 Found 288 hits with Last Name = 'diefenbacher' and Initial = 'c'
Found 288 hits with Last Name = 'diefenbacher' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50474994
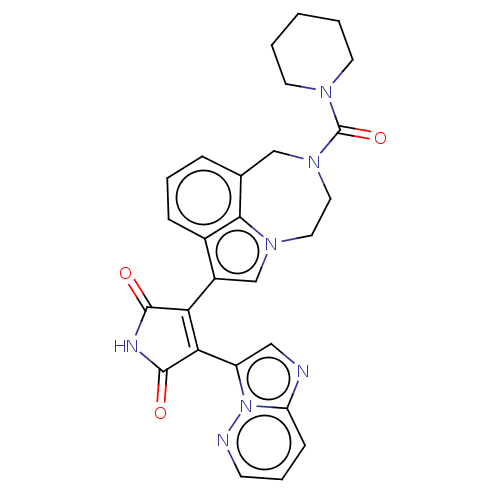
(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50251742

((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...)Show SMILES CC(C)C[C@H](NP(O)(=O)O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C23H34N3O10P/c1-11(2)8-16(26-37(33,34)36-23-20(29)19(28)18(27)12(3)35-23)21(30)25-17(22(31)32)9-13-10-24-15-7-5-4-6-14(13)15/h4-7,10-12,16-20,23-24,27-29H,8-9H2,1-3H3,(H,25,30)(H,31,32)(H2,26,33,34)/t12-,16-,17-,18-,19+,20+,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of enkephalinase activity in membranes prepared from rabbit |
J Med Chem 32: 2519-26 (1989)
BindingDB Entry DOI: 10.7270/Q2668DSV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Homo sapiens (Human)) | BDBM50291559
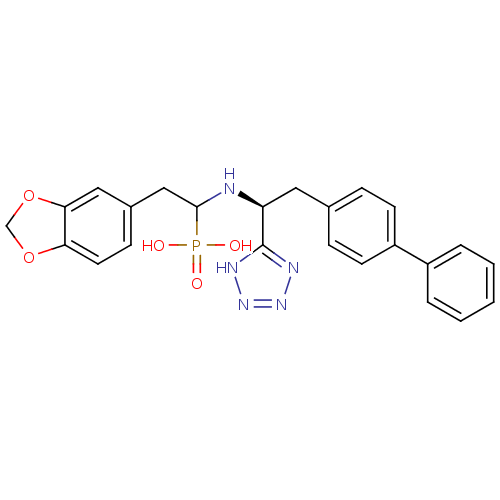
(CHEMBL268473 | {2-Benzo[1,3]dioxol-5-yl-1-[(S)-2-b...)Show SMILES OP(O)(=O)C(Cc1ccc2OCOc2c1)N[C@@H](Cc1ccc(cc1)-c1ccccc1)c1nnn[nH]1 Show InChI InChI=1S/C24H24N5O5P/c30-35(31,32)23(14-17-8-11-21-22(13-17)34-15-33-21)25-20(24-26-28-29-27-24)12-16-6-9-19(10-7-16)18-4-2-1-3-5-18/h1-11,13,20,23,25H,12,14-15H2,(H2,30,31,32)(H,26,27,28,29)/t20-,23?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase 24.11(NEP) |
Bioorg Med Chem Lett 7: 1059-1064 (1997)
Article DOI: 10.1016/S0960-894X(97)00159-5
BindingDB Entry DOI: 10.7270/Q2WH2QGK |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50251742

((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...)Show SMILES CC(C)C[C@H](NP(O)(=O)O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C23H34N3O10P/c1-11(2)8-16(26-37(33,34)36-23-20(29)19(28)18(27)12(3)35-23)21(30)25-17(22(31)32)9-13-10-24-15-7-5-4-6-14(13)15/h4-7,10-12,16-20,23-24,27-29H,8-9H2,1-3H3,(H,25,30)(H,31,32)(H2,26,33,34)/t12-,16-,17-,18-,19+,20+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum |
J Med Chem 32: 2519-26 (1989)
BindingDB Entry DOI: 10.7270/Q2668DSV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Homo sapiens (Human)) | BDBM50291555
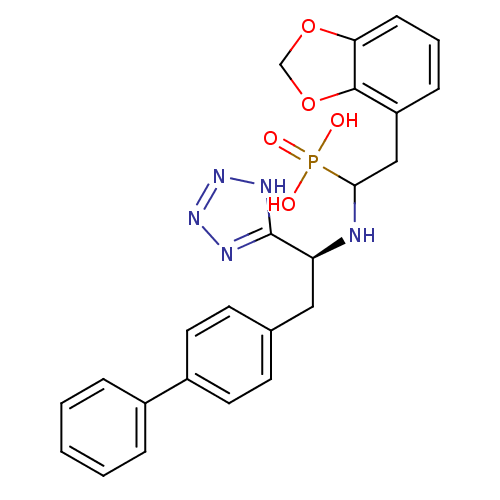
(CHEMBL276690 | {2-Benzo[1,3]dioxol-4-yl-1-[(S)-2-b...)Show SMILES OP(O)(=O)C(Cc1cccc2OCOc12)N[C@@H](Cc1ccc(cc1)-c1ccccc1)c1nnn[nH]1 Show InChI InChI=1S/C24H24N5O5P/c30-35(31,32)22(14-19-7-4-8-21-23(19)34-15-33-21)25-20(24-26-28-29-27-24)13-16-9-11-18(12-10-16)17-5-2-1-3-6-17/h1-12,20,22,25H,13-15H2,(H2,30,31,32)(H,26,27,28,29)/t20-,22?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase 24.11(NEP) |
Bioorg Med Chem Lett 7: 1059-1064 (1997)
Article DOI: 10.1016/S0960-894X(97)00159-5
BindingDB Entry DOI: 10.7270/Q2WH2QGK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50034841
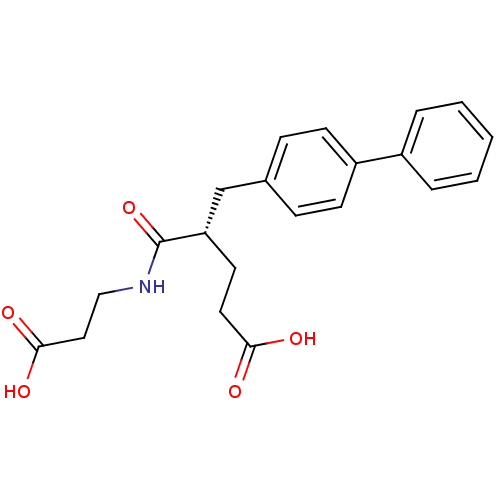
((S)-5-Biphenyl-4-yl-4-(2-carboxy-ethylcarbamoyl)-p...)Show SMILES OC(=O)CCNC(=O)[C@@H](CCC(O)=O)Cc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H23NO5/c23-19(24)11-10-18(21(27)22-13-12-20(25)26)14-15-6-8-17(9-7-15)16-4-2-1-3-5-16/h1-9,18H,10-14H2,(H,22,27)(H,23,24)(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate |
J Med Chem 38: 1689-700 (1995)
BindingDB Entry DOI: 10.7270/Q2Z89BD0 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50034846

((2R,4R)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...)Show SMILES C[C@H](C[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25NO5/c1-15(22(27)28)13-19(23-20(24)11-12-21(25)26)14-16-7-9-18(10-8-16)17-5-3-2-4-6-17/h2-10,15,19H,11-14H2,1H3,(H,23,24)(H,25,26)(H,27,28)/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate |
J Med Chem 38: 1689-700 (1995)
BindingDB Entry DOI: 10.7270/Q2Z89BD0 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50291558
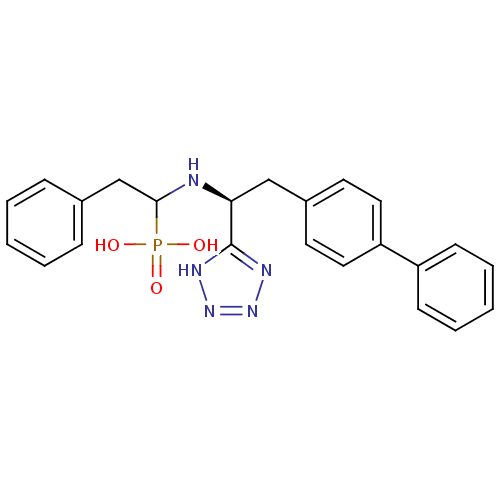
(CHEMBL276872 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetra...)Show SMILES OP(O)(=O)C(Cc1ccccc1)N[C@@H](Cc1ccc(cc1)-c1ccccc1)c1nnn[nH]1 Show InChI InChI=1S/C23H24N5O3P/c29-32(30,31)22(16-17-7-3-1-4-8-17)24-21(23-25-27-28-26-23)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,24H,15-16H2,(H2,29,30,31)(H,25,26,27,28)/t21-,22?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase 24.11(NEP) |
Bioorg Med Chem Lett 7: 1059-1064 (1997)
Article DOI: 10.1016/S0960-894X(97)00159-5
BindingDB Entry DOI: 10.7270/Q2WH2QGK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475025

(CHEMBL181339)Show SMILES O=C(C1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-23(24(27(35)30-26)21-14-29-22-6-1-2-9-33(21)22)20-16-31-10-11-32(28(36)17-7-12-37-13-8-17)15-18-4-3-5-19(20)25(18)31/h1-6,9,14,16-17H,7-8,10-13,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475007

(Bisarylmaleimide 2)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H23N5O5/c33-25-21(19-13-28-12-16-4-9-37-24(16)19)22(26(34)29-25)20-15-31-5-6-32(27(35)30-7-10-36-11-8-30)14-17-2-1-3-18(20)23(17)31/h1-4,9,12-13,15H,5-8,10-11,14H2,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50034851
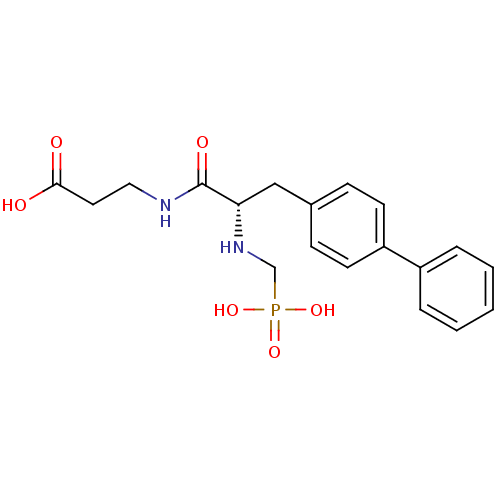
(3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...)Show SMILES OC(=O)CCNC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NCP(O)(O)=O Show InChI InChI=1S/C19H23N2O6P/c22-18(23)10-11-20-19(24)17(21-13-28(25,26)27)12-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-9,17,21H,10-13H2,(H,20,24)(H,22,23)(H2,25,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate |
J Med Chem 38: 1689-700 (1995)
BindingDB Entry DOI: 10.7270/Q2Z89BD0 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50064106
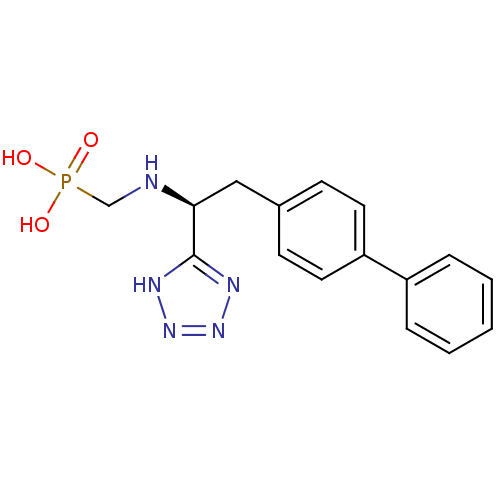
(CGS-26303 | CHEMBL290698 | {[(R)-2-Biphenyl-4-yl-1...)Show SMILES OP(O)(=O)CN[C@@H](Cc1ccc(cc1)-c1ccccc1)c1nnn[nH]1 Show InChI InChI=1S/C16H18N5O3P/c22-25(23,24)11-17-15(16-18-20-21-19-16)10-12-6-8-14(9-7-12)13-4-2-1-3-5-13/h1-9,15,17H,10-11H2,(H2,22,23,24)(H,18,19,20,21)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase 24.11(NEP) |
Bioorg Med Chem Lett 7: 1059-1064 (1997)
Article DOI: 10.1016/S0960-894X(97)00159-5
BindingDB Entry DOI: 10.7270/Q2WH2QGK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475024

(CHEMBL181371)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H18N4O4/c1-13(29)27-6-7-28-12-18(16-4-2-3-15(11-27)21(16)28)20-19(23(30)26-24(20)31)17-10-25-9-14-5-8-32-22(14)17/h2-5,8-10,12H,6-7,11H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475018
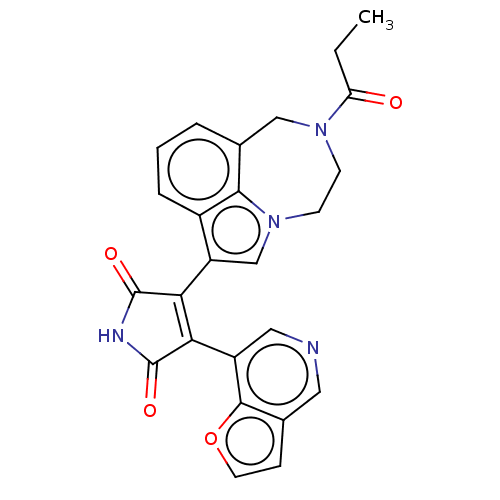
(CHEMBL181518)Show SMILES CCC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:10| Show InChI InChI=1S/C25H20N4O4/c1-2-19(30)28-7-8-29-13-18(16-5-3-4-15(12-28)22(16)29)21-20(24(31)27-25(21)32)17-11-26-10-14-6-9-33-23(14)17/h3-6,9-11,13H,2,7-8,12H2,1H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475031

(CHEMBL359871)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4CCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H21N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-7,13H,8-12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475029

(CHEMBL180779)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H21N5O4/c1-28(2)25(33)30-8-7-29-13-18(16-5-3-4-15(12-30)21(16)29)20-19(23(31)27-24(20)32)17-11-26-10-14-6-9-34-22(14)17/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475008

(CHEMBL369090)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3ccn4ncccc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(19-6-8-33-21(19)5-2-7-28-33)23(26(35)29-25)20-16-31-9-10-32(27(36)30-11-13-37-14-12-30)15-17-3-1-4-18(20)24(17)31/h1-8,16H,9-15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150698
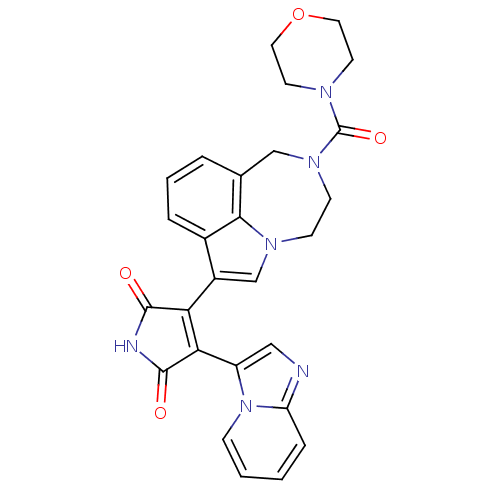
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700
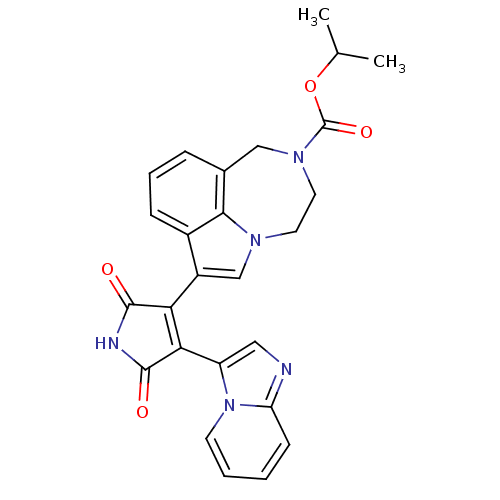
(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50017742
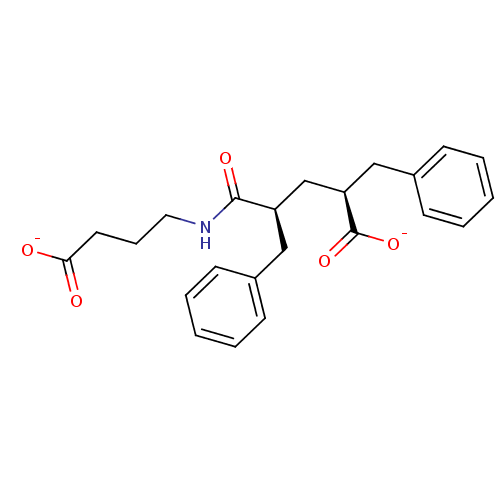
(CHEMBL316180 | disodium 2-benzyl-4-(3-carboxylatop...)Show SMILES [O-]C(=O)CCCNC(=O)[C@@H](C[C@@H](Cc1ccccc1)C([O-])=O)Cc1ccccc1 Show InChI InChI=1S/C23H27NO5/c25-21(26)12-7-13-24-22(27)19(14-17-8-3-1-4-9-17)16-20(23(28)29)15-18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,24,27)(H,25,26)(H,28,29)/p-2/t19-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of enkephalinase activity in membranes prepared from rabbit |
J Med Chem 32: 2519-26 (1989)
BindingDB Entry DOI: 10.7270/Q2668DSV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475022

(CHEMBL361765)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H19N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-8,11,13H,9-10,12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM21641

(2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate |
J Med Chem 38: 1689-700 (1995)
BindingDB Entry DOI: 10.7270/Q2Z89BD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Homo sapiens (Human)) | BDBM50291557
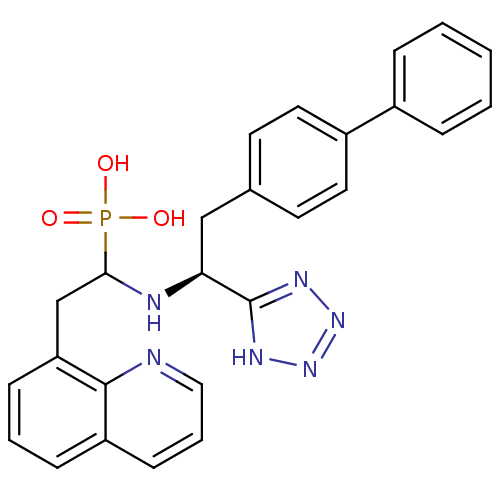
(CHEMBL268761 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetra...)Show SMILES OP(O)(=O)C(Cc1cccc2cccnc12)N[C@@H](Cc1ccc(cc1)-c1ccccc1)c1nnn[nH]1 Show InChI InChI=1S/C26H25N6O3P/c33-36(34,35)24(17-22-9-4-8-21-10-5-15-27-25(21)22)28-23(26-29-31-32-30-26)16-18-11-13-20(14-12-18)19-6-2-1-3-7-19/h1-15,23-24,28H,16-17H2,(H2,33,34,35)(H,29,30,31,32)/t23-,24?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase 24.11(NEP) |
Bioorg Med Chem Lett 7: 1059-1064 (1997)
Article DOI: 10.1016/S0960-894X(97)00159-5
BindingDB Entry DOI: 10.7270/Q2WH2QGK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701
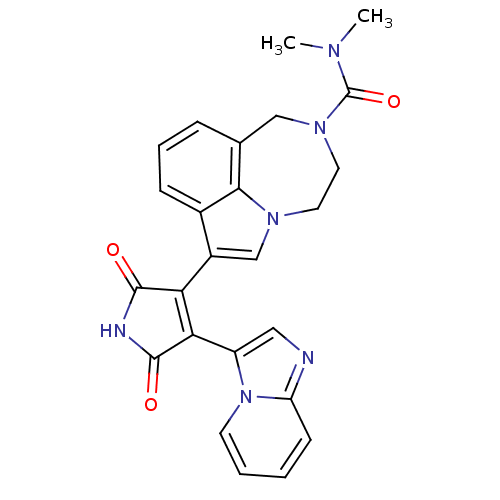
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50034851

(3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...)Show SMILES OC(=O)CCNC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NCP(O)(O)=O Show InChI InChI=1S/C19H23N2O6P/c22-18(23)10-11-20-19(24)17(21-13-28(25,26)27)12-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-9,17,21H,10-13H2,(H,20,24)(H,22,23)(H2,25,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Neutral endopeptidase by using GAAP as substrate |
J Med Chem 38: 1689-700 (1995)
BindingDB Entry DOI: 10.7270/Q2Z89BD0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474994

(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475010
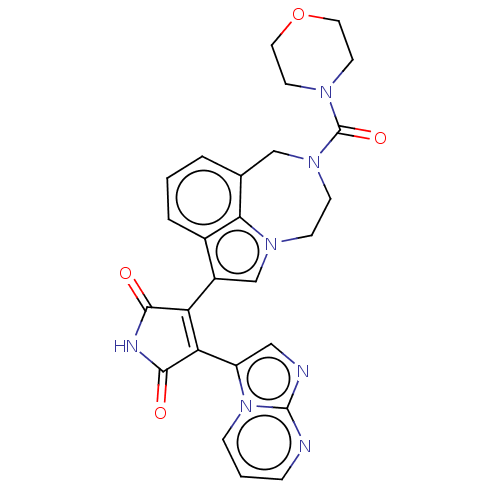
(CHEMBL369316)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-23-20(21(24(35)29-23)19-13-28-25-27-5-2-6-33(19)25)18-15-31-7-8-32(26(36)30-9-11-37-12-10-30)14-16-3-1-4-17(18)22(16)31/h1-6,13,15H,7-12,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475001
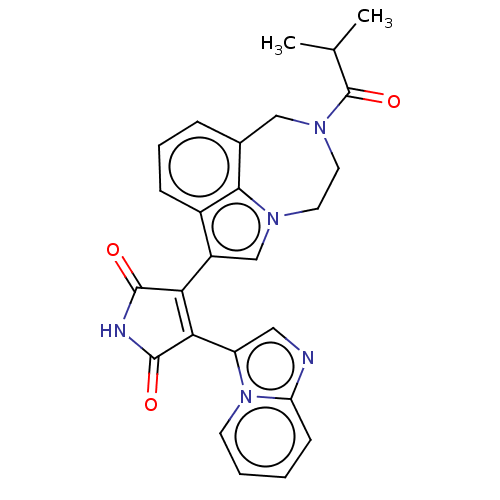
(CHEMBL368246)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475014
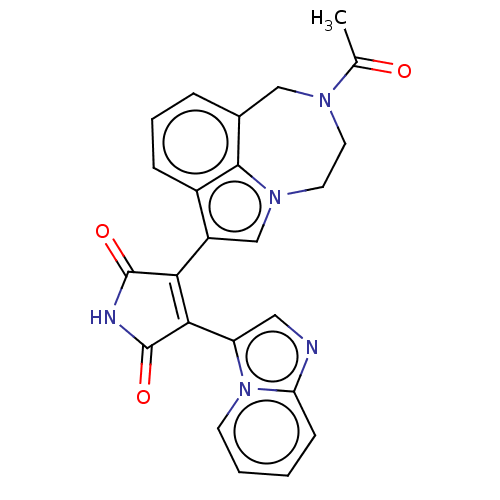
(CHEMBL361948)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N5O3/c1-14(30)27-9-10-28-13-17(16-6-4-5-15(12-27)22(16)28)20-21(24(32)26-23(20)31)18-11-25-19-7-2-3-8-29(18)19/h2-8,11,13H,9-10,12H2,1H3,(H,26,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475004

(CHEMBL369572)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-24-21(22(25(36)30-24)20-14-29-26-28-8-5-11-34(20)26)19-16-32-12-13-33(27(37)31-9-2-1-3-10-31)15-17-6-4-7-18(19)23(17)32/h4-8,11,14,16H,1-3,9-10,12-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50021153
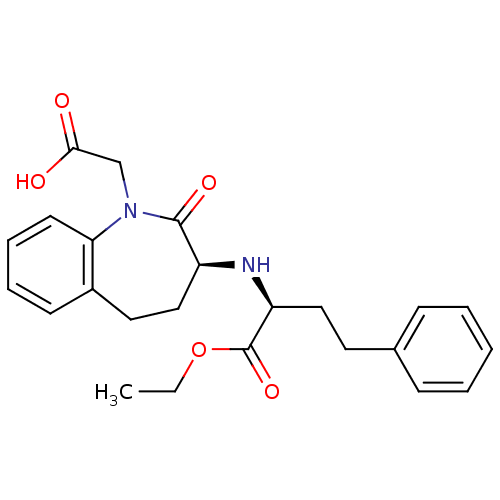
(1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) |
J Med Chem 37: 1823-32 (1994)
BindingDB Entry DOI: 10.7270/Q280537C |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50038591

(6-(5-Chloro-1-methyl-2-pyridin-3-yl-1H-indol-3-yl)...)Show InChI InChI=1S/C20H21ClN2O2/c1-23-18-10-9-15(21)12-17(18)16(7-3-2-4-8-19(24)25)20(23)14-6-5-11-22-13-14/h5-6,9-13H,2-4,7-8H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Tested for 50% inhibition of thromboxane synthase (TxS) in human platelets (in vitro) |
J Med Chem 37: 1823-32 (1994)
BindingDB Entry DOI: 10.7270/Q280537C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50017129

((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) |
J Med Chem 37: 1823-32 (1994)
BindingDB Entry DOI: 10.7270/Q280537C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50034851

(3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...)Show SMILES OC(=O)CCNC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NCP(O)(O)=O Show InChI InChI=1S/C19H23N2O6P/c22-18(23)10-11-20-19(24)17(21-13-28(25,26)27)12-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-9,17,21H,10-13H2,(H,20,24)(H,22,23)(H2,25,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of Neutral endopeptidase by using ANF(atrial natriuretic factor) as substrate |
J Med Chem 38: 1689-700 (1995)
BindingDB Entry DOI: 10.7270/Q2Z89BD0 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50017736
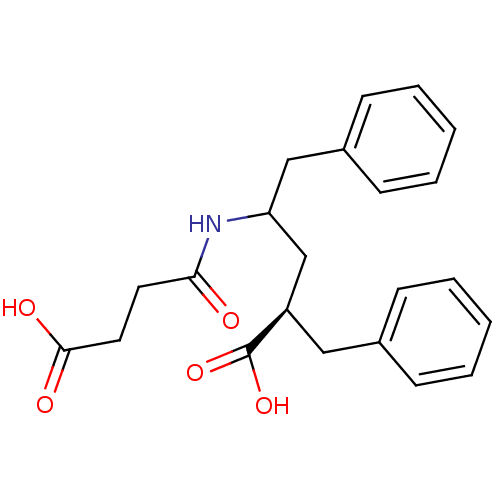
(2-Benzyl-4-(3-carboxy-propionylamino)-5-phenyl-pen...)Show SMILES OC(=O)CCC(=O)NC(C[C@@H](Cc1ccccc1)C(O)=O)Cc1ccccc1 Show InChI InChI=1S/C22H25NO5/c24-20(11-12-21(25)26)23-19(14-17-9-5-2-6-10-17)15-18(22(27)28)13-16-7-3-1-4-8-16/h1-10,18-19H,11-15H2,(H,23,24)(H,25,26)(H,27,28)/t18-,19?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of enkephalinase activity in membranes prepared from rabbit |
J Med Chem 32: 2519-26 (1989)
BindingDB Entry DOI: 10.7270/Q2668DSV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474996

(CHEMBL178646)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-22(20-14-29-13-17-7-12-37-25(17)20)23(27(35)30-26)21-16-32-10-11-33(28(36)31-8-2-1-3-9-31)15-18-5-4-6-19(21)24(18)32/h4-7,12-14,16H,1-3,8-11,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475006

(CHEMBL178851)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-13-29-21-14-28-7-10-34(20)21)19-16-32-11-12-33(27(37)31-8-2-1-3-9-31)15-17-5-4-6-18(19)24(17)32/h4-7,10,13-14,16H,1-3,8-9,11-12,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475000

(CHEMBL181296)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N3O5/c1-13(28)26-8-9-27-11-17(15-5-2-4-14(10-26)21(15)27)20-19(23(29)25-24(20)30)16-6-3-7-18-22(16)32-12-31-18/h2-7,11H,8-10,12H2,1H3,(H,25,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50291556
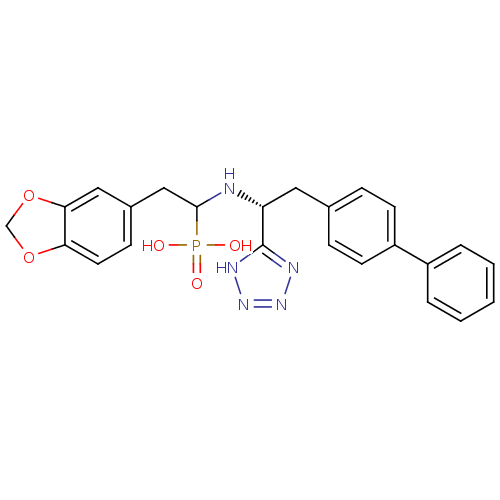
(CHEMBL273489 | {2-Benzo[1,3]dioxol-5-yl-1-[(R)-2-b...)Show SMILES OP(O)(=O)C(Cc1ccc2OCOc2c1)N[C@H](Cc1ccc(cc1)-c1ccccc1)c1nnn[nH]1 Show InChI InChI=1S/C24H24N5O5P/c30-35(31,32)23(14-17-8-11-21-22(13-17)34-15-33-21)25-20(24-26-28-29-27-24)12-16-6-9-19(10-7-16)18-4-2-1-3-5-18/h1-11,13,20,23,25H,12,14-15H2,(H2,30,31,32)(H,26,27,28,29)/t20-,23?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase 24.11(NEP) |
Bioorg Med Chem Lett 7: 1059-1064 (1997)
Article DOI: 10.1016/S0960-894X(97)00159-5
BindingDB Entry DOI: 10.7270/Q2WH2QGK |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475011

(CHEMBL178850)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H22N4O4/c1-28(2)26(33)30-11-10-29-14-19(17-7-4-6-16(13-30)22(17)29)21-20(24(31)27-25(21)32)18-8-3-5-15-9-12-34-23(15)18/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data