 Found 410 hits with Last Name = 'durroux' and Initial = 't'
Found 410 hits with Last Name = 'durroux' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50221813
(CHEMBL394602 | HO-LVA)Show SMILES [#6]-[#7](-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6@@H](-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6]-c1ccc(-[#8])cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)27-32-13-17-35(71)18-14-32)41(26-31-11-15-34(70)16-12-31)50(80)67-39(25-30-7-3-2-4-8-30)48(78)64-37(19-20-42(54)72)47(77)66-40(28-43(55)73)49(79)65-38(10-6-23-62-53(59)60)51(81)69-24-21-33(29-69)46(76)63-36(45(56)75)9-5-22-61-52(57)58/h2-4,7-8,11-18,33,36-41,70-71H,5-6,9-10,19-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,76)(H,64,78)(H,65,79)(H,66,77)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t33-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in CHO cells by polarisation binding assay using 384-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50221813

(CHEMBL394602 | HO-LVA)Show SMILES [#6]-[#7](-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6@@H](-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6]-c1ccc(-[#8])cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)27-32-13-17-35(71)18-14-32)41(26-31-11-15-34(70)16-12-31)50(80)67-39(25-30-7-3-2-4-8-30)48(78)64-37(19-20-42(54)72)47(77)66-40(28-43(55)73)49(79)65-38(10-6-23-62-53(59)60)51(81)69-24-21-33(29-69)46(76)63-36(45(56)75)9-5-22-61-52(57)58/h2-4,7-8,11-18,33,36-41,70-71H,5-6,9-10,19-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,76)(H,64,78)(H,65,79)(H,66,77)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t33-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in CHO cells by polarisation binding assay using 96-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075724
(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3cc(ccc23)C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)CCc2ccc(O)cc2)N(C)C)c1 Show InChI InChI=1S/C79H96N16O16/c1-91(2)49-24-31-55-64(42-49)110-65-43-50(92(3)4)25-32-56(65)79(55)54-30-23-48(41-53(54)77(109)111-79)74(106)95(61(69(83)101)16-9-10-36-80)76(108)62-17-12-38-94(62)75(107)58(15-11-37-86-78(84)85)88-72(104)60(44-67(82)99)89-70(102)57(33-34-66(81)98)87-71(103)59(39-46-13-7-6-8-14-46)90-73(105)63(40-47-20-28-52(97)29-21-47)93(5)68(100)35-22-45-18-26-51(96)27-19-45/h6-8,13-14,18-21,23-32,41-43,57-63,96-97H,9-12,15-17,22,33-40,44,80H2,1-5H3,(H2,81,98)(H2,82,99)(H2,83,101)(H,87,103)(H,88,104)(H,89,102)(H,90,105)(H4,84,85,86)/t57-,58-,59-,60-,61-,62-,63+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075720
((HO-LVA23)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-4-H...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)C(=O)Cc1ccc(O)cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)28-32-15-19-34(71)20-16-32)41(27-31-13-17-33(70)18-14-31)50(80)67-38(26-30-8-3-2-4-9-30)47(77)64-36(21-22-42(54)72)46(76)66-39(29-43(55)73)48(78)65-37(11-6-24-62-53(59)60)51(81)69-25-7-12-40(69)49(79)63-35(45(56)75)10-5-23-61-52(57)58/h2-4,8-9,13-20,35-41,70-71H,5-7,10-12,21-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,79)(H,64,77)(H,65,78)(H,66,76)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50221815
(4-({[(6-{N-[(1S)-5-amino-1-carbamoylpentyl]-1-[(3S...)Show SMILES [#6]-[#6]-1-[#6]C([#6])([#6])[#7]=[#6]-2-[#6]-1-[#6]-[#6]-1=[#6](-c3cc4-[#6](-[#6])-[#6]C([#6])([#6])[#7]-c4c(c3-[#8]-[#6]-1-[#6]-2S([#8-])(=O)=O)S([#8-])(=O)=O)-c1c(Cl)c(-[#16]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7](-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6@H]-2-[#6]-[#6]-[#7](-[#6]-2)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7](-[#6])-[#6](=O)-[#6]-[#6]-c2ccc(-[#8])cc2)c(Cl)c(Cl)c1-[#6](-[#8-])=O |c:6,t:11| Show InChI InChI=1S/C94H122Cl3N17O23S3/c1-48-44-93(3,4)110-77-56(48)41-58-71(59-42-57-49(2)45-94(5,6)111-78(57)83(140(134,135)136)80(59)137-79(58)82(77)139(131,132)133)72-73(91(129)130)74(95)76(97)81(75(72)96)138-47-68(119)104-36-15-9-12-21-70(121)114(64(84(101)122)20-13-14-35-98)89(127)53-34-38-113(46-53)90(128)61(19-16-37-105-92(102)103)107-87(125)63(43-67(100)118)108-85(123)60(31-32-66(99)117)106-86(124)62(39-51-17-10-8-11-18-51)109-88(126)65(40-52-24-29-55(116)30-25-52)112(7)69(120)33-26-50-22-27-54(115)28-23-50/h8,10-11,17-18,22-25,27-30,41,48-49,53,57,60-65,80,83,110,115-116H,9,12-16,19-21,26,31-40,42-47,98H2,1-7H3,(H2,99,117)(H2,100,118)(H2,101,122)(H,104,119)(H,106,124)(H,107,125)(H,108,123)(H,109,126)(H,129,130)(H4,102,103,105)(H,131,132,133)(H,134,135,136)/p-3/t48?,49?,53-,57?,60-,61-,62-,63-,64-,65+,80?,83?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1a receptor expressed in CHO cells |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370109

(CHEMBL1790723)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6](-[#8])-[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(-[#6]-2=[#6]-3-[#6]=[#6]-[#6](=[#6]-[#6]-3-[#8]-[#6]-3=[#6]\[#6](-[#6]=[#6]-[#6]-2-3)=[#7+](\[#6])-[#6])-[#7](-[#6])-[#6])c(c1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#7])-c1ccc(-[#8])cc1 |c:62,64,66,74,t:71| Show InChI InChI=1S/C65H84N14O16S2/c1-7-33(2)54-62(90)72-44(22-23-51(67)82)58(86)73-45(29-52(68)83)59(87)74-46(30-96-97-31-48(81)61(89)76-55(63(91)75-54)34-12-17-38(80)18-13-34)64(92)79-25-9-11-47(79)60(88)71-43(57(85)70-32-66)10-8-24-69-56(84)35-14-19-39(42(26-35)65(93)94)53-40-20-15-36(77(3)4)27-49(40)95-50-28-37(78(5)6)16-21-41(50)53/h12-21,26-28,33,40,43-48,50,54-55,81H,7-11,22-25,29-32,66H2,1-6H3,(H13-,67,68,69,70,71,72,73,74,75,76,80,82,83,84,85,86,87,88,89,90,91,93,94)/p+1/t33-,40?,43+,44-,45-,46-,47-,48?,50?,54-,55+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075722

(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)c1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)CCc1ccc(O)cc1 Show InChI InChI=1S/C75H86N14O18/c1-87(64(96)31-18-41-14-20-45(90)21-15-41)59(36-43-16-22-46(91)23-17-43)69(101)86-55(35-42-9-3-2-4-10-42)67(99)83-53(29-30-62(77)94)66(98)85-56(40-63(78)95)68(100)84-54(11-7-33-82-74(80)81)71(103)88-34-8-13-58(88)72(104)89(57(65(79)97)12-5-6-32-76)70(102)44-19-26-50-49(37-44)73(105)107-75(50)51-27-24-47(92)38-60(51)106-61-39-48(93)25-28-52(61)75/h2-4,9-10,14-17,19-28,37-39,53-59,90-93H,5-8,11-13,18,29-36,40,76H2,1H3,(H2,77,94)(H2,78,95)(H2,79,97)(H,83,99)(H,84,100)(H,85,98)(H,86,101)(H4,80,81,82)/t53-,54-,55-,56-,57-,58-,59+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370113

(CHEMBL1790719)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C60H71N11O18S2/c1-4-28(2)47-55(83)69-48(29(3)72)56(84)66-40(24-46(62)77)52(80)67-41(25-90-91-26-43(76)54(82)70-49(57(85)68-47)30-9-12-32(73)13-10-30)58(86)71-20-6-8-42(71)53(81)65-39(51(79)64-27-61)7-5-19-63-50(78)31-11-16-35-38(21-31)60(89-59(35)87)36-17-14-33(74)22-44(36)88-45-23-34(75)15-18-37(45)60/h9-18,21-23,28-29,39-43,47-49,72-76H,4-8,19-20,24-27,61H2,1-3H3,(H2,62,77)(H,63,78)(H,64,79)(H,65,81)(H,66,84)(H,67,80)(H,68,85)(H,69,83)(H,70,82)/t28-,29?,39+,40-,41-,42-,43?,47-,48-,49+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075725
(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(6C-...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3ccc(cc23)C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)CCc2ccc(O)cc2)N(C)C)c1 Show InChI InChI=1S/C79H96N16O16/c1-91(2)49-24-31-54-64(42-49)110-65-43-50(92(3)4)25-32-55(65)79(54)56-41-48(23-30-53(56)77(109)111-79)74(106)95(61(69(83)101)16-9-10-36-80)76(108)62-17-12-38-94(62)75(107)58(15-11-37-86-78(84)85)88-72(104)60(44-67(82)99)89-70(102)57(33-34-66(81)98)87-71(103)59(39-46-13-7-6-8-14-46)90-73(105)63(40-47-20-28-52(97)29-21-47)93(5)68(100)35-22-45-18-26-51(96)27-19-45/h6-8,13-14,18-21,23-32,41-43,57-63,96-97H,9-12,15-17,22,33-40,44,80H2,1-5H3,(H2,81,98)(H2,82,99)(H2,83,101)(H,87,103)(H,88,104)(H,89,102)(H,90,105)(H4,84,85,86)/t57-,58-,59-,60-,61-,62-,63+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370117

(CHEMBL1790720)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H72N12O18S2/c1-3-29(2)49-57(86)68-40(18-19-47(63)78)53(82)69-41(25-48(64)79)54(83)70-42(26-92-93-27-44(77)56(85)72-50(58(87)71-49)30-8-11-32(74)12-9-30)59(88)73-21-5-7-43(73)55(84)67-39(52(81)66-28-62)6-4-20-65-51(80)31-10-15-35-38(22-31)61(91-60(35)89)36-16-13-33(75)23-45(36)90-46-24-34(76)14-17-37(46)61/h8-17,22-24,29,39-44,49-50,74-77H,3-7,18-21,25-28,62H2,1-2H3,(H2,63,78)(H2,64,79)(H,65,80)(H,66,81)(H,67,84)(H,68,86)(H,69,82)(H,70,83)(H,71,87)(H,72,85)/t29-,39+,40-,41-,42-,43-,44?,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031
((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in COS7 cells by HTRF-FRET assay using 96-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50221813

(CHEMBL394602 | HO-LVA)Show SMILES [#6]-[#7](-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6@@H](-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6]-c1ccc(-[#8])cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)27-32-13-17-35(71)18-14-32)41(26-31-11-15-34(70)16-12-31)50(80)67-39(25-30-7-3-2-4-8-30)48(78)64-37(19-20-42(54)72)47(77)66-40(28-43(55)73)49(79)65-38(10-6-23-62-53(59)60)51(81)69-24-21-33(29-69)46(76)63-36(45(56)75)9-5-22-61-52(57)58/h2-4,7-8,11-18,33,36-41,70-71H,5-6,9-10,19-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,76)(H,64,78)(H,65,79)(H,66,77)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t33-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in COS7 cells by HTRF-FRET assay using 384-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370106

(CHEMBL1790712)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@](C)(NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@H](C(N)=O)c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C52H75N11O13S2/c1-5-28(2)40-47(73)59-41(29(3)64)48(74)57-35(25-38(54)67)45(71)58-36(27-77-78-52(21-7-6-8-22-52)26-39(68)62-51(4,50(76)61-40)31-15-19-33(66)20-16-31)49(75)63-24-10-12-37(63)46(72)56-34(11-9-23-53)44(70)60-42(43(55)69)30-13-17-32(65)18-14-30/h13-20,28-29,34-37,40-42,64-66H,5-12,21-27,53H2,1-4H3,(H2,54,67)(H2,55,69)(H,56,72)(H,57,74)(H,58,71)(H,59,73)(H,60,70)(H,61,76)(H,62,68)/t28-,29-,34+,35-,36-,37-,40-,41-,42+,51-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50221813

(CHEMBL394602 | HO-LVA)Show SMILES [#6]-[#7](-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6@@H](-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6]-c1ccc(-[#8])cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)27-32-13-17-35(71)18-14-32)41(26-31-11-15-34(70)16-12-31)50(80)67-39(25-30-7-3-2-4-8-30)48(78)64-37(19-20-42(54)72)47(77)66-40(28-43(55)73)49(79)65-38(10-6-23-62-53(59)60)51(81)69-24-21-33(29-69)46(76)63-36(45(56)75)9-5-22-61-52(57)58/h2-4,7-8,11-18,33,36-41,70-71H,5-6,9-10,19-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,76)(H,64,78)(H,65,79)(H,66,77)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t33-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in COS7 cells by HTRF-FRET assay using 96-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077141
(CHEMBL3416758)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)C(N)C2(CCCCC2)SSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)[C@@H](C)O |r| Show InChI InChI=1S/C57H78N12O13S2/c1-32(70)46-54(79)65-42(30-45(59)72)51(76)67-43(56(81)69-26-10-14-44(69)53(78)62-38(13-9-25-58)49(74)63-39(48(61)73)27-34-15-19-36(71)20-16-34)31-83-84-57(23-7-4-8-24-57)47(60)55(80)66-40(29-35-17-21-37(82-2)22-18-35)50(75)64-41(52(77)68-46)28-33-11-5-3-6-12-33/h3,5-6,11-12,15-22,32,38-44,46-47,70-71H,4,7-10,13-14,23-31,58,60H2,1-2H3,(H2,59,72)(H2,61,73)(H,62,78)(H,63,74)(H,64,75)(H,65,79)(H,66,80)(H,67,76)(H,68,77)/t32-,38+,39+,40+,41+,42+,43+,44+,46+,47?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Competitive binding to human oxytocin receptor by radioligand binding assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075728
(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(6C-...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)CCc1ccc(O)cc1 Show InChI InChI=1S/C75H86N14O18/c1-87(64(96)31-18-41-14-20-45(90)21-15-41)59(36-43-16-22-46(91)23-17-43)69(101)86-55(35-42-9-3-2-4-10-42)67(99)83-53(29-30-62(77)94)66(98)85-56(40-63(78)95)68(100)84-54(11-7-33-82-74(80)81)71(103)88-34-8-13-58(88)72(104)89(57(65(79)97)12-5-6-32-76)70(102)44-19-26-49-52(37-44)75(107-73(49)105)50-27-24-47(92)38-60(50)106-61-39-48(93)25-28-51(61)75/h2-4,9-10,14-17,19-28,37-39,53-59,90-93H,5-8,11-13,18,29-36,40,76H2,1H3,(H2,77,94)(H2,78,95)(H2,79,97)(H,83,99)(H,84,100)(H,85,98)(H,86,101)(H4,80,81,82)/t53-,54-,55-,56-,57-,58-,59+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370110

(CHEMBL1790721)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H72N12O17S2/c1-3-30(2)50-57(85)68-41(18-19-47(63)77)54(82)69-42(27-48(64)78)55(83)70-43(28-92-91-23-20-49(79)71-51(58(86)72-50)31-8-11-33(74)12-9-31)59(87)73-22-5-7-44(73)56(84)67-40(53(81)66-29-62)6-4-21-65-52(80)32-10-15-36-39(24-32)61(90-60(36)88)37-16-13-34(75)25-45(37)89-46-26-35(76)14-17-38(46)61/h8-17,24-26,30,40-44,50-51,74-76H,3-7,18-23,27-29,62H2,1-2H3,(H2,63,77)(H2,64,78)(H,65,80)(H,66,81)(H,67,84)(H,68,85)(H,69,82)(H,70,83)(H,71,79)(H,72,86)/t30-,40+,41-,42-,43-,44-,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50221813

(CHEMBL394602 | HO-LVA)Show SMILES [#6]-[#7](-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6@@H](-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6]-c1ccc(-[#8])cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)27-32-13-17-35(71)18-14-32)41(26-31-11-15-34(70)16-12-31)50(80)67-39(25-30-7-3-2-4-8-30)48(78)64-37(19-20-42(54)72)47(77)66-40(28-43(55)73)49(79)65-38(10-6-23-62-53(59)60)51(81)69-24-21-33(29-69)46(76)63-36(45(56)75)9-5-22-61-52(57)58/h2-4,7-8,11-18,33,36-41,70-71H,5-6,9-10,19-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,76)(H,64,78)(H,65,79)(H,66,77)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t33-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in COS7 cells by HTRF-FRET assay using 96-well plate cells |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370114
(CHEMBL1790718)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C62H74N12O17S2/c1-3-31(2)51-58(86)69-42(19-20-48(64)78)55(83)70-43(28-49(65)79)56(84)71-44(29-93-92-24-21-50(80)72-52(59(87)73-51)32-9-12-34(75)13-10-32)60(88)74-23-6-8-45(74)57(85)68-41(54(82)67-30-63)7-4-5-22-66-53(81)33-11-16-37-40(25-33)62(91-61(37)89)38-17-14-35(76)26-46(38)90-47-27-36(77)15-18-39(47)62/h9-18,25-27,31,41-45,51-52,75-77H,3-8,19-24,28-30,63H2,1-2H3,(H2,64,78)(H2,65,79)(H,66,81)(H,67,82)(H,68,85)(H,69,86)(H,70,83)(H,71,84)(H,72,80)(H,73,87)/t31-,41+,42-,43-,44-,45-,51-,52+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50459408
(CHEMBL3307200)Show SMILES Cc1occc1C(=O)Nc1ccc(cc1)C(=O)N1CCC(F)(F)\C(=C/C(=O)N2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C35H38F2N4O4/c1-24-28(15-22-45-24)33(43)38-26-11-9-25(10-12-26)34(44)41-21-16-35(36,37)30(29-7-3-4-8-31(29)41)23-32(42)40-19-13-27(14-20-40)39-17-5-2-6-18-39/h3-4,7-12,15,22-23,27H,2,5-6,13-14,16-21H2,1H3,(H,38,43)/b30-23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor expressed in CHO cell membranes |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370112

(CHEMBL1790729)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H73N11O18S2/c1-4-29(2)48-56(84)70-49(30(3)73)57(85)67-41(25-47(63)78)53(81)68-42(26-91-92-27-44(77)55(83)71-50(58(86)69-48)31-10-13-33(74)14-11-31)59(87)72-21-7-9-43(72)54(82)66-40(52(80)65-28-62)8-5-6-20-64-51(79)32-12-17-36-39(22-32)61(90-60(36)88)37-18-15-34(75)23-45(37)89-46-24-35(76)16-19-38(46)61/h10-19,22-24,29-30,40-44,48-50,73-77H,4-9,20-21,25-28,62H2,1-3H3,(H2,63,78)(H,64,79)(H,65,80)(H,66,82)(H,67,85)(H,68,81)(H,69,86)(H,70,84)(H,71,83)/t29-,30?,40+,41-,42-,43-,44?,48-,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370115
(CHEMBL1790717)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C62H74N12O18S2/c1-3-30(2)50-58(87)69-41(19-20-48(64)79)54(83)70-42(26-49(65)80)55(84)71-43(27-93-94-28-45(78)57(86)73-51(59(88)72-50)31-9-12-33(75)13-10-31)60(89)74-22-6-8-44(74)56(85)68-40(53(82)67-29-63)7-4-5-21-66-52(81)32-11-16-36-39(23-32)62(92-61(36)90)37-17-14-34(76)24-46(37)91-47-25-35(77)15-18-38(47)62/h9-18,23-25,30,40-45,50-51,75-78H,3-8,19-22,26-29,63H2,1-2H3,(H2,64,79)(H2,65,80)(H,66,81)(H,67,82)(H,68,85)(H,69,87)(H,70,83)(H,71,84)(H,72,88)(H,73,86)/t30-,40+,41-,42-,43-,44-,45?,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370111

(CHEMBL1790711)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)[C@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C60H71N11O17S2/c1-4-29(2)48-55(82)70-49(30(3)72)56(83)66-41(26-46(62)76)53(80)67-42(27-90-89-22-19-47(77)68-50(57(84)69-48)31-9-12-33(73)13-10-31)58(85)71-21-6-8-43(71)54(81)65-40(52(79)64-28-61)7-5-20-63-51(78)32-11-16-36-39(23-32)60(88-59(36)86)37-17-14-34(74)24-44(37)87-45-25-35(75)15-18-38(45)60/h9-18,23-25,29-30,40-43,48-50,72-75H,4-8,19-22,26-28,61H2,1-3H3,(H2,62,76)(H,63,78)(H,64,79)(H,65,81)(H,66,83)(H,67,80)(H,68,77)(H,69,84)(H,70,82)/t29-,30+,40+,41-,42-,43-,48-,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077141

(CHEMBL3416758)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)C(N)C2(CCCCC2)SSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)[C@@H](C)O |r| Show InChI InChI=1S/C57H78N12O13S2/c1-32(70)46-54(79)65-42(30-45(59)72)51(76)67-43(56(81)69-26-10-14-44(69)53(78)62-38(13-9-25-58)49(74)63-39(48(61)73)27-34-15-19-36(71)20-16-34)31-83-84-57(23-7-4-8-24-57)47(60)55(80)66-40(29-35-17-21-37(82-2)22-18-35)50(75)64-41(52(77)68-46)28-33-11-5-3-6-12-33/h3,5-6,11-12,15-22,32,38-44,46-47,70-71H,4,7-10,13-14,23-31,58,60H2,1-2H3,(H2,59,72)(H2,61,73)(H,62,78)(H,63,74)(H,64,75)(H,65,79)(H,66,80)(H,67,76)(H,68,77)/t32-,38+,39+,40+,41+,42+,43+,44+,46+,47?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Competitive binding to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells incubated for 1 hr at RT followed by 4 hrs at 4 degC ... |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095
(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from V1A receptor in Wistar rat liver membranes incubated for 60 mins by microplate scintillation counting method |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1b receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031

((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in COS7 cells by HTRF-FRET assay using 384-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V2 receptor expressed in CHO cell membranes by radioligand binding assay |
J Med Chem 61: 8670-8692 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00697
BindingDB Entry DOI: 10.7270/Q24J0HRF |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50307117

(CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCCc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C30H26N2O2/c33-29(27-15-6-5-14-26(27)22-10-2-1-3-11-22)31-25-19-17-24(18-20-25)30(34)32-21-9-8-13-23-12-4-7-16-28(23)32/h1-7,10-12,14-20H,8-9,13,21H2,(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells |
J Med Chem 53: 1546-62 (2010)
Article DOI: 10.1021/jm901084f
BindingDB Entry DOI: 10.7270/Q2FX7BD4 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50077141

(CHEMBL3416758)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)C(N)C2(CCCCC2)SSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)[C@@H](C)O |r| Show InChI InChI=1S/C57H78N12O13S2/c1-32(70)46-54(79)65-42(30-45(59)72)51(76)67-43(56(81)69-26-10-14-44(69)53(78)62-38(13-9-25-58)49(74)63-39(48(61)73)27-34-15-19-36(71)20-16-34)31-83-84-57(23-7-4-8-24-57)47(60)55(80)66-40(29-35-17-21-37(82-2)22-18-35)50(75)64-41(52(77)68-46)28-33-11-5-3-6-12-33/h3,5-6,11-12,15-22,32,38-44,46-47,70-71H,4,7-10,13-14,23-31,58,60H2,1-2H3,(H2,59,72)(H2,61,73)(H,62,78)(H,63,74)(H,64,75)(H,65,79)(H,66,80)(H,67,76)(H,68,77)/t32-,38+,39+,40+,41+,42+,43+,44+,46+,47?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Competitive binding to SNAP-tagged oxytocin receptor (unknown origin) expressed in HEK293 cells incubated for 1 hr at RT followed by 4 hrs at 4 degC ... |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031

((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in CHO cells by polarisation binding assay using 96-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1a receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Competitive binding to oxytocin receptor (unknown origin) by radioligand binding assay |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Binding affinity to OTR (unknown origin) |
J Med Chem 58: 2547-52 (2015)
Article DOI: 10.1021/jm501395b
BindingDB Entry DOI: 10.7270/Q2TB18KT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075721
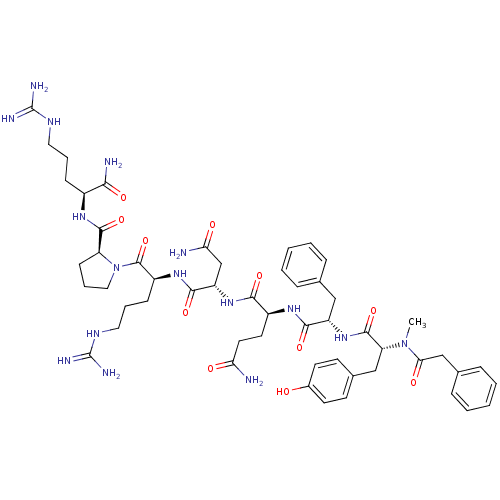
((LVA)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-phenylac...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)C(=O)Cc1ccccc1 Show InChI InChI=1S/C53H74N16O11/c1-68(44(73)29-32-13-6-3-7-14-32)41(28-33-18-20-34(70)21-19-33)50(79)67-38(27-31-11-4-2-5-12-31)47(76)64-36(22-23-42(54)71)46(75)66-39(30-43(55)72)48(77)65-37(16-9-25-62-53(59)60)51(80)69-26-10-17-40(69)49(78)63-35(45(56)74)15-8-24-61-52(57)58/h2-7,11-14,18-21,35-41,70H,8-10,15-17,22-30H2,1H3,(H2,54,71)(H2,55,72)(H2,56,74)(H,63,78)(H,64,76)(H,65,77)(H,66,75)(H,67,79)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397209

(CHEMBL2172291)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C83H100N10O17S2/c1-6-91-73-36-34-64(111(99,100)101)56-69(73)82(3,4)75(91)27-12-9-13-28-76-83(5,70-57-65(112(102,103)104)35-37-74(70)92(76)7-2)40-18-29-77(94)84-41-44-105-46-48-107-50-52-109-54-55-110-53-51-108-49-47-106-45-43-90-59-63(88-89-90)58-85-78(95)38-39-79(96)87-71-25-19-42-93(72-26-17-16-24-68(71)72)81(98)61-30-32-62(33-31-61)86-80(97)67-23-15-14-22-66(67)60-20-10-8-11-21-60/h8-17,20-24,26-28,30-37,56-57,59,71H,6-7,18-19,25,29,38-55,58H2,1-5H3,(H5-,84,85,86,87,94,95,96,97,98,99,100,101,102,103,104) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assay |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031

((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in COS7 cells by HTRF-FRET assay using 96-well plate cells |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50221815

(4-({[(6-{N-[(1S)-5-amino-1-carbamoylpentyl]-1-[(3S...)Show SMILES [#6]-[#6]-1-[#6]C([#6])([#6])[#7]=[#6]-2-[#6]-1-[#6]-[#6]-1=[#6](-c3cc4-[#6](-[#6])-[#6]C([#6])([#6])[#7]-c4c(c3-[#8]-[#6]-1-[#6]-2S([#8-])(=O)=O)S([#8-])(=O)=O)-c1c(Cl)c(-[#16]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7](-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O)-[#6](=O)-[#6@H]-2-[#6]-[#6]-[#7](-[#6]-2)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#7](-[#6])-[#6](=O)-[#6]-[#6]-c2ccc(-[#8])cc2)c(Cl)c(Cl)c1-[#6](-[#8-])=O |c:6,t:11| Show InChI InChI=1S/C94H122Cl3N17O23S3/c1-48-44-93(3,4)110-77-56(48)41-58-71(59-42-57-49(2)45-94(5,6)111-78(57)83(140(134,135)136)80(59)137-79(58)82(77)139(131,132)133)72-73(91(129)130)74(95)76(97)81(75(72)96)138-47-68(119)104-36-15-9-12-21-70(121)114(64(84(101)122)20-13-14-35-98)89(127)53-34-38-113(46-53)90(128)61(19-16-37-105-92(102)103)107-87(125)63(43-67(100)118)108-85(123)60(31-32-66(99)117)106-86(124)62(39-51-17-10-8-11-18-51)109-88(126)65(40-52-24-29-55(116)30-25-52)112(7)69(120)33-26-50-22-27-54(115)28-23-50/h8,10-11,17-18,22-25,27-30,41,48-49,53,57,60-65,80,83,110,115-116H,9,12-16,19-21,26,31-40,42-47,98H2,1-7H3,(H2,99,117)(H2,100,118)(H2,101,122)(H,104,119)(H,106,124)(H,107,125)(H,108,123)(H,109,126)(H,129,130)(H4,102,103,105)(H,131,132,133)(H,134,135,136)/p-3/t48?,49?,53-,57?,60-,61-,62-,63-,64-,65+,80?,83?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human oxytocin receptor expressed in CHO cells |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50075721

((LVA)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-phenylac...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)C(=O)Cc1ccccc1 Show InChI InChI=1S/C53H74N16O11/c1-68(44(73)29-32-13-6-3-7-14-32)41(28-33-18-20-34(70)21-19-33)50(79)67-38(27-31-11-4-2-5-12-31)47(76)64-36(22-23-42(54)71)46(75)66-39(30-43(55)72)48(77)65-37(16-9-25-62-53(59)60)51(80)69-26-10-17-40(69)49(78)63-35(45(56)74)15-8-24-61-52(57)58/h2-7,11-14,18-21,35-41,70H,8-10,15-17,22-30H2,1H3,(H2,54,71)(H2,55,72)(H2,56,74)(H,63,78)(H,64,76)(H,65,77)(H,66,75)(H,67,79)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50075724

(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3cc(ccc23)C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)CCc2ccc(O)cc2)N(C)C)c1 Show InChI InChI=1S/C79H96N16O16/c1-91(2)49-24-31-55-64(42-49)110-65-43-50(92(3)4)25-32-56(65)79(55)54-30-23-48(41-53(54)77(109)111-79)74(106)95(61(69(83)101)16-9-10-36-80)76(108)62-17-12-38-94(62)75(107)58(15-11-37-86-78(84)85)88-72(104)60(44-67(82)99)89-70(102)57(33-34-66(81)98)87-71(103)59(39-46-13-7-6-8-14-46)90-73(105)63(40-47-20-28-52(97)29-21-47)93(5)68(100)35-22-45-18-26-51(96)27-19-45/h6-8,13-14,18-21,23-32,41-43,57-63,96-97H,9-12,15-17,22,33-40,44,80H2,1-5H3,(H2,81,98)(H2,82,99)(H2,83,101)(H,87,103)(H,88,104)(H,89,102)(H,90,105)(H4,84,85,86)/t57-,58-,59-,60-,61-,62-,63+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075727

(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Lys(5C-Rhm)-Pro...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3cc(ccc23)C(=O)N([C@@H](CCCCN)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)CCc2ccc(O)cc2)N(C)C)c1 Show InChI InChI=1S/C79H96N16O16/c1-91(2)49-24-31-55-64(42-49)110-65-43-50(92(3)4)25-32-56(65)79(55)54-30-23-48(41-53(54)77(109)111-79)74(106)95(62(16-9-10-36-80)76(108)94-38-12-17-61(94)72(104)87-57(69(83)101)15-11-37-86-78(84)85)75(107)60(44-67(82)99)90-70(102)58(33-34-66(81)98)88-71(103)59(39-46-13-7-6-8-14-46)89-73(105)63(40-47-20-28-52(97)29-21-47)93(5)68(100)35-22-45-18-26-51(96)27-19-45/h6-8,13-14,18-21,23-32,41-43,57-63,96-97H,9-12,15-17,22,33-40,44,80H2,1-5H3,(H2,81,98)(H2,82,99)(H2,83,101)(H,87,104)(H,88,103)(H,89,105)(H,90,102)(H4,84,85,86)/t57-,58-,59-,60-,61-,62-,63+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins by saturation binding assay |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50114031

((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)N1[C@@H](C(=O)N2CCC[C@H]2C(N)=O)[C@](O)(c2cc(Cl)ccc12)c1ccccc1Cl |r| Show InChI InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human vasopressin V1a receptor expressed in CHO cells by polarisation binding assay using 384-well plate membranes |
J Med Chem 50: 4976-85 (2007)
Article DOI: 10.1021/jm061404q
BindingDB Entry DOI: 10.7270/Q24X58NW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50075722

(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)c1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)CCc1ccc(O)cc1 Show InChI InChI=1S/C75H86N14O18/c1-87(64(96)31-18-41-14-20-45(90)21-15-41)59(36-43-16-22-46(91)23-17-43)69(101)86-55(35-42-9-3-2-4-10-42)67(99)83-53(29-30-62(77)94)66(98)85-56(40-63(78)95)68(100)84-54(11-7-33-82-74(80)81)71(103)88-34-8-13-58(88)72(104)89(57(65(79)97)12-5-6-32-76)70(102)44-19-26-50-49(37-44)73(105)107-75(50)51-27-24-47(92)38-60(51)106-61-39-48(93)25-28-52(61)75/h2-4,9-10,14-17,19-28,37-39,53-59,90-93H,5-8,11-13,18,29-36,40,76H2,1H3,(H2,77,94)(H2,78,95)(H2,79,97)(H,83,99)(H,84,100)(H,85,98)(H,86,101)(H4,80,81,82)/t53-,54-,55-,56-,57-,58-,59+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The inhibition constant (Ki(nM)) of the compound was determined by displacement of [125I]- HO-LVA radiolabeled ligand using membranes of CHO cells of... |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human oxytocin receptor expressed in CHO cells after 30 mins |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370107
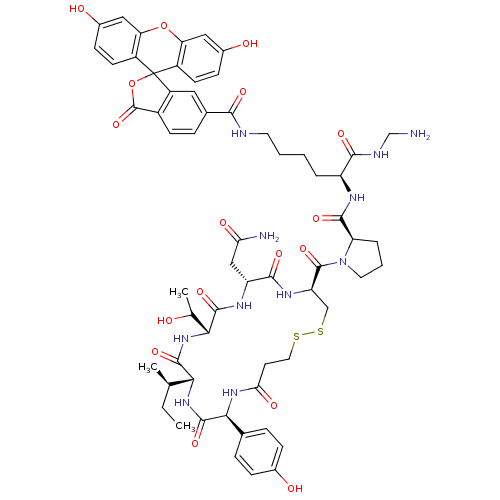
(CHEMBL1790728)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H73N11O17S2/c1-4-30(2)49-56(83)71-50(31(3)73)57(84)67-42(27-47(63)77)54(81)68-43(28-91-90-23-20-48(78)69-51(58(85)70-49)32-10-13-34(74)14-11-32)59(86)72-22-7-9-44(72)55(82)66-41(53(80)65-29-62)8-5-6-21-64-52(79)33-12-17-37-40(24-33)61(89-60(37)87)38-18-15-35(75)25-45(38)88-46-26-36(76)16-19-39(46)61/h10-19,24-26,30-31,41-44,49-51,73-76H,4-9,20-23,27-29,62H2,1-3H3,(H2,63,77)(H,64,79)(H,65,80)(H,66,82)(H,67,84)(H,68,81)(H,69,78)(H,70,85)(H,71,83)/t30-,31?,41+,42-,43-,44-,49-,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50397210
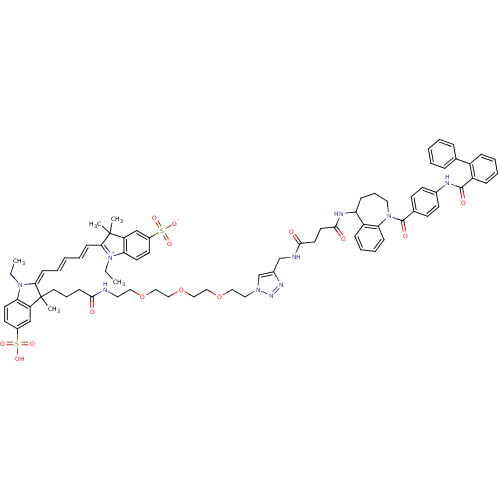
(CHEMBL2172290)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CC)c3ccc(cc3C2(C)C)S([O-])(=O)=O)C(C)(CCCC(=O)NCCOCCOCCOCCn2cc(CNC(=O)CCC(=O)NC3CCCN(C(=O)c4ccc(NC(=O)c5ccccc5-c5ccccc5)cc4)c4ccccc34)nn2)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C77H88N10O14S2/c1-6-85-67-36-34-58(102(93,94)95)50-63(67)76(3,4)69(85)27-12-9-13-28-70-77(5,64-51-59(103(96,97)98)35-37-68(64)86(70)7-2)40-18-29-71(88)78-41-44-99-46-48-101-49-47-100-45-43-84-53-57(82-83-84)52-79-72(89)38-39-73(90)81-65-25-19-42-87(66-26-17-16-24-62(65)66)75(92)55-30-32-56(33-31-55)80-74(91)61-23-15-14-22-60(61)54-20-10-8-11-21-54/h8-17,20-24,26-28,30-37,50-51,53,65H,6-7,18-19,25,29,38-49,52H2,1-5H3,(H5-,78,79,80,81,88,89,90,91,92,93,94,95,96,97,98) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assay |
J Med Chem 55: 8588-602 (2012)
Article DOI: 10.1021/jm3006146
BindingDB Entry DOI: 10.7270/Q28G8MT5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































