 Found 424 hits with Last Name = 'duan' and Initial = 'l'
Found 424 hits with Last Name = 'duan' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM129823
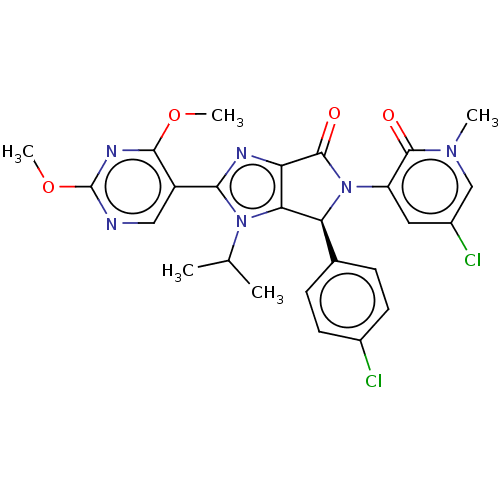
(US8815926, 102)Show SMILES COc1ncc(-c2nc3C(=O)N([C@H](c3n2C(C)C)c2ccc(Cl)cc2)c2cc(Cl)cn(C)c2=O)c(OC)n1 |r| Show InChI InChI=1S/C26H24Cl2N6O4/c1-13(2)33-21-19(30-22(33)17-11-29-26(38-5)31-23(17)37-4)25(36)34(18-10-16(28)12-32(3)24(18)35)20(21)14-6-8-15(27)9-7-14/h6-13,20H,1-5H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Institute for Translational Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) |
Eur J Med Chem 159: 1-9 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.044
BindingDB Entry DOI: 10.7270/Q2SB48FV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50433561
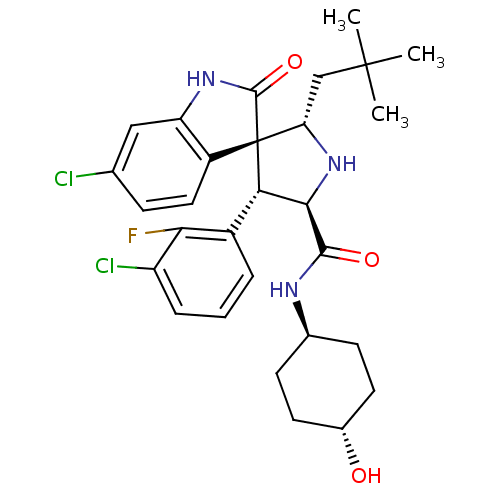
(CHEMBL2381408)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:8.8,5.4,31.34,wD:17.29,7.31,34.38,(40.88,-57.18,;39.34,-57.18,;38.57,-58.51,;40.11,-58.5,;38.57,-55.84,;37.04,-55.84,;37.52,-54.37,;36.27,-53.47,;35.03,-54.37,;33.51,-54.09,;32.52,-55.27,;31.01,-54.99,;30.49,-53.53,;31.49,-52.36,;30.98,-50.9,;33,-52.64,;34.01,-51.48,;35.49,-55.84,;36.38,-57.1,;37.93,-57.11,;35.48,-58.34,;34.01,-57.86,;32.69,-58.61,;31.36,-57.84,;30.03,-58.6,;31.37,-56.3,;32.71,-55.54,;34.03,-56.32,;36.26,-51.93,;34.93,-51.16,;37.6,-51.15,;37.6,-49.61,;36.26,-48.86,;36.26,-47.31,;37.6,-46.54,;37.6,-45,;38.93,-47.32,;38.93,-48.85,)| Show InChI InChI=1S/C29H34Cl2FN3O3/c1-28(2,3)14-22-29(19-12-7-15(30)13-21(19)34-27(29)38)23(18-5-4-6-20(31)24(18)32)25(35-22)26(37)33-16-8-10-17(36)11-9-16/h4-7,12-13,16-17,22-23,25,35-36H,8-11,14H2,1-3H3,(H,33,37)(H,34,38)/t16-,17-,22-,23-,25+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
International Institute for Translational Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) |
Eur J Med Chem 159: 1-9 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.044
BindingDB Entry DOI: 10.7270/Q2SB48FV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448936
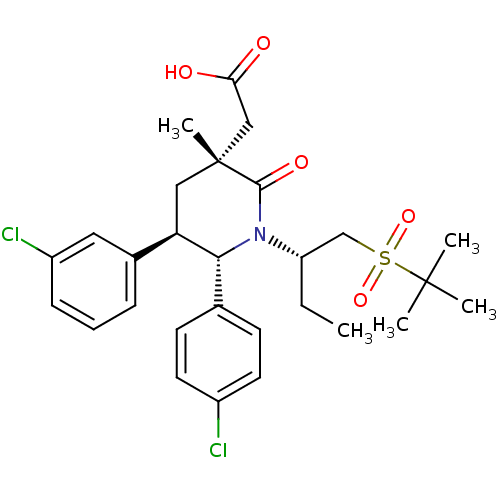
(CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C(C)(C)C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H35Cl2NO5S/c1-6-22(17-37(35,36)27(2,3)4)31-25(18-10-12-20(29)13-11-18)23(19-8-7-9-21(30)14-19)15-28(5,26(31)34)16-24(32)33/h7-14,22-23,25H,6,15-17H2,1-5H3,(H,32,33)/t22-,23+,25+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
International Institute for Translational Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p53-MDM2 interaction (unknown origin) by HTRF assay |
Eur J Med Chem 159: 1-9 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.044
BindingDB Entry DOI: 10.7270/Q2SB48FV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604493
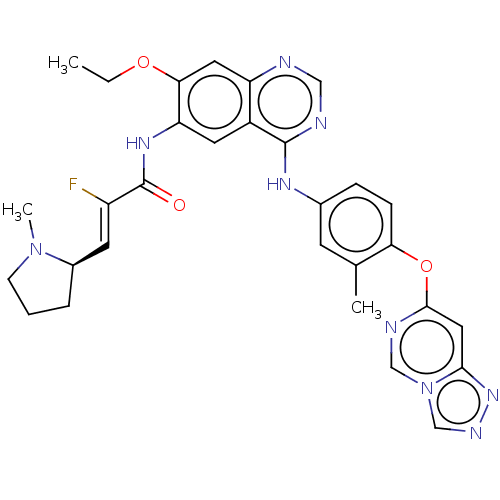
(CHEMBL5178703)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C\[C@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448969
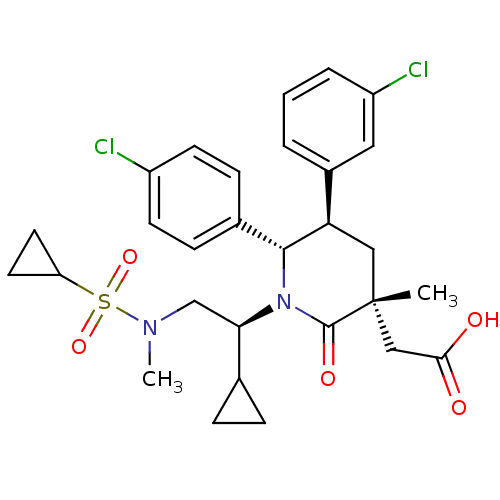
(CHEMBL3125517 | US9296736, 256 | US9593129, Exampl...)Show SMILES CN(C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(16-26(34)35)15-24(20-4-3-5-22(31)14-20)27(19-8-10-21(30)11-9-19)33(28(29)36)25(18-6-7-18)17-32(2)39(37,38)23-12-13-23/h3-5,8-11,14,18,23-25,27H,6-7,12-13,15-17H2,1-2H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
International Institute for Translational Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p53-MDM2 interaction (unknown origin) by HTRF assay |
Eur J Med Chem 159: 1-9 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.044
BindingDB Entry DOI: 10.7270/Q2SB48FV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161957
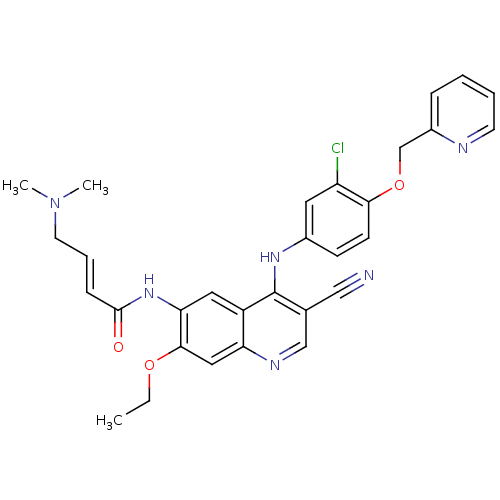
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604493

(CHEMBL5178703)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C\[C@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604493

(CHEMBL5178703)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C\[C@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM139991

(US8901140, 5 | US9358227, 5)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\[C@H]1CCCN1C |r| Show InChI InChI=1S/C32H31ClN6O3/c1-3-41-30-17-27-25(16-28(30)38-31(40)12-10-24-8-6-14-39(24)2)32(21(18-34)19-36-27)37-22-9-11-29(26(33)15-22)42-20-23-7-4-5-13-35-23/h4-5,7,9-13,15-17,19,24H,3,6,8,14,20H2,1-2H3,(H,36,37)(H,38,40)/b12-10+/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604493

(CHEMBL5178703)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C\[C@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161957

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM139991

(US8901140, 5 | US9358227, 5)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\[C@H]1CCCN1C |r| Show InChI InChI=1S/C32H31ClN6O3/c1-3-41-30-17-27-25(16-28(30)38-31(40)12-10-24-8-6-14-39(24)2)32(21(18-34)19-36-27)37-22-9-11-29(26(33)15-22)42-20-23-7-4-5-13-35-23/h4-5,7,9-13,15-17,19,24H,3,6,8,14,20H2,1-2H3,(H,36,37)(H,38,40)/b12-10+/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604485

(CHEMBL5190132)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4cnc5ccnn5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604486

(CHEMBL5176920)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4cc5nccn5cn4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604486

(CHEMBL5176920)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4cc5nccn5cn4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604497
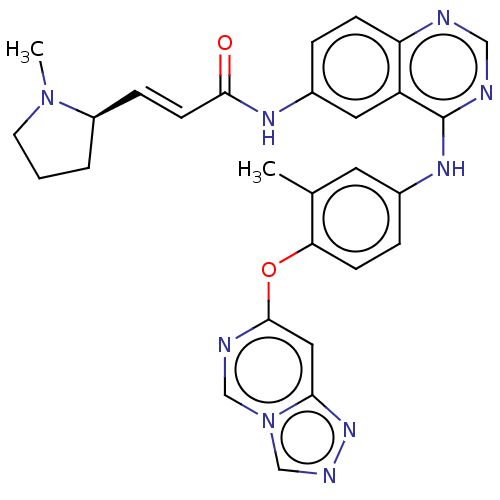
(CHEMBL5179954)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604494

(CHEMBL5184610)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)\C=C\[C@H]1CCCN1C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604483
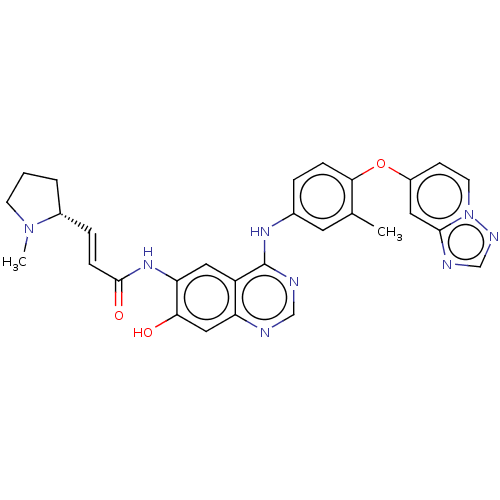
(CHEMBL5193451)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4ccn5ncnc5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604497

(CHEMBL5179954)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604495
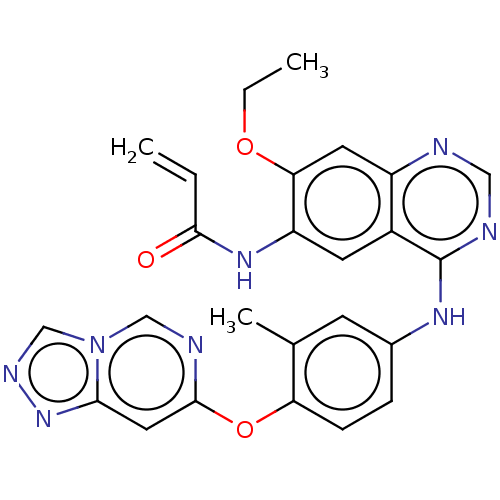
(CHEMBL5175489)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C=C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604494

(CHEMBL5184610)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)\C=C\[C@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604482

(CHEMBL5192750)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4ccn5ccnc5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50161957

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50430862
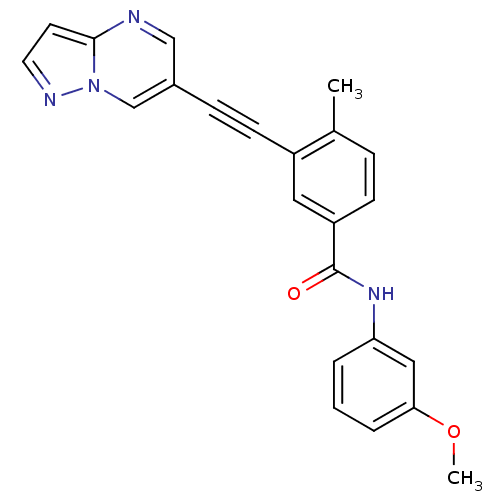
(CHEMBL2336040)Show SMILES COc1cccc(NC(=O)c2ccc(C)c(c2)C#Cc2cnc3ccnn3c2)c1 Show InChI InChI=1S/C23H18N4O2/c1-16-6-8-19(23(28)26-20-4-3-5-21(13-20)29-2)12-18(16)9-7-17-14-24-22-10-11-25-27(22)15-17/h3-6,8,10-15H,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT (unknown origin) using Ser/Thr 6 peptide as substrate incubated for 1 hr prior to substrate addition measured after 2 hrs by FRET... |
J Med Chem 56: 3281-95 (2013)
Article DOI: 10.1021/jm301824k
BindingDB Entry DOI: 10.7270/Q22V2HHJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604495

(CHEMBL5175489)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C=C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604499
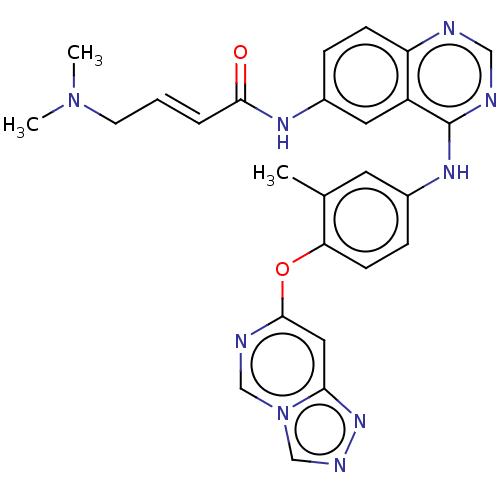
(CHEMBL5175873)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604484
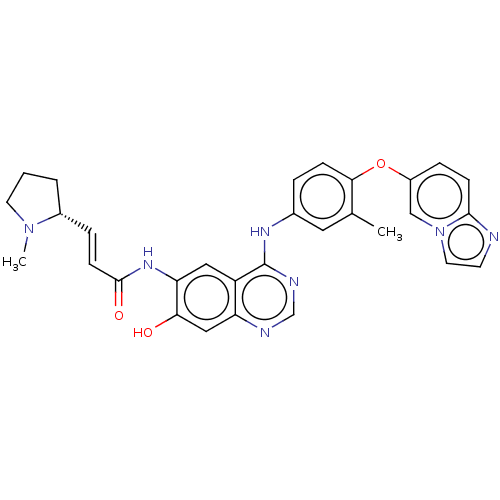
(CHEMBL5201973)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4ccc5nccn5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604482

(CHEMBL5192750)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4ccn5ccnc5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50430865
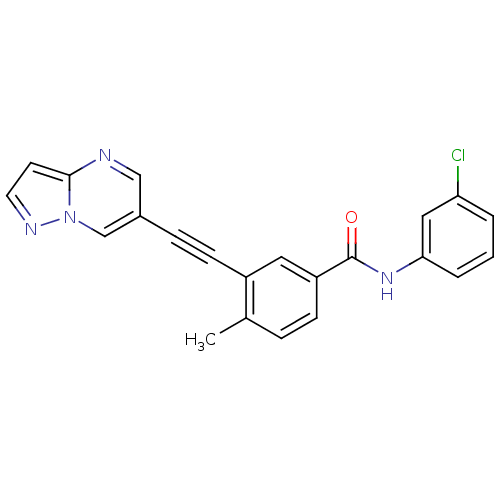
(CHEMBL2336037)Show SMILES Cc1ccc(cc1C#Cc1cnc2ccnn2c1)C(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C22H15ClN4O/c1-15-5-7-18(22(28)26-20-4-2-3-19(23)12-20)11-17(15)8-6-16-13-24-21-9-10-25-27(21)14-16/h2-5,7,9-14H,1H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) using fluorescein-labeled poly GAT as substrate incubated for 1 hr prior to substrate addition measured after 1 h... |
J Med Chem 56: 3281-95 (2013)
Article DOI: 10.1021/jm301824k
BindingDB Entry DOI: 10.7270/Q22V2HHJ |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT (unknown origin) using Ser/Thr 6 peptide as substrate incubated for 1 hr prior to substrate addition measured after 2 hrs by FRET... |
J Med Chem 56: 3281-95 (2013)
Article DOI: 10.1021/jm301824k
BindingDB Entry DOI: 10.7270/Q22V2HHJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604485

(CHEMBL5190132)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4cnc5ccnn5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM139991

(US8901140, 5 | US9358227, 5)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\[C@H]1CCCN1C |r| Show InChI InChI=1S/C32H31ClN6O3/c1-3-41-30-17-27-25(16-28(30)38-31(40)12-10-24-8-6-14-39(24)2)32(21(18-34)19-36-27)37-22-9-11-29(26(33)15-22)42-20-23-7-4-5-13-35-23/h4-5,7,9-13,15-17,19,24H,3,6,8,14,20H2,1-2H3,(H,36,37)(H,38,40)/b12-10+/t24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604500
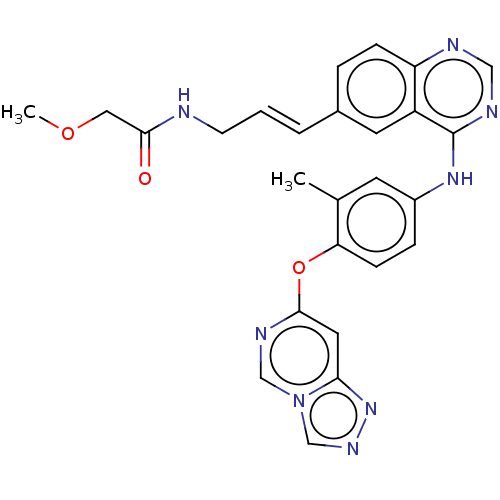
(CHEMBL5203179)Show SMILES COCC(=O)NC\C=C\c1ccc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50430867
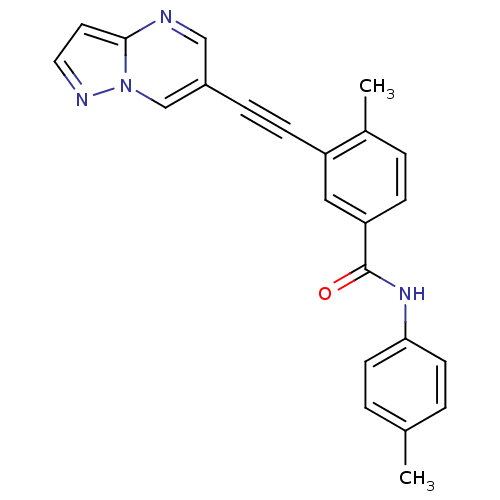
(CHEMBL2336035)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C#Cc2cnc3ccnn3c2)cc1 Show InChI InChI=1S/C23H18N4O/c1-16-3-9-21(10-4-16)26-23(28)20-7-5-17(2)19(13-20)8-6-18-14-24-22-11-12-25-27(22)15-18/h3-5,7,9-15H,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT (unknown origin) using Ser/Thr 6 peptide as substrate incubated for 1 hr prior to substrate addition measured after 2 hrs by FRET... |
J Med Chem 56: 3281-95 (2013)
Article DOI: 10.1021/jm301824k
BindingDB Entry DOI: 10.7270/Q22V2HHJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50091138

(CHEMBL3582168)Show SMILES COc1cc(OC)c2C[C@H](OC(=O)c3ccc(F)c(NC(=O)c4cc(OC)c(OC)c(OC)c4)c3)[C@H](Oc2c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C37H38FNO12/c1-42-22-16-26(43-2)23-18-32(33(50-27(23)17-22)20-12-28(44-3)34(48-7)29(13-20)45-4)51-37(41)19-9-10-24(38)25(11-19)39-36(40)21-14-30(46-5)35(49-8)31(15-21)47-6/h9-17,32-33H,18H2,1-8H3,(H,39,40)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for pa... |
J Med Chem 58: 4529-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00085
BindingDB Entry DOI: 10.7270/Q2N29ZP5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604483

(CHEMBL5193451)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4ccn5ncnc5c4)c(C)c3)ncnc2cc1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604499

(CHEMBL5175873)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50091264

(CHEMBL3582155)Show SMILES COc1cc(OC)c2C[C@@H](OC(=O)c3ccc(F)c(NC(=O)c4ccc(OC)c(OC)c4)c3)[C@H](Oc2c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C36H36FNO11/c1-41-22-16-27(43-3)23-18-32(33(48-28(23)17-22)21-14-30(45-5)34(47-7)31(15-21)46-6)49-36(40)20-8-10-24(37)25(12-20)38-35(39)19-9-11-26(42-2)29(13-19)44-4/h8-17,32-33H,18H2,1-7H3,(H,38,39)/t32-,33-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for pa... |
J Med Chem 58: 4529-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00085
BindingDB Entry DOI: 10.7270/Q2N29ZP5 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50430868
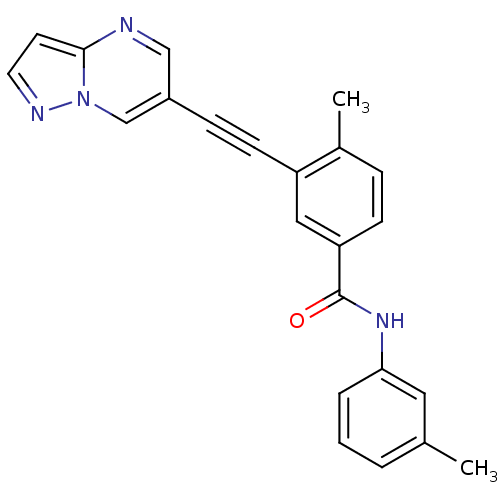
(CHEMBL2331602)Show SMILES Cc1cccc(NC(=O)c2ccc(C)c(c2)C#Cc2cnc3ccnn3c2)c1 Show InChI InChI=1S/C23H18N4O/c1-16-4-3-5-21(12-16)26-23(28)20-8-6-17(2)19(13-20)9-7-18-14-24-22-10-11-25-27(22)15-18/h3-6,8,10-15H,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-KIT (unknown origin) using Ser/Thr 6 peptide as substrate incubated for 1 hr prior to substrate addition measured after 2 hrs by FRET... |
J Med Chem 56: 3281-95 (2013)
Article DOI: 10.1021/jm301824k
BindingDB Entry DOI: 10.7270/Q22V2HHJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50604496

(CHEMBL5209046)Show SMILES CN1CCC[C@@H]1\C=C\C(=O)Nc1ccc2ncc(C#N)c(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50091087
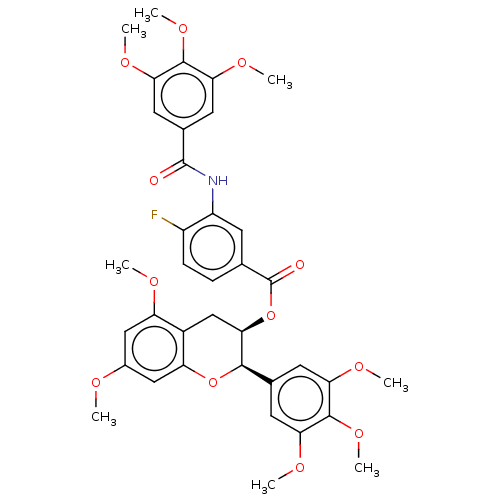
(CHEMBL3582156)Show SMILES COc1cc(OC)c2C[C@@H](OC(=O)c3ccc(F)c(NC(=O)c4cc(OC)c(OC)c(OC)c4)c3)[C@H](Oc2c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C37H38FNO12/c1-42-22-16-26(43-2)23-18-32(33(50-27(23)17-22)20-12-28(44-3)34(48-7)29(13-20)45-4)51-37(41)19-9-10-24(38)25(11-19)39-36(40)21-14-30(46-5)35(49-8)31(15-21)47-6/h9-17,32-33H,18H2,1-8H3,(H,39,40)/t32-,33-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for pa... |
J Med Chem 58: 4529-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00085
BindingDB Entry DOI: 10.7270/Q2N29ZP5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604490

(CHEMBL5176854)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C\[C@@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604493

(CHEMBL5178703)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C\[C@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604491

(CHEMBL5181694)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(\F)=C/[C@@H]1CCCN1C |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50091265

(CHEMBL3582149)Show SMILES COc1cc(OC)c2C[C@@H](OC(=O)\C=C\c3cc(OC)c(OC)c(OC)c3)[C@H](Oc2c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C32H36O11/c1-34-20-15-22(35-2)21-17-28(42-29(33)10-9-18-11-24(36-3)31(40-7)25(12-18)37-4)30(43-23(21)16-20)19-13-26(38-5)32(41-8)27(14-19)39-6/h9-16,28,30H,17H2,1-8H3/b10-9+/t28-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for pa... |
J Med Chem 58: 4529-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00085
BindingDB Entry DOI: 10.7270/Q2N29ZP5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM139991

(US8901140, 5 | US9358227, 5)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\[C@H]1CCCN1C |r| Show InChI InChI=1S/C32H31ClN6O3/c1-3-41-30-17-27-25(16-28(30)38-31(40)12-10-24-8-6-14-39(24)2)32(21(18-34)19-36-27)37-22-9-11-29(26(33)15-22)42-20-23-7-4-5-13-35-23/h4-5,7,9-13,15-17,19,24H,3,6,8,14,20H2,1-2H3,(H,36,37)(H,38,40)/b12-10+/t24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50430862

(CHEMBL2336040)Show SMILES COc1cccc(NC(=O)c2ccc(C)c(c2)C#Cc2cnc3ccnn3c2)c1 Show InChI InChI=1S/C23H18N4O2/c1-16-6-8-19(23(28)26-20-4-3-5-21(13-20)29-2)12-18(16)9-7-17-14-24-22-10-11-25-27(22)15-17/h3-6,8,10-15H,1-2H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) using fluorescein-labeled poly GAT as substrate incubated for 1 hr prior to substrate addition measured after 1 h... |
J Med Chem 56: 3281-95 (2013)
Article DOI: 10.1021/jm301824k
BindingDB Entry DOI: 10.7270/Q22V2HHJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50604492
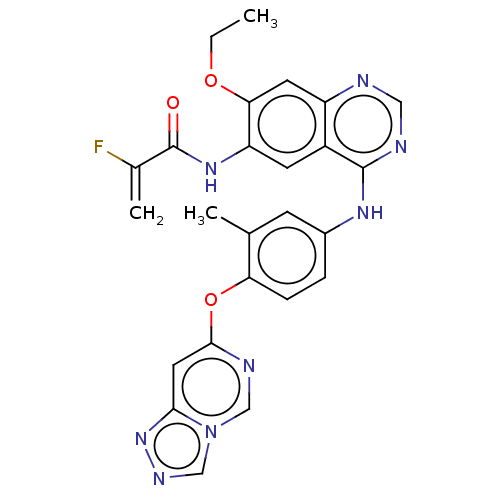
(CHEMBL5183924)Show SMILES CCOc1cc2ncnc(Nc3ccc(Oc4cc5nncn5cn4)c(C)c3)c2cc1NC(=O)C(F)=C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50091161

(CHEMBL3582170)Show SMILES COc1cc(OC)c2C[C@H](OC(=O)c3ccc(F)c(NC(=O)\C=C\c4cc(OC)c(OC)c(OC)c4)c3)[C@H](Oc2c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C24H25ClN3O2S/c1-28(2,17-18-8-11-20(12-9-18)27(29)30)15-5-14-26-21-6-3-4-7-23(21)31-24-13-10-19(25)16-22(24)26/h3-4,6-13,16H,5,14-15,17H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hong Kong Polytechnic University
Curated by ChEMBL
| Assay Description
Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for pa... |
J Med Chem 58: 4529-49 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00085
BindingDB Entry DOI: 10.7270/Q2N29ZP5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50161957

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00710
BindingDB Entry DOI: 10.7270/Q2CC14RQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data