

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233628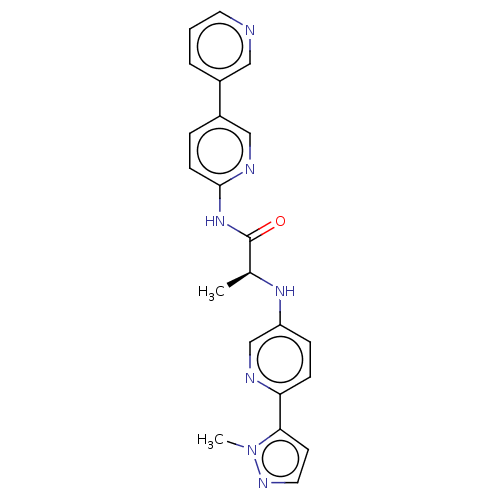 (CHEMBL4088716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233629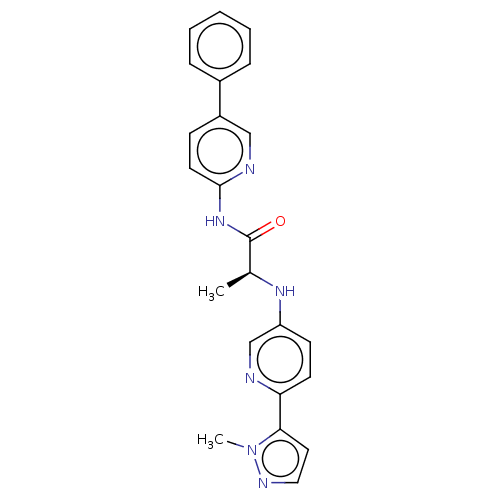 (CHEMBL4080842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233636 (CHEMBL4070649) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233635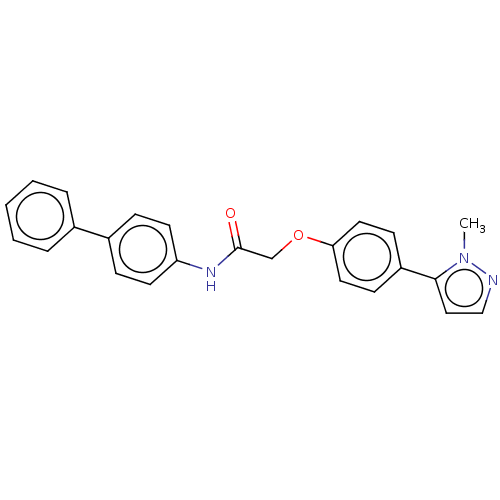 (CHEMBL4099279) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233630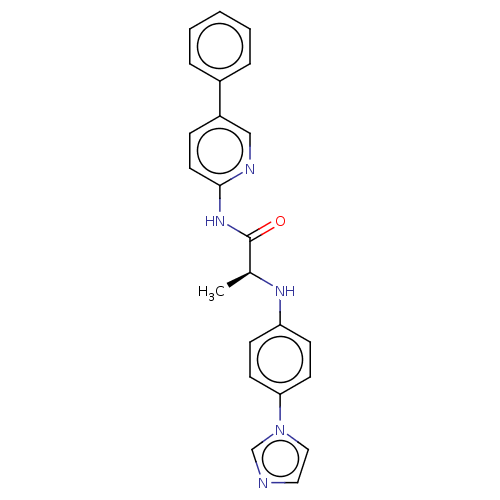 (CHEMBL4084739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233633 (CHEMBL4089128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255003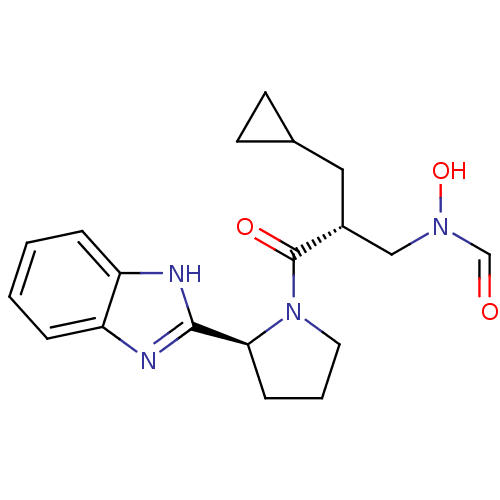 (CHEMBL519613 | N-((R)-3-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255000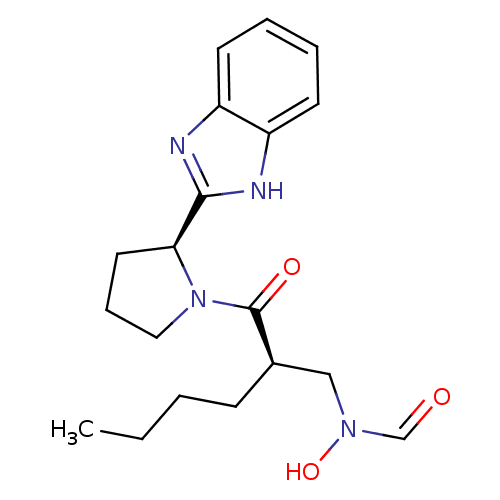 (CHEMBL443904 | N-((R)-2-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233640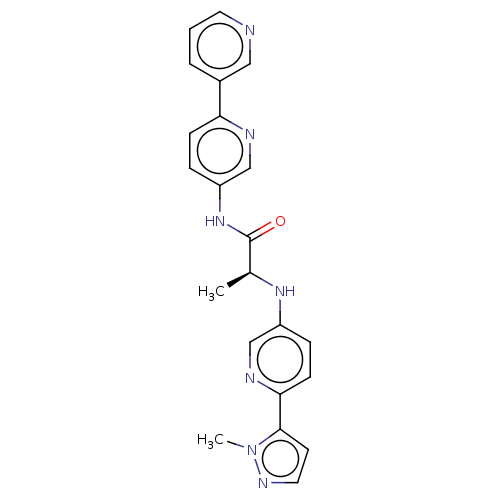 (CHEMBL4098880) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255030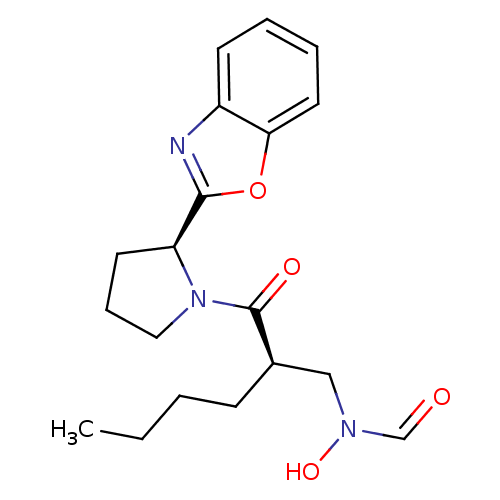 (CHEMBL506649 | N-((R)-2-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255032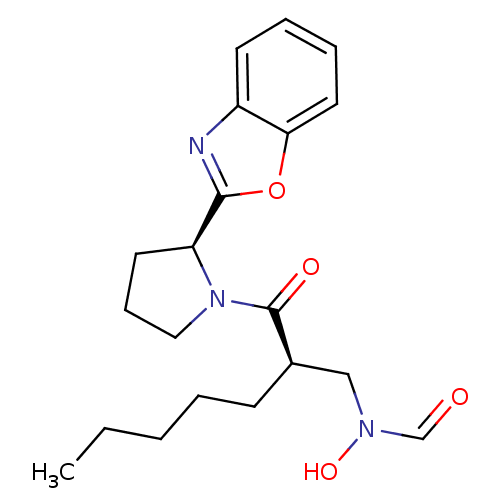 (CHEMBL465740 | N-((R)-2-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255002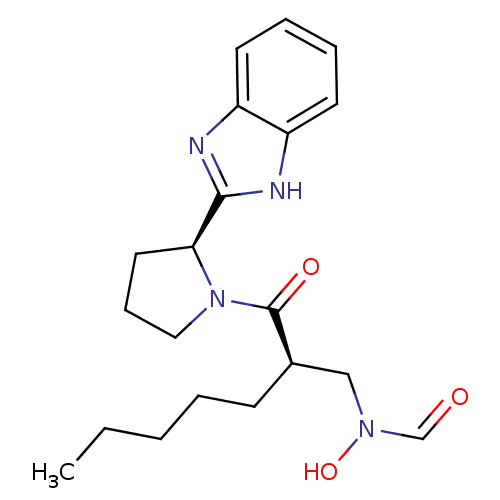 (CHEMBL482180 | N-((R)-2-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233639 (CHEMBL4097104) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255065 ((S)-2-((S)-2-(1H-benzo[d]imidazol-2-yl)pyrrolidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50233639 (CHEMBL4097104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255033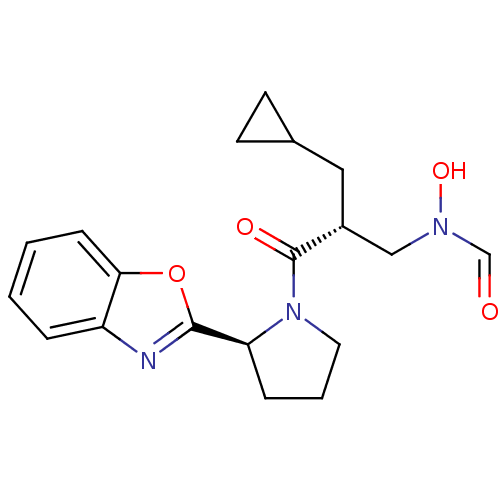 (CHEMBL463859 | N-((R)-3-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255001 (CHEMBL482179 | N-((R)-2-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255031 (CHEMBL480220 | N-((R)-2-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233634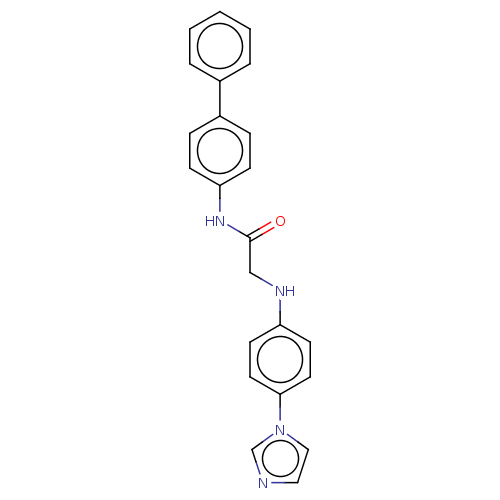 (CHEMBL4081262) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233641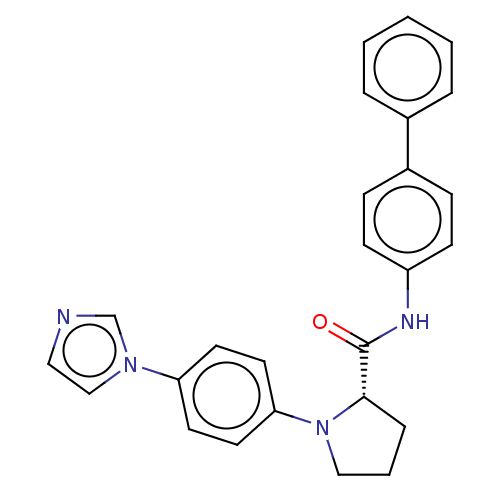 (CHEMBL4096832) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233637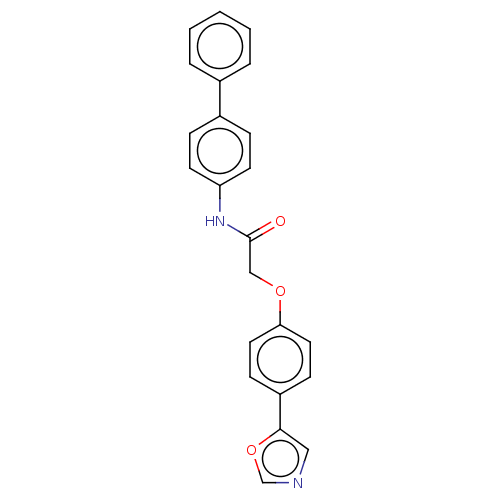 (CHEMBL4063419) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255066 ((S)-2-((S)-2-(benzo[d]oxazol-2-yl)pyrrolidine-1-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255004 (CHEMBL481391 | N-((R)-3-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50233639 (CHEMBL4097104) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375537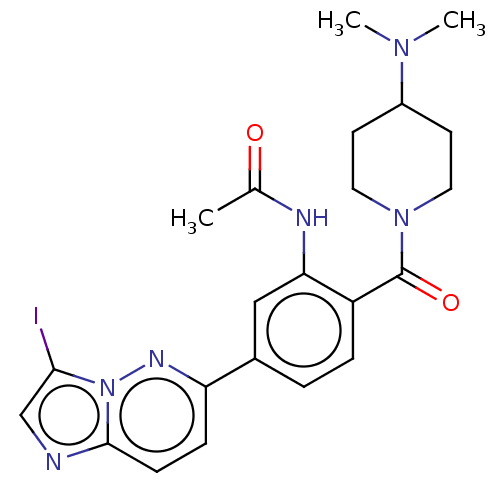 (N-(5-(3-(4-cyanophenyl)imidazo[1,2-b]pyridazin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375537 (N-(5-(3-(4-cyanophenyl)imidazo[1,2-b]pyridazin-6-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375538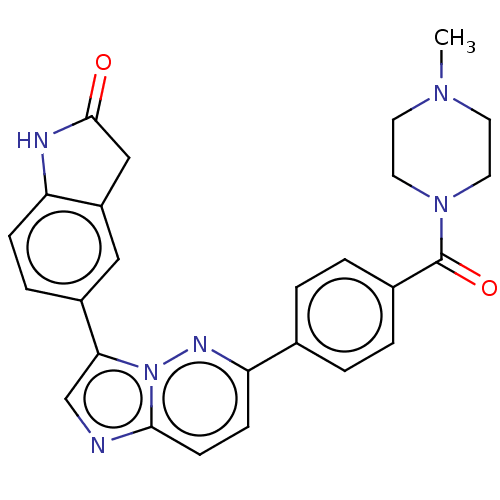 (5-(6-(4-(4-methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375538 (5-(6-(4-(4-methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375539 ((4-(3-(1H-indazol-5-yl)imidazo[1,2-b]pyridazin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375539 ((4-(3-(1H-indazol-5-yl)imidazo[1,2-b]pyridazin-6-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375540 ((4-(3-(1H-benzo[d]imidazol-5-yl)imidazo[1,2-b]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375540 ((4-(3-(1H-benzo[d]imidazol-5-yl)imidazo[1,2-b]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375541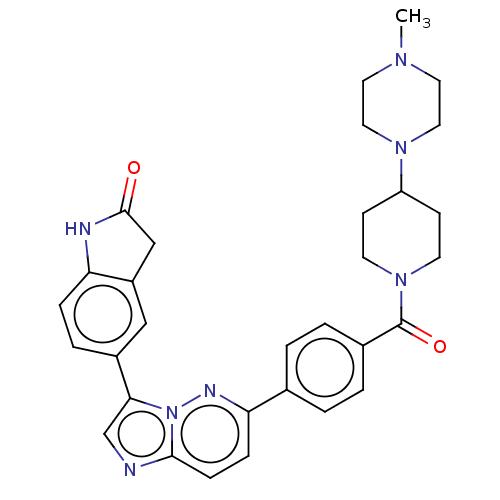 (5-(6-(4-(4-(4-methylpiperazin-1-yl)piperidine-1-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375541 (5-(6-(4-(4-(4-methylpiperazin-1-yl)piperidine-1-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375542 (5-(6-(4-(4-morpholinopiperidine-1-carbonyl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375542 (5-(6-(4-(4-morpholinopiperidine-1-carbonyl)phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375543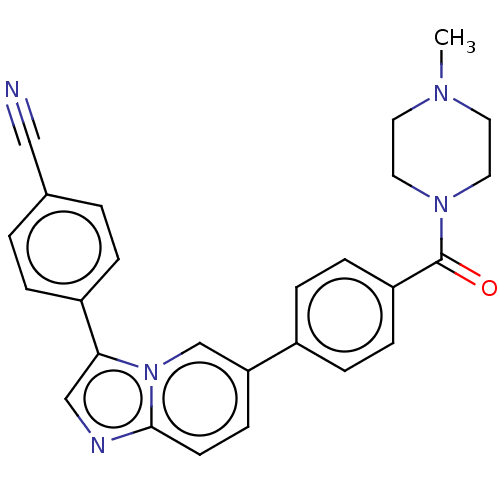 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375543 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375544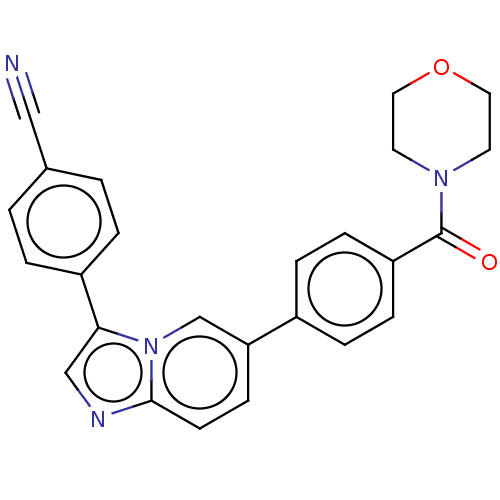 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375544 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375546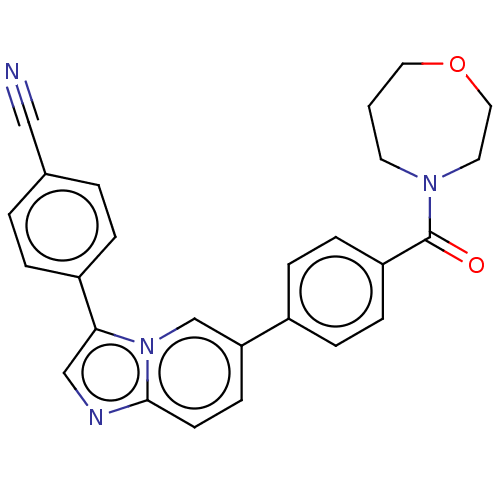 (4-(6-(4-(1,4-oxazepane-4-carbonyl)phenyl imidazo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375546 (4-(6-(4-(1,4-oxazepane-4-carbonyl)phenyl imidazo[1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375547 (4-(6-(4-(4-ethylpiperazine-1-carbonyl)phenyl)imida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375547 (4-(6-(4-(4-ethylpiperazine-1-carbonyl)phenyl)imida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375548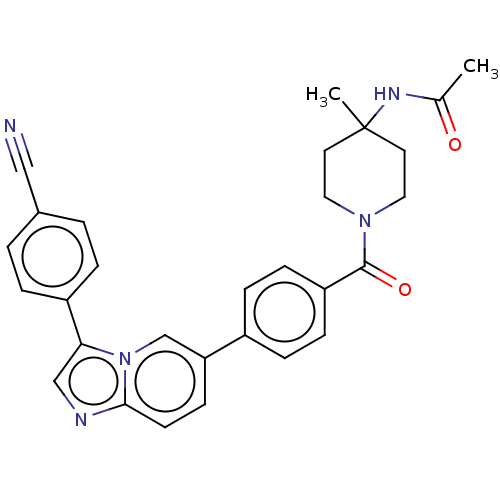 (N-(1-(4-(3-(4-cyanophenyl)imidazo[1,2-a]pyridin-6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375548 (N-(1-(4-(3-(4-cyanophenyl)imidazo[1,2-a]pyridin-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375549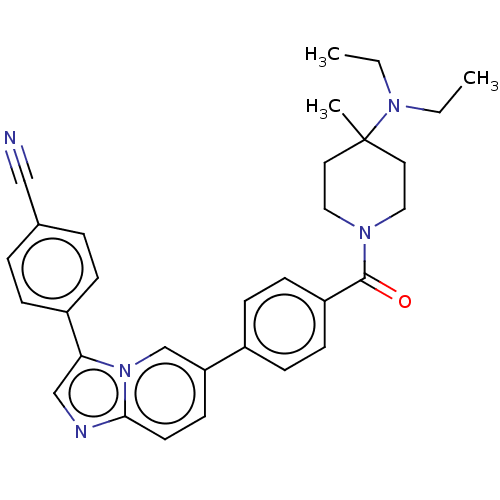 (4-(6-(4-(4-(diethylamino)-4-methylpiperidine-1-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375549 (4-(6-(4-(4-(diethylamino)-4-methylpiperidine-1-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375550 (4-(3-(4-cyanophenyl)imidazo[1,2-a]pyridin-6-yl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375550 (4-(3-(4-cyanophenyl)imidazo[1,2-a]pyridin-6-yl)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi | Assay Description MNK1 and MNK2 inhibitor activity was determined using recombinant kinase domains expressed in E. coli. MNK1 and MNK2 were expressed as GST fusion pro... | J Med Chem 50: 3841-50 (2007) BindingDB Entry DOI: 10.7270/Q2XP777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 679 total ) | Next | Last >> |