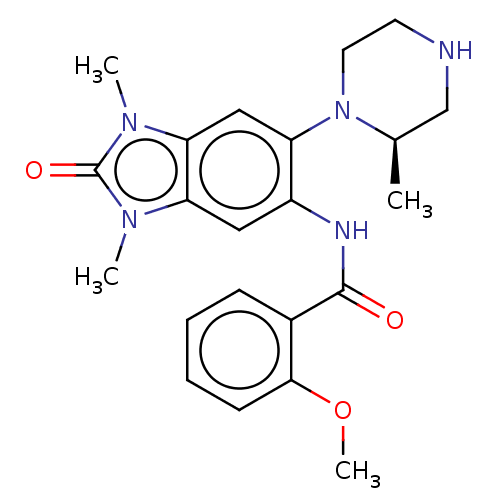
(CHEMBL3828191)Show SMILES COc1ccccc1C(=O)Nc1cc2n(C)c(=O)n(C)c2cc1N1CCNC[C@H]1C |r| Show InChI InChI=1S/C22H27N5O3/c1-14-13-23-9-10-27(14)17-12-19-18(25(2)22(29)26(19)3)11-16(17)24-21(28)15-7-5-6-8-20(15)30-4/h5-8,11-12,14,23H,9-10,13H2,1-4H3,(H,24,28)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length BRPF1 in human HUT78 cell nuclear/chromatin extract after 45 mins by chemoproteomic competition binding assay |
ACS Med Chem Lett 7: 552-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00092
BindingDB Entry DOI: 10.7270/Q2S184G2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain-containing protein 2
(Homo sapiens (Human)) | BDBM50576438

(CHEMBL4869149)Show SMILES [H][C@@]12C[C@@H](O)C[C@]1([H])[C@H]2NC(=O)c1cc2c(OC[C@@]2(C)c2ccccc2)c(c1)C(=O)NC |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BRD2 BD2 (348 to 455 residues) expressed in bacterial expression system by bromoscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00344
BindingDB Entry DOI: 10.7270/Q2S46WSG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322882
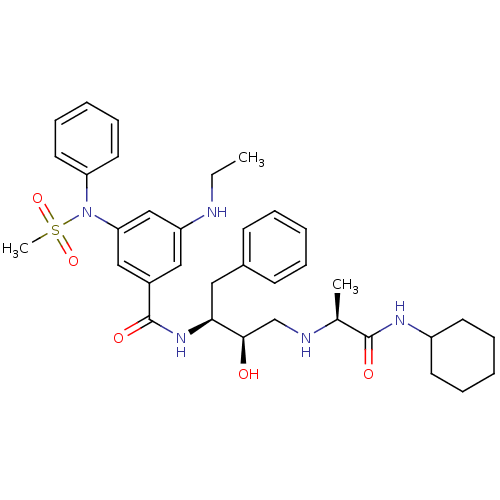
(CHEMBL1210359 | N-((1S,2R)-3-(((1S)-2-(CYCLOHEXYLA...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N(c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C35H47N5O5S/c1-4-36-29-21-27(22-31(23-29)40(46(3,44)45)30-18-12-7-13-19-30)35(43)39-32(20-26-14-8-5-9-15-26)33(41)24-37-25(2)34(42)38-28-16-10-6-11-17-28/h5,7-9,12-15,18-19,21-23,25,28,32-33,36-37,41H,4,6,10-11,16-17,20,24H2,1-3H3,(H,38,42)(H,39,43)/t25-,32-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 4639-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.111
BindingDB Entry DOI: 10.7270/Q2RB75K7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data