 Found 712 hits with Last Name = 'dunn' and Initial = 'd'
Found 712 hits with Last Name = 'dunn' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36608

(Rapamycin C-7, analog 1)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42-,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36612
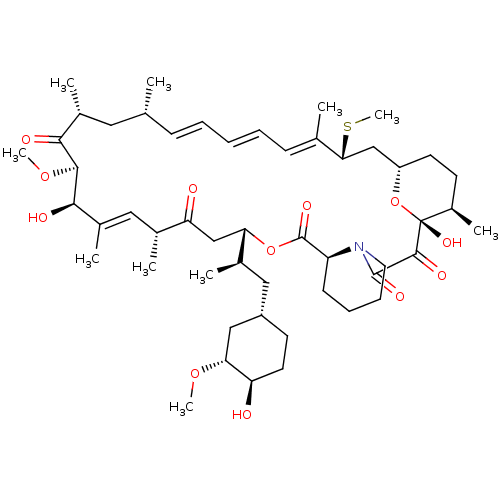
(Rapamycin C-7, analog 6a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
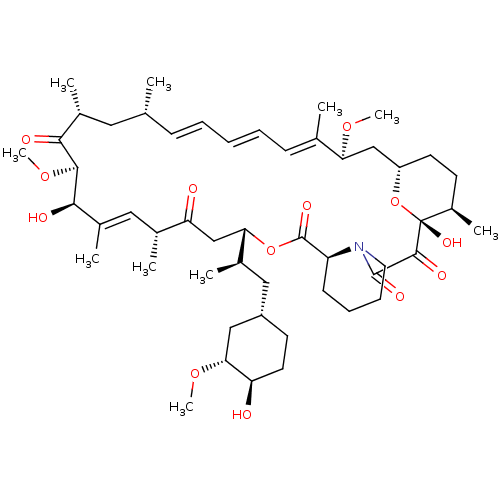
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36613
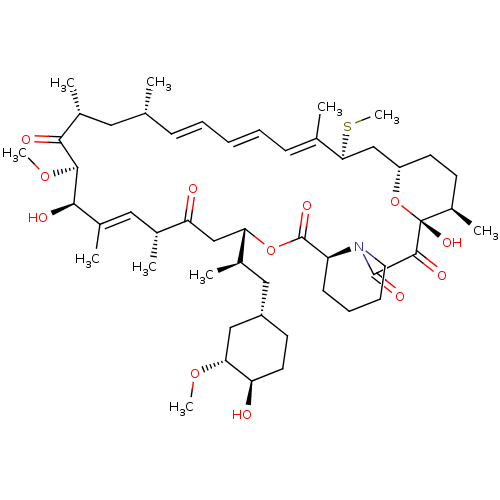
(Rapamycin C-7, analog 6b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)SC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO12S/c1-30-16-12-11-13-17-31(2)44(65-10)28-38-21-19-36(7)51(60,64-38)48(57)49(58)52-23-15-14-18-39(52)50(59)63-42(33(4)26-37-20-22-40(53)43(27-37)61-8)29-41(54)32(3)25-35(6)46(56)47(62-9)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43-,44+,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36623
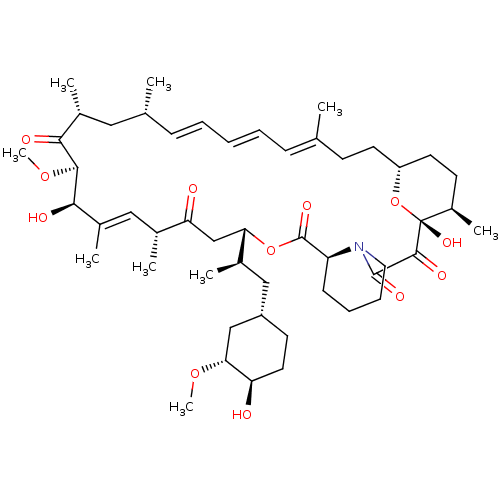
(Rapamycin C-7, analog 12)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\CC[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C50H77NO12/c1-30-15-11-10-12-16-31(2)25-34(5)44(54)46(61-9)45(55)35(6)26-32(3)41(53)29-42(33(4)27-37-20-23-40(52)43(28-37)60-8)62-49(58)39-17-13-14-24-51(39)48(57)47(56)50(59)36(7)19-22-38(63-50)21-18-30/h10-12,15-16,26,31-34,36-40,42-43,45-46,52,55,59H,13-14,17-25,27-29H2,1-9H3/b11-10+,16-12+,30-15+,35-26+/t31-,32-,33-,34-,36-,37+,38-,39+,40-,42+,43-,45-,46+,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36627

(Rapamycin C-7, analog 16a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccc(Cl)cc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H82ClNO13/c1-34-15-11-10-12-16-35(2)48(70-33-41-19-22-43(58)23-20-41)31-44-24-18-40(7)57(67,72-44)54(64)55(65)59-26-14-13-17-45(59)56(66)71-49(37(4)29-42-21-25-46(60)50(30-42)68-8)32-47(61)36(3)28-39(6)52(63)53(69-9)51(62)38(5)27-34/h10-12,15-16,19-20,22-23,28,34,36-38,40,42,44-46,48-50,52-53,60,63,67H,13-14,17-18,21,24-27,29-33H2,1-9H3/b12-10+,15-11+,35-16+,39-28+/t34-,36-,37-,38-,40-,42+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353165
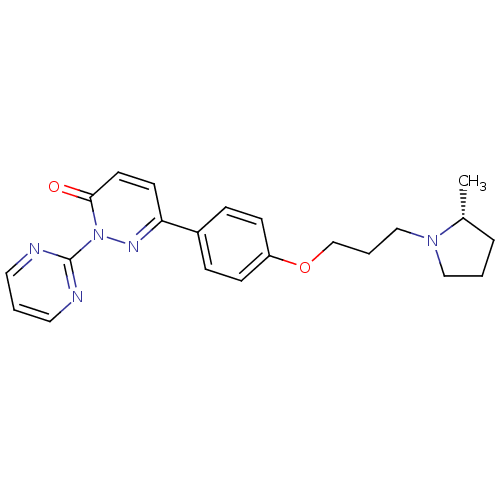
(CHEMBL1829472)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ncccn1 |r| Show InChI InChI=1S/C22H25N5O2/c1-17-5-2-14-26(17)15-4-16-29-19-8-6-18(7-9-19)20-10-11-21(28)27(25-20)22-23-12-3-13-24-22/h3,6-13,17H,2,4-5,14-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350022
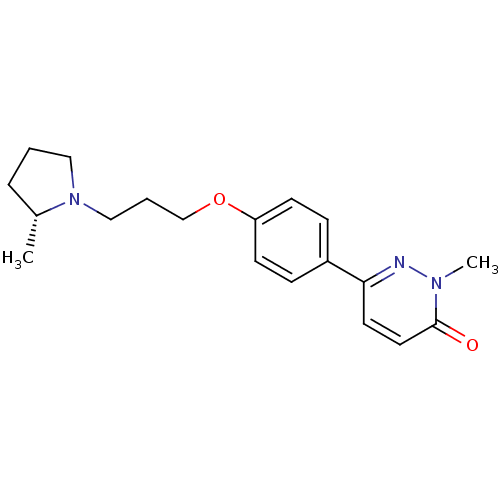
(CHEMBL1813057)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-11,15H,3-5,12-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36622

(Rapamycin C-7, analog 11b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccc[nH]2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H80N2O12/c1-32-16-11-10-12-17-33(2)41(42-18-15-24-55-42)30-40-22-20-38(7)54(64,68-40)51(61)52(62)56-25-14-13-19-43(56)53(63)67-46(35(4)28-39-21-23-44(57)47(29-39)65-8)31-45(58)34(3)27-37(6)49(60)50(66-9)48(59)36(5)26-32/h10-12,15-18,24,27,32,34-36,38-41,43-44,46-47,49-50,55,57,60,64H,13-14,19-23,25-26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43+,44-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361051
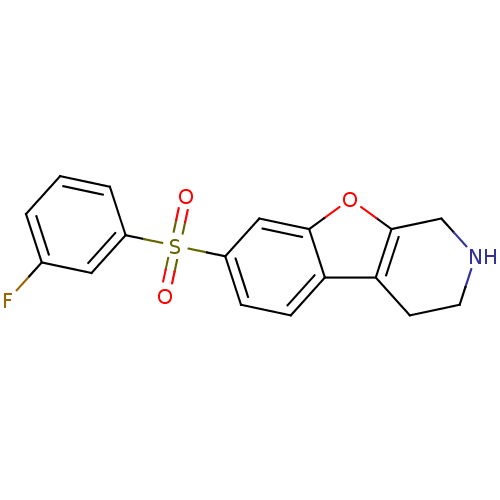
(CHEMBL1935590)Show InChI InChI=1S/C17H14FNO3S/c18-11-2-1-3-12(8-11)23(20,21)13-4-5-14-15-6-7-19-10-17(15)22-16(14)9-13/h1-5,8-9,19H,6-7,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360899
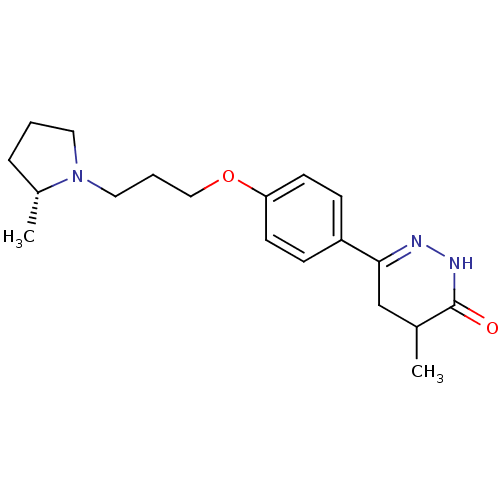
(CHEMBL1935110)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)C(C)C1 |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-14-13-18(20-21-19(14)23)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,14-15H,3-5,10-13H2,1-2H3,(H,21,23)/t14?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350021
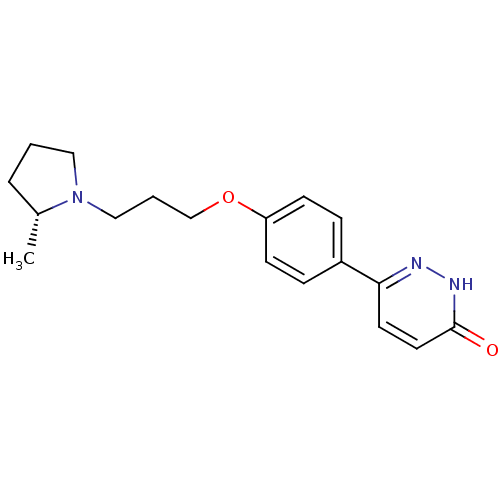
(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50065404
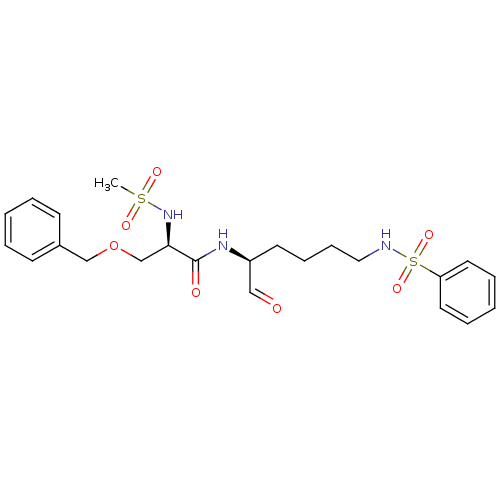
((R)-N-((S)-5-Benzenesulfonylamino-1-formyl-pentyl)...)Show SMILES CS(=O)(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](CCCCNS(=O)(=O)c1ccccc1)C=O Show InChI InChI=1S/C23H31N3O7S2/c1-34(29,30)26-22(18-33-17-19-10-4-2-5-11-19)23(28)25-20(16-27)12-8-9-15-24-35(31,32)21-13-6-3-7-14-21/h2-7,10-11,13-14,16,20,22,24,26H,8-9,12,15,17-18H2,1H3,(H,25,28)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Calpain-I receptor |
J Med Chem 41: 2663-6 (1998)
Article DOI: 10.1021/jm980035y
BindingDB Entry DOI: 10.7270/Q2S46SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360897
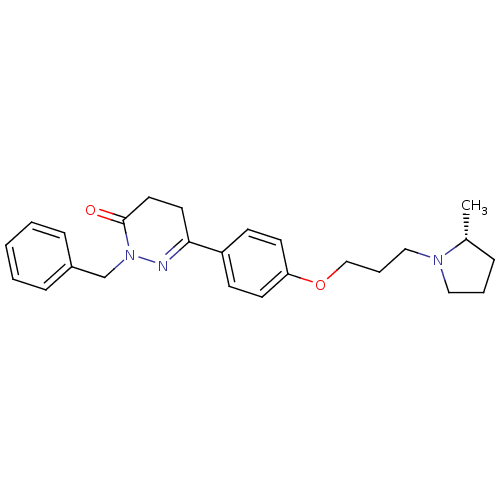
(CHEMBL1935108)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NN(Cc2ccccc2)C(=O)CC1 |r,t:18| Show InChI InChI=1S/C25H31N3O2/c1-20-7-5-16-27(20)17-6-18-30-23-12-10-22(11-13-23)24-14-15-25(29)28(26-24)19-21-8-3-2-4-9-21/h2-4,8-13,20H,5-7,14-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361055
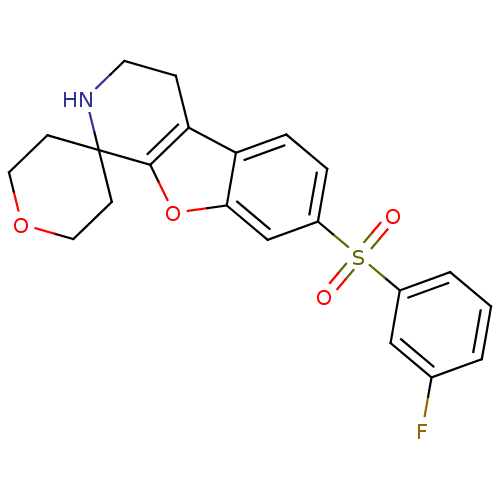
(CHEMBL1935599)Show SMILES Fc1cccc(c1)S(=O)(=O)c1ccc2c3CCNC4(CCOCC4)c3oc2c1 Show InChI InChI=1S/C21H20FNO4S/c22-14-2-1-3-15(12-14)28(24,25)16-4-5-17-18-6-9-23-21(7-10-26-11-8-21)20(18)27-19(17)13-16/h1-5,12-13,23H,6-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353181
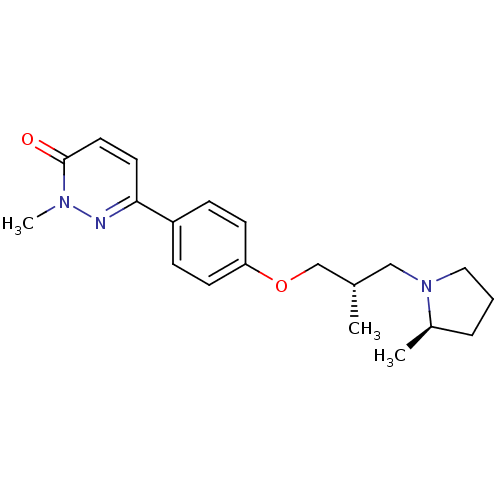
(CHEMBL1829485)Show SMILES C[C@H](COc1ccc(cc1)-c1ccc(=O)n(C)n1)CN1CCC[C@H]1C |r| Show InChI InChI=1S/C20H27N3O2/c1-15(13-23-12-4-5-16(23)2)14-25-18-8-6-17(7-9-18)19-10-11-20(24)22(3)21-19/h6-11,15-16H,4-5,12-14H2,1-3H3/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350030
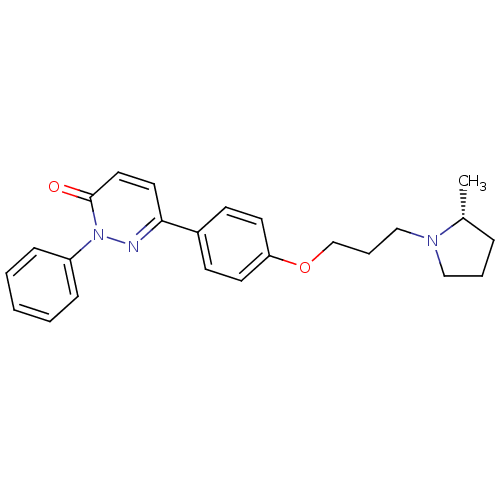
(CHEMBL1813060)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccccc1 |r| Show InChI InChI=1S/C24H27N3O2/c1-19-7-5-16-26(19)17-6-18-29-22-12-10-20(11-13-22)23-14-15-24(28)27(25-23)21-8-3-2-4-9-21/h2-4,8-15,19H,5-7,16-18H2,1H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50012572

(CHEMBL3260795)Show SMILES O=C1CC(CN1c1cnc2c(cccc2c1)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C23H24N4O/c28-22-14-19(17-5-2-1-3-6-17)16-27(22)20-13-18-7-4-8-21(23(18)25-15-20)26-11-9-24-10-12-26/h1-8,13,15,19,24H,9-12,14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO-K1 cell membrane after 3 hrs by liquid scintillation counting analysis |
Bioorg Med Chem Lett 24: 2094-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.049
BindingDB Entry DOI: 10.7270/Q2W95BQ7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361052
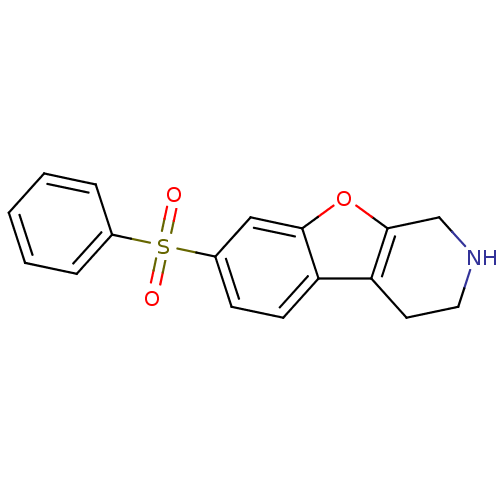
(CHEMBL1935589 | CHEMBL1949758)Show InChI InChI=1S/C17H15NO3S/c19-22(20,12-4-2-1-3-5-12)13-6-7-14-15-8-9-18-11-17(15)21-16(14)10-13/h1-7,10,18H,8-9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361058
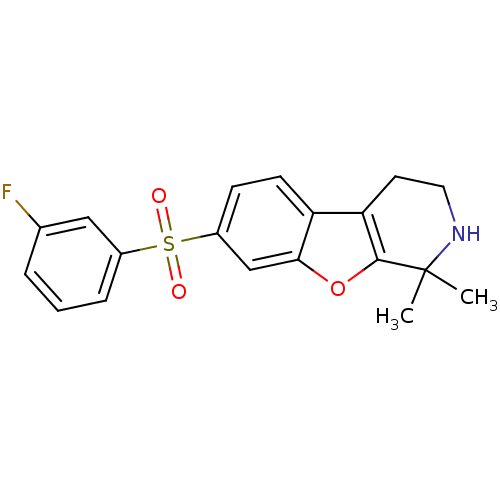
(CHEMBL1935594)Show SMILES CC1(C)NCCc2c1oc1cc(ccc21)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C19H18FNO3S/c1-19(2)18-16(8-9-21-19)15-7-6-14(11-17(15)24-18)25(22,23)13-5-3-4-12(20)10-13/h3-7,10-11,21H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353167
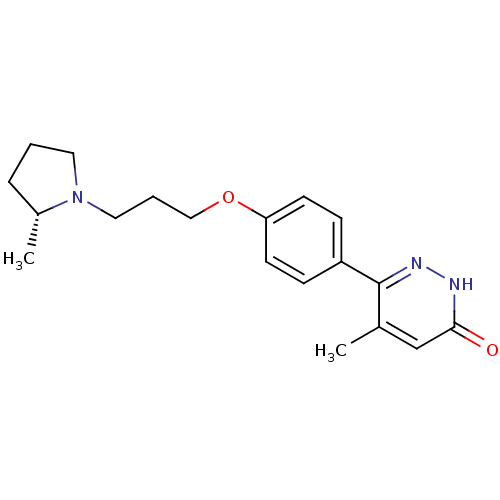
(CHEMBL1829475)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1n[nH]c(=O)cc1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,20,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353167

(CHEMBL1829475)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1n[nH]c(=O)cc1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,20,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353164
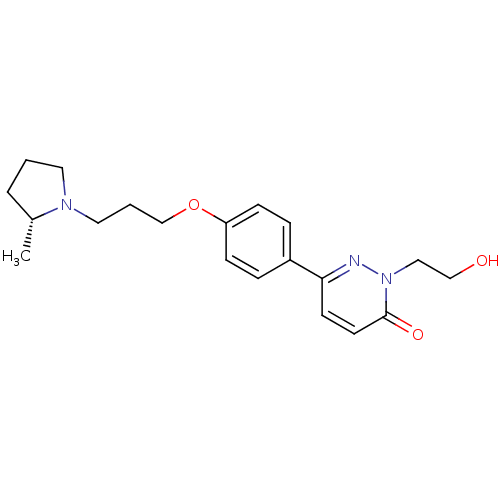
(CHEMBL1829469)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CCO)n1 |r| Show InChI InChI=1S/C20H27N3O3/c1-16-4-2-11-22(16)12-3-15-26-18-7-5-17(6-8-18)19-9-10-20(25)23(21-19)13-14-24/h5-10,16,24H,2-4,11-15H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36619
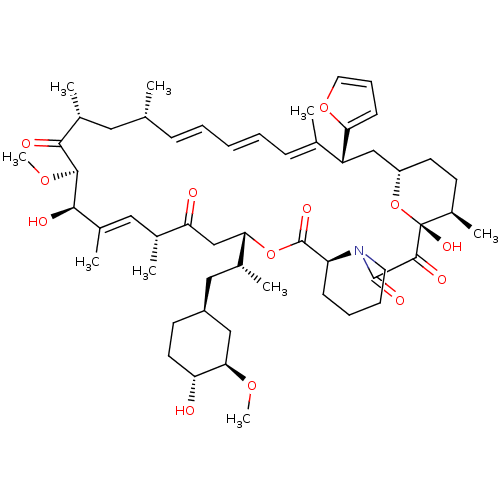
(Rapamycin C-7, analog 10a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)c2ccco2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C54H79NO13/c1-32-16-11-10-12-17-33(2)41(45-19-15-25-66-45)30-40-22-20-38(7)54(63,68-40)51(60)52(61)55-24-14-13-18-42(55)53(62)67-46(35(4)28-39-21-23-43(56)47(29-39)64-8)31-44(57)34(3)27-37(6)49(59)50(65-9)48(58)36(5)26-32/h10-12,15-17,19,25,27,32,34-36,38-43,46-47,49-50,56,59,63H,13-14,18,20-24,26,28-31H2,1-9H3/b12-10+,16-11+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41-,42+,43-,46+,47-,49-,50+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353163
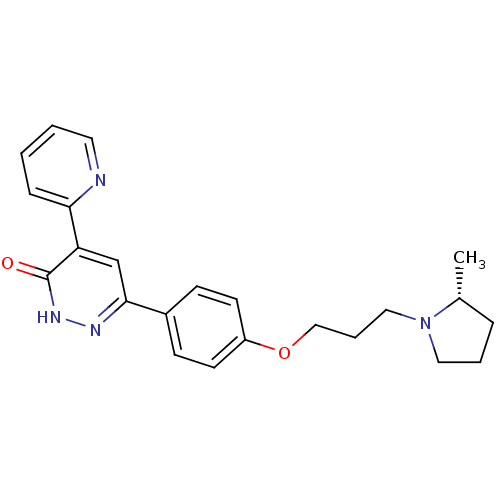
(CHEMBL1829474)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(-c2ccccn2)c(=O)[nH]n1 |r| Show InChI InChI=1S/C23H26N4O2/c1-17-6-4-13-27(17)14-5-15-29-19-10-8-18(9-11-19)22-16-20(23(28)26-25-22)21-7-2-3-12-24-21/h2-3,7-12,16-17H,4-6,13-15H2,1H3,(H,26,28)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360889
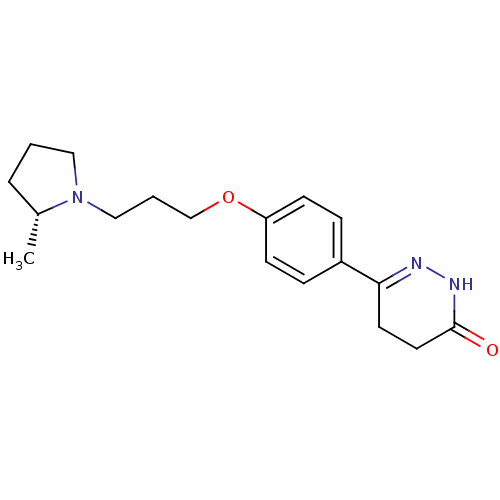
(CHEMBL1935100)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)CC1 |r,t:18| Show InChI InChI=1S/C18H25N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-8,14H,2-4,9-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353166

(CHEMBL1829473)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(C)c(=O)[nH]n1 |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(20-21-19(14)23)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,21,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353168
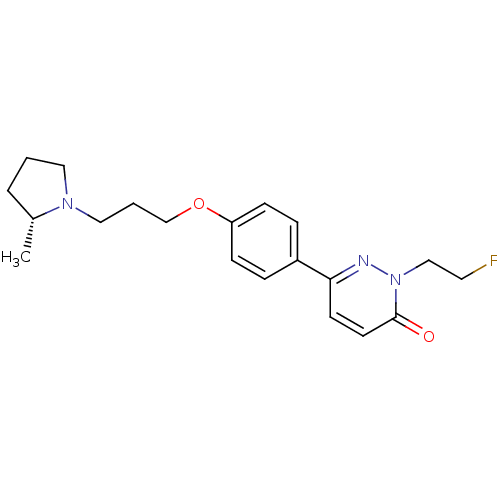
(CHEMBL1829337)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CCF)n1 |r| Show InChI InChI=1S/C20H26FN3O2/c1-16-4-2-12-23(16)13-3-15-26-18-7-5-17(6-8-18)19-9-10-20(25)24(22-19)14-11-21/h5-10,16H,2-4,11-15H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36610
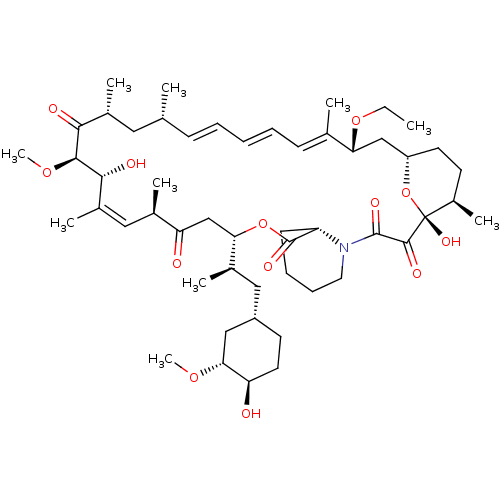
(Rapamycin C-7, analog 5a)Show SMILES CCO[C@@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43-,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36615
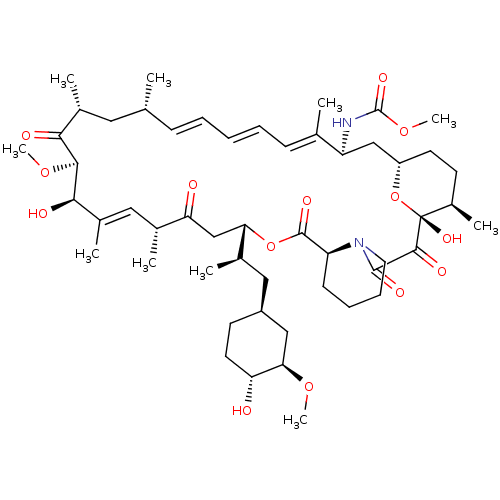
(Rapamycin C-7, analog 7b)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50360899

(CHEMBL1935110)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)C(C)C1 |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-14-13-18(20-21-19(14)23)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,14-15H,3-5,10-13H2,1-2H3,(H,21,23)/t14?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50065398
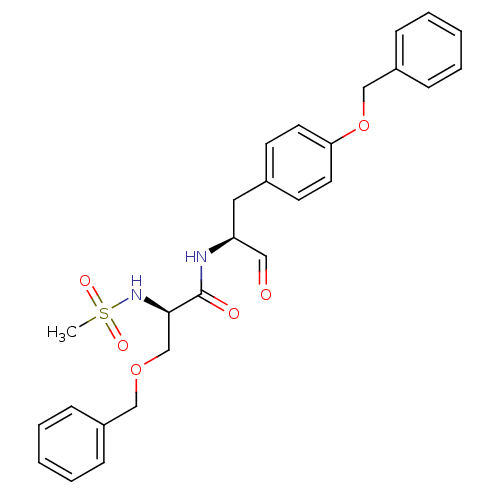
((R)-3-Benzyloxy-N-[(S)-1-(4-benzyloxy-benzyl)-2-ox...)Show SMILES CS(=O)(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C=O Show InChI InChI=1S/C27H30N2O6S/c1-36(32,33)29-26(20-34-18-22-8-4-2-5-9-22)27(31)28-24(17-30)16-21-12-14-25(15-13-21)35-19-23-10-6-3-7-11-23/h2-15,17,24,26,29H,16,18-20H2,1H3,(H,28,31)/t24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Calpain-I receptor |
J Med Chem 41: 2663-6 (1998)
Article DOI: 10.1021/jm980035y
BindingDB Entry DOI: 10.7270/Q2S46SNF |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50065404

((R)-N-((S)-5-Benzenesulfonylamino-1-formyl-pentyl)...)Show SMILES CS(=O)(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](CCCCNS(=O)(=O)c1ccccc1)C=O Show InChI InChI=1S/C23H31N3O7S2/c1-34(29,30)26-22(18-33-17-19-10-4-2-5-11-19)23(28)25-20(16-27)12-8-9-15-24-35(31,32)21-13-6-3-7-14-21/h2-7,10-11,13-14,16,20,22,24,26H,8-9,12,15,17-18H2,1H3,(H,25,28)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B receptor in human liver |
J Med Chem 41: 2663-6 (1998)
Article DOI: 10.1021/jm980035y
BindingDB Entry DOI: 10.7270/Q2S46SNF |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36611

(Rapamycin C-7, analog 5b)Show SMILES CCO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:34,t:49,51,53| Show InChI InChI=1S/C52H81NO13/c1-11-64-43-29-39-22-20-37(8)52(61,66-39)49(58)50(59)53-24-16-15-19-40(53)51(60)65-44(34(5)27-38-21-23-41(54)45(28-38)62-9)30-42(55)33(4)26-36(7)47(57)48(63-10)46(56)35(6)25-31(2)17-13-12-14-18-32(43)3/h12-14,17-18,26,31,33-35,37-41,43-45,47-48,54,57,61H,11,15-16,19-25,27-30H2,1-10H3/b14-12+,17-13+,32-18+,36-26+/t31-,33-,34-,35-,37-,38+,39+,40+,41-,43+,44+,45-,47-,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360902
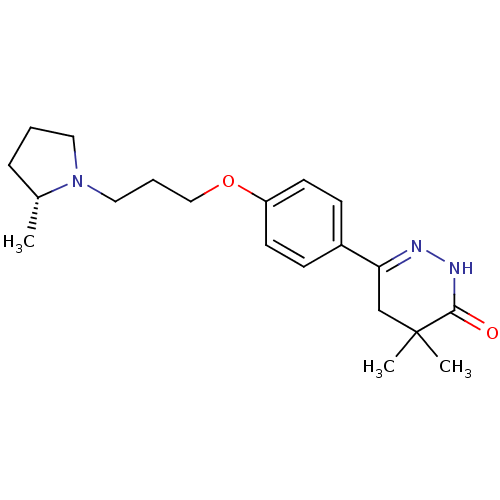
(CHEMBL1935114)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)C(C)(C)C1 |r,t:18| Show InChI InChI=1S/C20H29N3O2/c1-15-6-4-11-23(15)12-5-13-25-17-9-7-16(8-10-17)18-14-20(2,3)19(24)22-21-18/h7-10,15H,4-6,11-14H2,1-3H3,(H,22,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361053
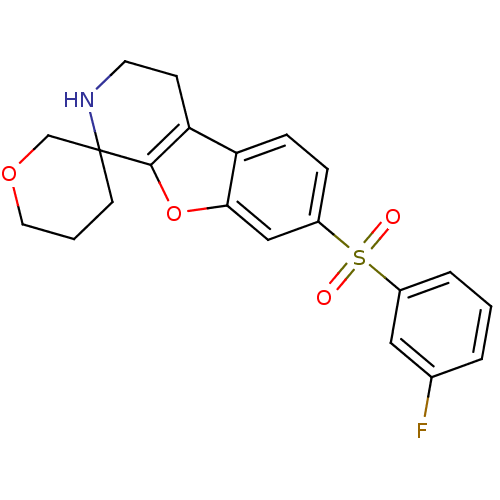
(CHEMBL1935601)Show SMILES Fc1cccc(c1)S(=O)(=O)c1ccc2c3CCNC4(CCCOC4)c3oc2c1 Show InChI InChI=1S/C21H20FNO4S/c22-14-3-1-4-15(11-14)28(24,25)16-5-6-17-18-7-9-23-21(8-2-10-26-13-21)20(18)27-19(17)12-16/h1,3-6,11-12,23H,2,7-10,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353169
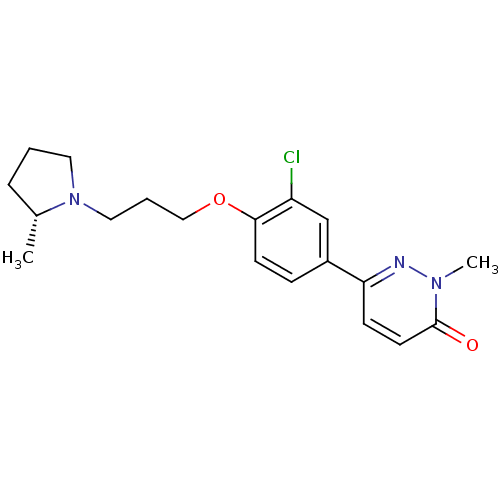
(CHEMBL1829479)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1Cl)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-14-5-3-10-23(14)11-4-12-25-18-8-6-15(13-16(18)20)17-7-9-19(24)22(2)21-17/h6-9,13-14H,3-5,10-12H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36614
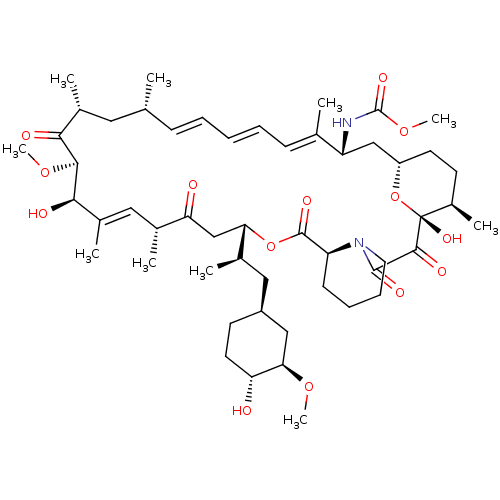
(Rapamycin C-7, analog 7a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)NC(=O)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H80N2O14/c1-30-16-12-11-13-17-31(2)39(53-51(62)66-10)28-38-21-19-36(7)52(63,68-38)48(59)49(60)54-23-15-14-18-40(54)50(61)67-43(33(4)26-37-20-22-41(55)44(27-37)64-8)29-42(56)32(3)25-35(6)46(58)47(65-9)45(57)34(5)24-30/h11-13,16-17,25,30,32-34,36-41,43-44,46-47,55,58,63H,14-15,18-24,26-29H2,1-10H3,(H,53,62)/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39-,40+,41-,43+,44-,46-,47+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50353181

(CHEMBL1829485)Show SMILES C[C@H](COc1ccc(cc1)-c1ccc(=O)n(C)n1)CN1CCC[C@H]1C |r| Show InChI InChI=1S/C20H27N3O2/c1-15(13-23-12-4-5-16(23)2)14-25-18-8-6-17(7-9-18)19-10-11-20(24)22(3)21-19/h6-11,15-16H,4-5,12-14H2,1-3H3/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50065407
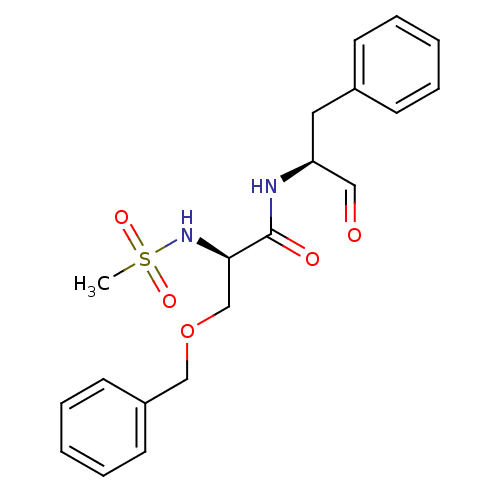
((R)-N-((S)-1-Benzyl-2-oxo-ethyl)-3-benzyloxy-2-met...)Show SMILES CS(=O)(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C20H24N2O5S/c1-28(25,26)22-19(15-27-14-17-10-6-3-7-11-17)20(24)21-18(13-23)12-16-8-4-2-5-9-16/h2-11,13,18-19,22H,12,14-15H2,1H3,(H,21,24)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calpain-II receptor in porcine kidney |
J Med Chem 41: 2663-6 (1998)
Article DOI: 10.1021/jm980035y
BindingDB Entry DOI: 10.7270/Q2S46SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360892
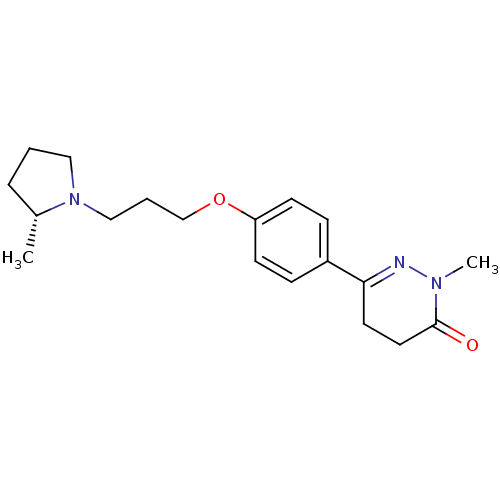
(CHEMBL1935103)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NN(C)C(=O)CC1 |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-15-5-3-12-22(15)13-4-14-24-17-8-6-16(7-9-17)18-10-11-19(23)21(2)20-18/h6-9,15H,3-5,10-14H2,1-2H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360894
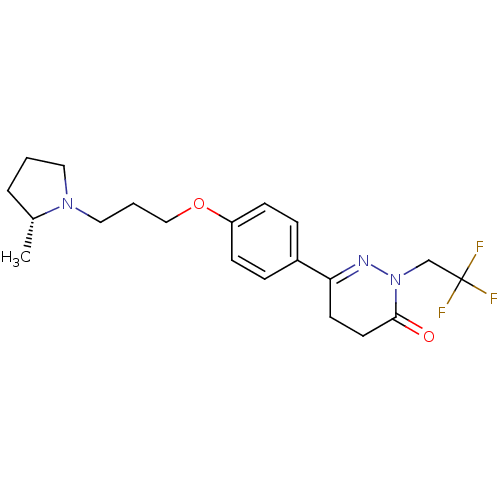
(CHEMBL1935105)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NN(CC(F)(F)F)C(=O)CC1 |r,t:18| Show InChI InChI=1S/C20H26F3N3O2/c1-15-4-2-11-25(15)12-3-13-28-17-7-5-16(6-8-17)18-9-10-19(27)26(24-18)14-20(21,22)23/h5-8,15H,2-4,9-14H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36625
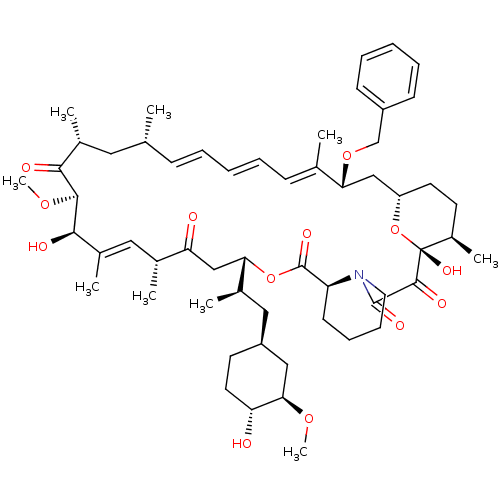
(Rapamycin C-7, analog 14a)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OCc2ccccc2)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C57H83NO13/c1-35-18-12-10-13-19-36(2)48(69-34-42-20-14-11-15-21-42)32-44-25-23-41(7)57(66,71-44)54(63)55(64)58-27-17-16-22-45(58)56(65)70-49(38(4)30-43-24-26-46(59)50(31-43)67-8)33-47(60)37(3)29-40(6)52(62)53(68-9)51(61)39(5)28-35/h10-15,18-21,29,35,37-39,41,43-46,48-50,52-53,59,62,66H,16-17,22-28,30-34H2,1-9H3/b13-10+,18-12+,36-19+,40-29+/t35-,37-,38-,39-,41-,43+,44+,45+,46-,48-,49+,50-,52-,53+,57-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
FKBP12 assay using rapamycin analogs. |
Chem Biol 2: 471-81 (1995)
Article DOI: 10.1016/1074-5521(95)90264-3
BindingDB Entry DOI: 10.7270/Q2CZ35J2 |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50065398

((R)-3-Benzyloxy-N-[(S)-1-(4-benzyloxy-benzyl)-2-ox...)Show SMILES CS(=O)(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C=O Show InChI InChI=1S/C27H30N2O6S/c1-36(32,33)29-26(20-34-18-22-8-4-2-5-9-22)27(31)28-24(17-30)16-21-12-14-25(15-13-21)35-19-23-10-6-3-7-11-23/h2-15,17,24,26,29H,16,18-20H2,1H3,(H,28,31)/t24-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calpain-II receptor in porcine kidney |
J Med Chem 41: 2663-6 (1998)
Article DOI: 10.1021/jm980035y
BindingDB Entry DOI: 10.7270/Q2S46SNF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50365043

(CHEMBL1951055)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C(=O)CN1CCOCC1 |r| Show InChI InChI=1S/C20H30N2O3/c1-17-4-2-9-22(17)10-3-13-25-19-7-5-18(6-8-19)20(23)16-21-11-14-24-15-12-21/h5-8,17H,2-4,9-16H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor |
Bioorg Med Chem Lett 22: 1546-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.004
BindingDB Entry DOI: 10.7270/Q2C24WWN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360888
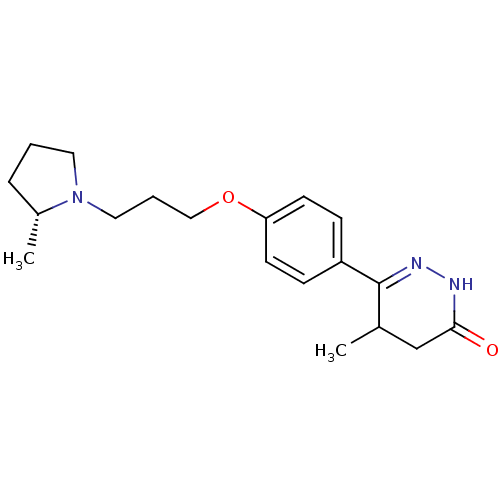
(CHEMBL1935111)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)CC1C |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,14-15H,3-5,10-13H2,1-2H3,(H,20,23)/t14?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50361050
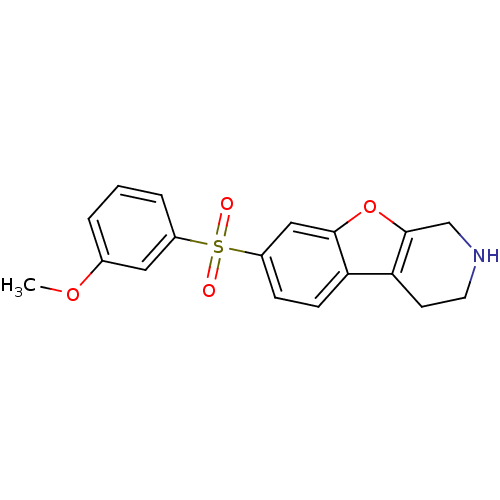
(CHEMBL1935592)Show InChI InChI=1S/C18H17NO4S/c1-22-12-3-2-4-13(9-12)24(20,21)14-5-6-15-16-7-8-19-11-18(16)23-17(15)10-14/h2-6,9-10,19H,7-8,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from full length human 5HT6 receptor |
Bioorg Med Chem Lett 22: 120-3 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.050
BindingDB Entry DOI: 10.7270/Q24Q7VFX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360888

(CHEMBL1935111)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)CC1C |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,14-15H,3-5,10-13H2,1-2H3,(H,20,23)/t14?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353170
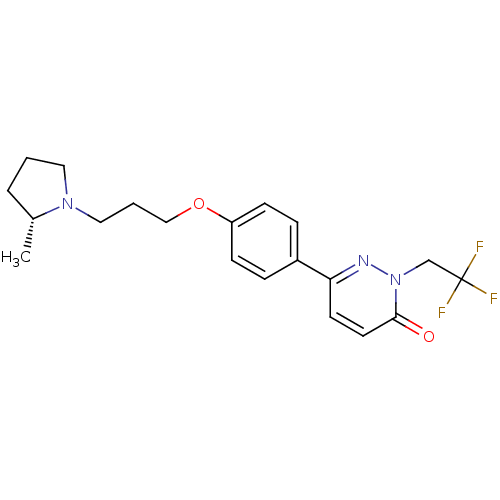
(CHEMBL1829468)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(CC(F)(F)F)n1 |r| Show InChI InChI=1S/C20H24F3N3O2/c1-15-4-2-11-25(15)12-3-13-28-17-7-5-16(6-8-17)18-9-10-19(27)26(24-18)14-20(21,22)23/h5-10,15H,2-4,11-14H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353172
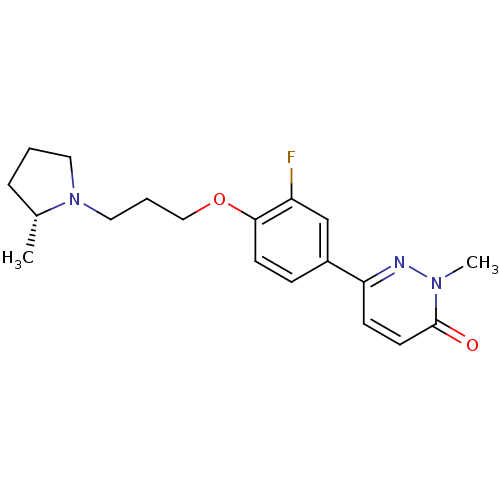
(CHEMBL1829477)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1F)-c1ccc(=O)n(C)n1 |r| Show InChI InChI=1S/C19H24FN3O2/c1-14-5-3-10-23(14)11-4-12-25-18-8-6-15(13-16(18)20)17-7-9-19(24)22(2)21-17/h6-9,13-14H,3-5,10-12H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human cloned histamine 3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 5493-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.108
BindingDB Entry DOI: 10.7270/Q2KP8359 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data