 Found 150 hits with Last Name = 'earley' and Initial = 'wg'
Found 150 hits with Last Name = 'earley' and Initial = 'wg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334454

(CHEMBL1643895 | Ramosetron | US9045501, Ramosetron)Show SMILES Cn1cc(C(=O)[C@@H]2CCc3nc[nH]c3C2)c2ccccc12 |r| Show InChI InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014549
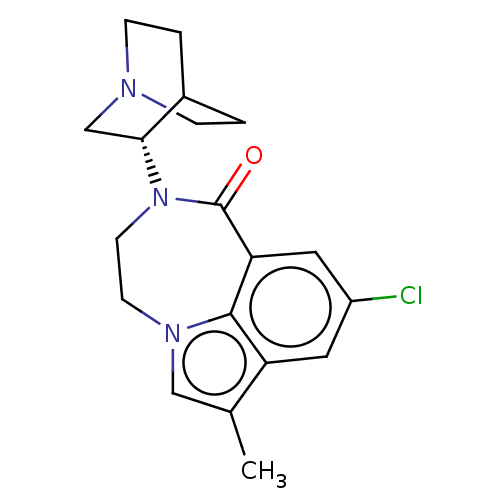
(CHEMBL3261480 | US9045501, 4)Show SMILES Cc1cn2CCN([C@@H]3CN4CCC3CC4)C(=O)c3cc(Cl)cc1c23 |r,wD:7.6,(8.92,-16.19,;8.45,-14.73,;9.36,-13.48,;8.42,-12.13,;8.87,-10.66,;7.99,-9.38,;6.46,-9.26,;5.89,-7.83,;4.36,-7.61,;3.8,-6.17,;4.77,-4.97,;6.29,-5.2,;6.84,-6.63,;5.89,-5.6,;5.13,-6.93,;5.4,-10.41,;3.93,-9.96,;5.64,-11.94,;4.31,-12.71,;4.31,-14.25,;2.98,-15.02,;5.64,-15.02,;6.97,-14.25,;6.98,-12.7,)| Show InChI InChI=1S/C19H22ClN3O/c1-12-10-22-6-7-23(17-11-21-4-2-13(17)3-5-21)19(24)16-9-14(20)8-15(12)18(16)22/h8-10,13,17H,2-7,11H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM93624
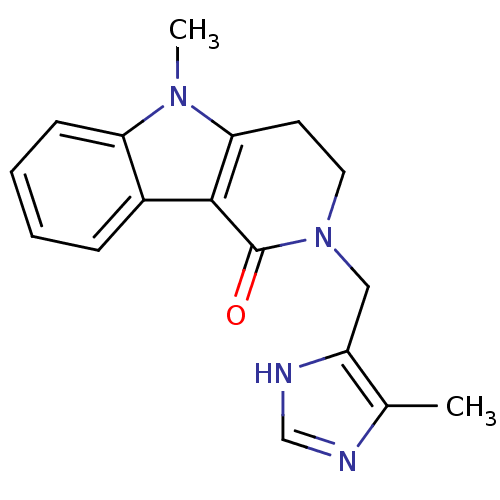
(5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014558
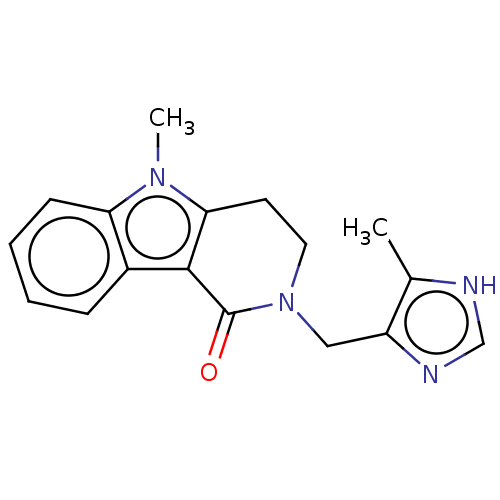
(ALOSETRON | CHEBI:253342 | Lotronex | US9045501, A...)Show InChI InChI=1S/C17H18N4O/c1-11-13(19-10-18-11)9-21-8-7-15-16(17(21)22)12-5-3-4-6-14(12)20(15)2/h3-6,10H,7-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014552
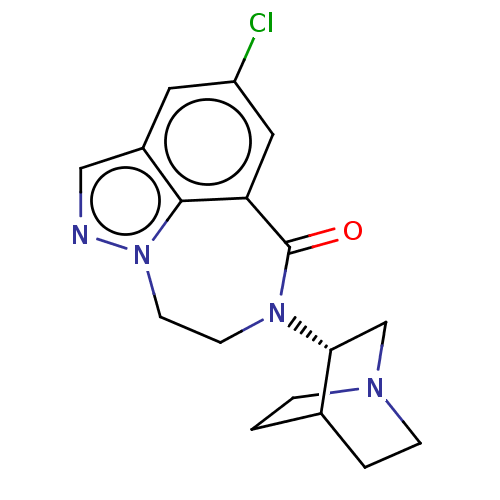
(CHEMBL3261483 | US9045501, 8)Show SMILES Clc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(22.97,-13.82,;24.31,-13.05,;24.31,-11.51,;25.64,-10.74,;25.4,-9.21,;23.93,-8.77,;26.45,-8.07,;27.99,-8.19,;28.86,-9.46,;28.42,-10.93,;29.36,-12.28,;28.45,-13.53,;26.97,-13.05,;25.64,-13.82,;26.98,-11.51,;25.89,-6.64,;24.36,-6.41,;23.8,-4.97,;24.76,-3.77,;26.29,-4,;26.84,-5.43,;25.89,-4.4,;25.13,-5.74,)| Show InChI InChI=1S/C17H19ClN4O/c18-13-7-12-9-19-22-6-5-21(17(23)14(8-13)16(12)22)15-10-20-3-1-11(15)2-4-20/h7-9,11,15H,1-6,10H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334442
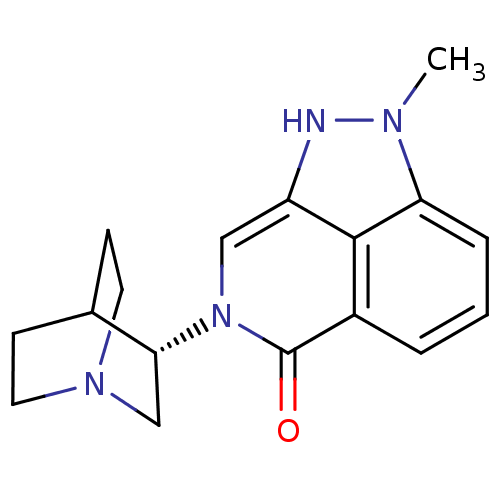
((S)-2-methyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES Cn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:6.5,(21.53,-4.9,;20.98,-3.46,;22.01,-1.72,;20.85,-.68,;20.86,.86,;19.52,1.63,;19.51,3.17,;18.18,3.93,;18.18,5.47,;19.52,6.24,;20.85,5.47,;20.85,3.94,;19.34,4.44,;19.66,5.08,;18.19,.86,;16.85,1.65,;18.19,-.68,;16.86,-1.45,;16.85,-2.99,;18.19,-3.76,;19.52,-2.99,;19.52,-1.45,)| Show InChI InChI=1S/C17H20N4O/c1-19-14-4-2-3-12-16(14)13(18-19)9-21(17(12)22)15-10-20-7-5-11(15)6-8-20/h2-4,9,11,15,18H,5-8,10H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334453

((S)-5-methyl-2-(quinuclidin-3-yl)-2,3-dihydropyrro...)Show SMILES Cn1cc2CN([C@@H]3CN4CCC3CC4)C(=O)c3cccc1c23 |r,wD:6.5,(4.39,-34,;3.85,-32.56,;4.88,-30.82,;3.72,-29.78,;3.73,-28.24,;2.39,-27.47,;2.38,-25.93,;1.05,-25.17,;1.05,-23.63,;2.39,-22.86,;3.72,-23.63,;3.72,-25.16,;2.21,-24.66,;2.53,-24.02,;1.06,-28.24,;-.28,-27.45,;1.06,-29.78,;-.27,-30.55,;-.28,-32.09,;1.06,-32.86,;2.39,-32.09,;2.39,-30.55,)| Show InChI InChI=1S/C18H21N3O/c1-19-9-13-10-21(16-11-20-7-5-12(16)6-8-20)18(22)14-3-2-4-15(19)17(13)14/h2-4,9,12,16H,5-8,10-11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014553

(CHEMBL3261484)Show SMILES Cc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(32.02,-14.44,;33.35,-13.67,;33.36,-12.13,;34.68,-11.36,;34.44,-9.83,;32.97,-9.38,;35.5,-8.68,;37.04,-8.8,;37.91,-10.07,;37.47,-11.55,;38.4,-12.89,;37.49,-14.15,;36.02,-13.67,;34.69,-14.44,;36.02,-12.12,;34.94,-7.25,;33.41,-7.03,;32.85,-5.59,;33.81,-4.38,;35.33,-4.62,;35.89,-6.05,;34.94,-5.01,;34.17,-6.35,)| Show InChI InChI=1S/C18H22N4O/c1-12-8-14-10-19-22-7-6-21(18(23)15(9-12)17(14)22)16-11-20-4-2-13(16)3-5-20/h8-10,13,16H,2-7,11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334452
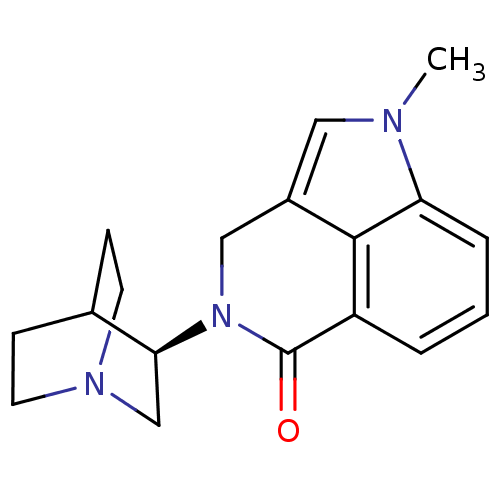
((R)-5-methyl-2-(quinuclidin-3-yl)-2,3-dihydropyrro...)Show SMILES Cn1cc2CN([C@H]3CN4CCC3CC4)C(=O)c3cccc1c23 |r,wU:6.5,(-3.65,-33.57,;-4.2,-32.13,;-3.17,-30.38,;-4.32,-29.35,;-4.32,-27.8,;-5.66,-27.04,;-5.66,-25.5,;-7,-24.74,;-6.99,-23.19,;-5.66,-22.42,;-4.33,-23.2,;-4.33,-24.73,;-5.83,-24.22,;-5.51,-23.59,;-6.98,-27.8,;-8.33,-27.02,;-6.99,-29.35,;-8.32,-30.12,;-8.32,-31.66,;-6.98,-32.43,;-5.66,-31.66,;-5.65,-30.12,)| Show InChI InChI=1S/C18H21N3O/c1-19-9-13-10-21(16-11-20-7-5-12(16)6-8-20)18(22)14-3-2-4-15(19)17(13)14/h2-4,9,12,16H,5-8,10-11H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334445
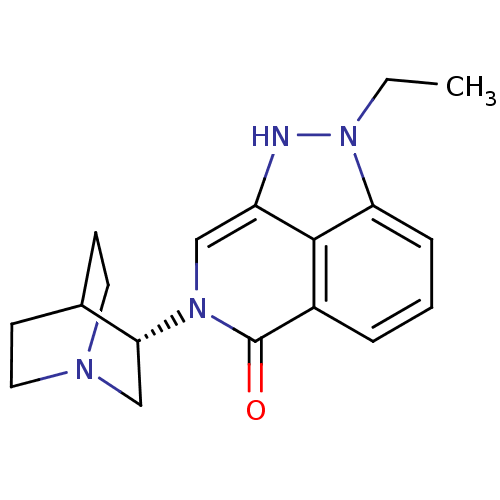
((S)-2-ethyl-7-(quinuclidin-3-yl)-7,8-dihydropyrazo...)Show SMILES CCn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:7.6,(47.4,-4.88,;48.37,-3.68,;47.82,-2.25,;48.85,-.5,;47.7,.54,;47.7,2.08,;46.36,2.85,;46.36,4.39,;45.02,5.15,;45.03,6.69,;46.36,7.46,;47.69,6.69,;47.69,5.16,;46.19,5.66,;46.5,6.3,;45.04,2.08,;43.69,2.87,;45.03,.54,;43.7,-.23,;43.7,-1.78,;45.04,-2.55,;46.36,-1.78,;46.36,-.23,)| Show InChI InChI=1S/C18H22N4O/c1-2-22-15-5-3-4-13-17(15)14(19-22)10-21(18(13)23)16-11-20-8-6-12(16)7-9-20/h3-5,10,12,16,19H,2,6-9,11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334439
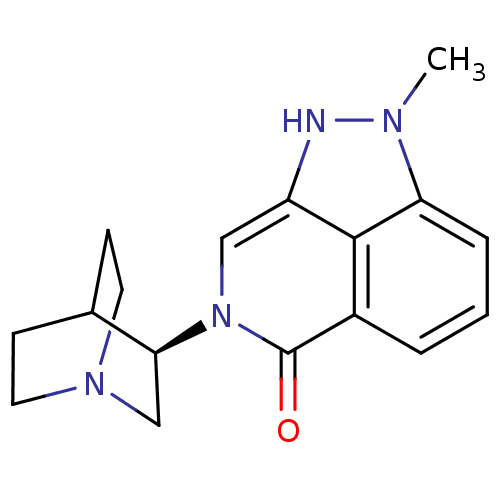
((R)-2-methyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES Cn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:6.5,(12.29,-5.2,;11.75,-3.77,;12.77,-2.02,;11.62,-.98,;11.62,.56,;10.28,1.33,;10.28,2.87,;8.95,3.63,;8.95,5.17,;10.28,5.94,;11.61,5.17,;11.61,3.64,;10.11,4.14,;10.43,4.78,;8.96,.56,;7.61,1.35,;8.96,-.99,;7.62,-1.76,;7.62,-3.3,;8.96,-4.07,;10.29,-3.3,;10.29,-1.75,)| Show InChI InChI=1S/C17H20N4O/c1-19-14-4-2-3-12-16(14)13(18-19)9-21(17(12)22)15-10-20-7-5-11(15)6-8-20/h2-4,9,11,15,18H,5-8,10H2,1H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334443

((R)-4-fluoro-2-methyl-7-(quinuclidin-3-yl)-7,8-dih...)Show SMILES Cn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cc(F)cc1c23 |r,wU:6.5,(31.37,-5.57,;30.82,-4.13,;31.85,-2.38,;30.7,-1.35,;30.7,.2,;29.36,.96,;29.36,2.5,;28.02,3.26,;28.03,4.81,;29.36,5.58,;30.69,4.8,;30.69,3.27,;29.19,3.77,;29.51,4.41,;28.04,.2,;26.69,.98,;28.03,-1.35,;26.7,-2.12,;26.7,-3.66,;25.37,-4.43,;28.04,-4.43,;29.36,-3.66,;29.37,-2.12,)| Show InChI InChI=1S/C17H19FN4O/c1-20-14-7-11(18)6-12-16(14)13(19-20)8-22(17(12)23)15-9-21-4-2-10(15)3-5-21/h6-8,10,15,19H,2-5,9H2,1H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334444
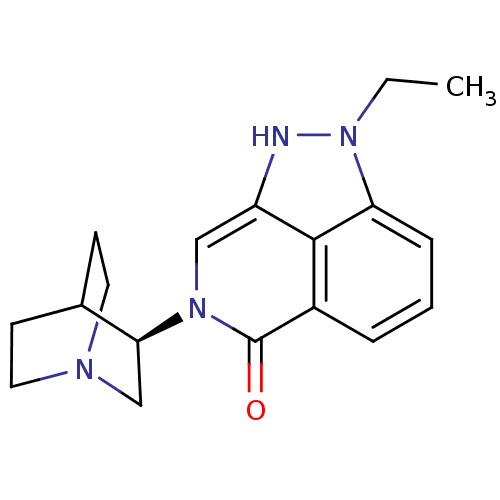
((R)-2-ethyl-7-(quinuclidin-3-yl)-7,8-dihydropyrazo...)Show SMILES CCn1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:7.6,(39.2,-5.65,;40.17,-4.46,;39.63,-3.02,;40.65,-1.27,;39.5,-.24,;39.5,1.31,;38.16,2.07,;38.16,3.61,;36.83,4.37,;36.83,5.92,;38.16,6.69,;39.49,5.91,;39.49,4.38,;37.99,4.89,;38.31,5.52,;36.84,1.31,;35.49,2.09,;36.84,-.24,;35.5,-1.01,;35.5,-2.55,;36.84,-3.32,;38.16,-2.55,;38.17,-1.01,)| Show InChI InChI=1S/C18H22N4O/c1-2-22-15-5-3-4-13-17(15)14(19-22)10-21(18(13)23)16-11-20-8-6-12(16)7-9-20/h3-5,10,12,16,19H,2,6-9,11H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334447
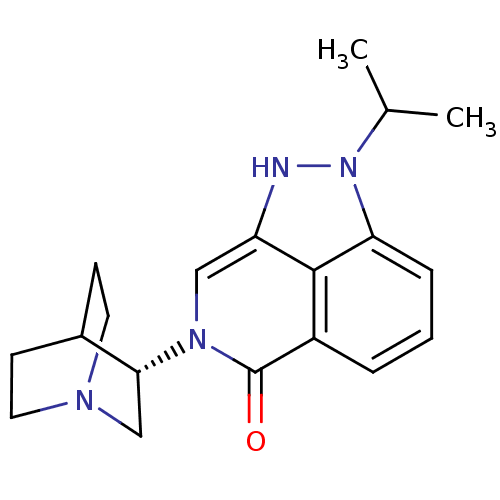
((S)-2-isopropyl-7-(quinuclidin-3-yl)-7,8-dihydropy...)Show SMILES CC(C)n1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:8.7,(5.55,-19.44,;6.52,-18.25,;8.03,-18.49,;5.97,-16.81,;7,-15.06,;5.85,-14.02,;5.85,-12.48,;4.51,-11.71,;4.5,-10.18,;3.17,-9.41,;3.17,-7.87,;4.51,-7.1,;5.84,-7.87,;5.84,-9.4,;4.33,-8.9,;4.65,-8.27,;3.18,-12.48,;1.84,-11.7,;3.18,-14.03,;1.85,-14.8,;1.84,-16.34,;3.18,-17.11,;4.51,-16.34,;4.51,-14.8,)| Show InChI InChI=1S/C19H24N4O/c1-12(2)23-16-5-3-4-14-18(16)15(20-23)10-22(19(14)24)17-11-21-8-6-13(17)7-9-21/h3-5,10,12-13,17,20H,6-9,11H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334448

((S)-2-isobutyl-7-(quinuclidin-3-yl)-7,8-dihydropyr...)Show SMILES CC(C)Cn1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:9.8,(15.89,-21.07,;15.34,-19.64,;13.82,-19.39,;16.31,-18.44,;15.77,-17.01,;16.79,-15.26,;15.64,-14.22,;15.64,-12.68,;14.3,-11.91,;14.3,-10.37,;12.97,-9.61,;12.97,-8.07,;14.3,-7.3,;15.63,-8.07,;15.63,-9.6,;14.13,-9.1,;14.45,-8.46,;12.98,-12.68,;11.63,-11.89,;12.98,-14.22,;11.64,-14.99,;11.64,-16.54,;12.98,-17.31,;14.31,-16.54,;14.31,-14.99,)| Show InChI InChI=1S/C20H26N4O/c1-13(2)10-24-17-5-3-4-15-19(17)16(21-24)11-23(20(15)25)18-12-22-8-6-14(18)7-9-22/h3-5,11,13-14,18,21H,6-10,12H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014548

(CHEMBL3261479 | US9045501, 2)Show SMILES O=C1N(CCn2ccc3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wD:14.16,(3.92,-9.95,;5.4,-10.4,;6.45,-9.26,;7.99,-9.38,;8.86,-10.65,;8.41,-12.12,;9.35,-13.46,;8.44,-14.72,;6.97,-14.24,;5.64,-15.01,;4.3,-14.24,;4.31,-12.7,;5.63,-11.93,;6.97,-12.69,;5.89,-7.83,;4.36,-7.6,;3.8,-6.16,;4.76,-4.96,;6.28,-5.2,;6.84,-6.62,;5.89,-5.59,;5.13,-6.93,)| Show InChI InChI=1S/C18H21N3O/c22-18-15-3-1-2-14-6-9-20(17(14)15)10-11-21(18)16-12-19-7-4-13(16)5-8-19/h1-3,6,9,13,16H,4-5,7-8,10-12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334446

((R)-2-isopropyl-7-(quinuclidin-3-yl)-7,8-dihydropy...)Show SMILES CC(C)n1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:8.7,(-3.99,-20.22,;-3.02,-19.03,;-1.5,-19.27,;-3.56,-17.59,;-2.53,-15.84,;-3.69,-14.8,;-3.68,-13.26,;-5.02,-12.49,;-5.03,-10.95,;-6.36,-10.19,;-6.36,-8.65,;-5.02,-7.88,;-3.69,-8.65,;-3.69,-10.18,;-5.2,-9.68,;-4.88,-9.04,;-6.35,-13.26,;-7.7,-12.48,;-6.35,-14.81,;-7.69,-15.58,;-7.69,-17.12,;-6.35,-17.89,;-5.02,-17.12,;-5.02,-15.58,)| Show InChI InChI=1S/C19H24N4O/c1-12(2)23-16-5-3-4-14-18(16)15(20-23)10-22(19(14)24)17-11-21-8-6-13(17)7-9-21/h3-5,10,12-13,17,20H,6-9,11H2,1-2H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334440

((R)-7-(quinuclidin-3-yl)-7,8-dihydropyrazolo[3,4,5...)Show SMILES O=c1n(cc2[nH][nH]c3cccc1c23)[C@H]1CN2CCC1CC2 |r,wU:13.15,(-7.86,1.16,;-6.52,.38,;-5.19,1.14,;-3.85,.37,;-3.86,-1.17,;-2.7,-2.21,;-3.73,-3.95,;-5.19,-3.48,;-6.52,-4.25,;-7.86,-3.48,;-7.85,-1.94,;-6.52,-1.17,;-5.19,-1.94,;-5.2,2.68,;-6.53,3.44,;-6.53,4.98,;-5.19,5.75,;-3.86,4.98,;-3.86,3.45,;-5.37,3.95,;-5.05,4.59,)| Show InChI InChI=1S/C16H18N4O/c21-16-11-2-1-3-12-15(11)13(18-17-12)8-20(16)14-9-19-6-4-10(14)5-7-19/h1-3,8,10,14,17-18H,4-7,9H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334441

((S)-7-(quinuclidin-3-yl)-7,8-dihydropyrazolo[3,4,5...)Show SMILES O=c1n(cc2[nH][nH]c3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wD:13.15,(-.61,1.94,;.74,1.15,;2.06,1.92,;3.4,1.15,;3.4,-.39,;4.55,-1.43,;3.52,-3.18,;2.06,-2.71,;.74,-3.48,;-.6,-2.71,;-.6,-1.16,;.73,-.4,;2.07,-1.16,;2.06,3.46,;.72,4.22,;.73,5.76,;2.06,6.53,;3.39,5.76,;3.39,4.23,;1.89,4.73,;2.21,5.37,)| Show InChI InChI=1S/C16H18N4O/c21-16-11-2-1-3-12-15(11)13(18-17-12)8-20(16)14-9-19-6-4-10(14)5-7-19/h1-3,8,10,14,17-18H,4-7,9H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014556

(CHEMBL3261486 | US9045501, 25)Show SMILES O=C1N(CCn2c3c1cccc3[nH]c2=O)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(7.11,-8.33,;8.59,-8.78,;9.64,-7.63,;11.18,-7.75,;12.05,-9.02,;11.61,-10.5,;10.17,-11.07,;8.83,-10.31,;7.5,-11.08,;7.5,-12.62,;8.83,-13.39,;10.16,-12.62,;11.64,-13.1,;12.55,-11.84,;14.09,-11.86,;9.08,-6.2,;7.55,-5.98,;6.99,-4.54,;7.95,-3.33,;9.47,-3.57,;10.03,-5,;9.08,-3.96,;8.32,-5.3,)| Show InChI InChI=1S/C17H20N4O2/c22-16-12-2-1-3-13-15(12)21(17(23)18-13)9-8-20(16)14-10-19-6-4-11(14)5-7-19/h1-3,11,14H,4-10H2,(H,18,23)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014551
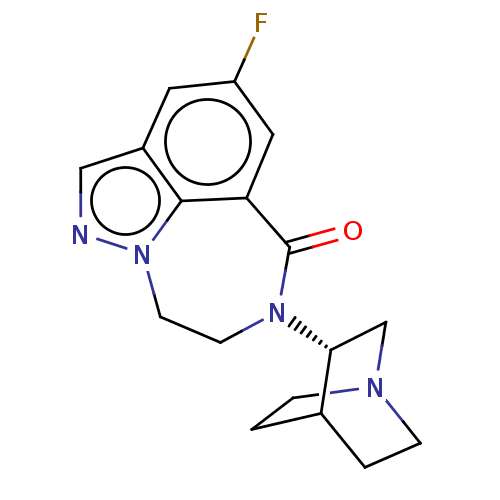
(CHEMBL3261482)Show SMILES Fc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:15.17,(13.49,-14.18,;14.83,-13.41,;14.83,-11.87,;16.16,-11.1,;15.92,-9.57,;14.45,-9.12,;16.97,-8.43,;18.51,-8.55,;19.38,-9.82,;18.94,-11.29,;19.88,-12.64,;18.97,-13.89,;17.49,-13.41,;16.16,-14.18,;17.5,-11.87,;16.41,-6.99,;14.88,-6.77,;14.32,-5.33,;15.28,-4.13,;16.81,-4.36,;17.36,-5.79,;16.41,-4.76,;15.65,-6.09,)| Show InChI InChI=1S/C17H19FN4O/c18-13-7-12-9-19-22-6-5-21(17(23)14(8-13)16(12)22)15-10-20-3-1-11(15)2-4-20/h7-9,11,15H,1-6,10H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334449
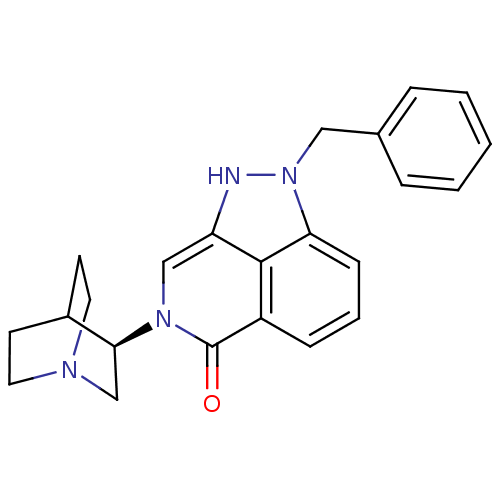
((R)-2-benzyl-7-(quinuclidin-3-yl)-7,8-dihydropyraz...)Show SMILES O=c1n(cc2[nH]n(Cc3ccccc3)c3cccc1c23)[C@H]1CN2CCC1CC2 |r,wU:20.23,(21.21,-11.04,;22.56,-11.82,;23.88,-11.05,;25.22,-11.82,;25.22,-13.37,;26.37,-14.4,;25.34,-16.15,;26.1,-17.48,;25.33,-18.81,;23.79,-18.79,;23.01,-20.12,;23.77,-21.46,;25.32,-21.46,;26.09,-20.13,;23.88,-15.68,;22.56,-16.45,;21.22,-15.68,;21.22,-14.14,;22.56,-13.37,;23.89,-14.14,;23.88,-9.52,;22.55,-8.75,;22.55,-7.21,;23.88,-6.44,;25.21,-7.21,;25.21,-8.75,;23.71,-8.24,;24.03,-7.61,)| Show InChI InChI=1S/C23H24N4O/c28-23-18-7-4-8-20-22(18)19(24-27(20)13-16-5-2-1-3-6-16)14-26(23)21-15-25-11-9-17(21)10-12-25/h1-8,14,17,21,24H,9-13,15H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334451
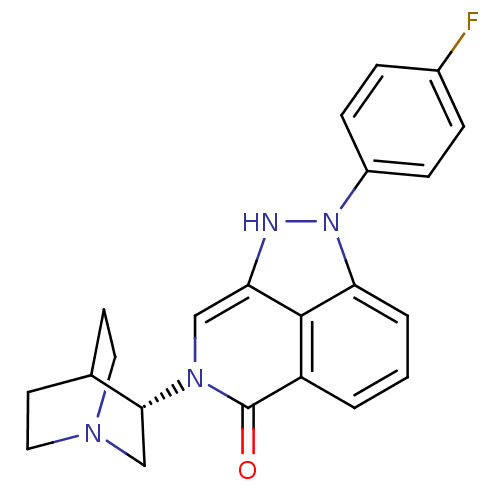
((S)-2-(4-fluorophenyl)-7-(quinuclidin-3-yl)-7,8-di...)Show SMILES Fc1ccc(cc1)-n1[nH]c2cn([C@@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wD:12.12,(46.59,-23.52,;46.04,-22.08,;44.52,-21.83,;43.97,-20.4,;44.95,-19.21,;46.46,-19.44,;47.01,-20.88,;44.4,-17.77,;45.43,-16.02,;44.28,-14.98,;44.28,-13.44,;42.94,-12.67,;42.94,-11.13,;41.6,-10.37,;41.61,-8.83,;42.94,-8.06,;44.27,-8.83,;44.27,-10.36,;42.77,-9.86,;43.09,-9.22,;41.62,-13.44,;40.27,-12.65,;41.61,-14.99,;40.28,-15.76,;40.28,-17.3,;41.62,-18.07,;42.94,-17.3,;42.95,-15.75,)| Show InChI InChI=1S/C22H21FN4O/c23-15-4-6-16(7-5-15)27-19-3-1-2-17-21(19)18(24-27)12-26(22(17)28)20-13-25-10-8-14(20)9-11-25/h1-7,12,14,20,24H,8-11,13H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014550
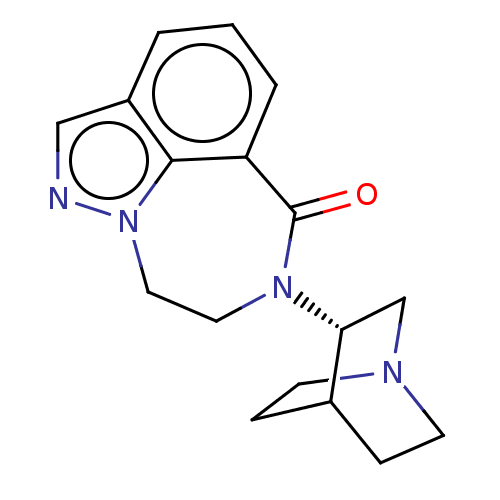
(CHEMBL3261481 | US9045501, 7)Show SMILES O=C1N(CCn2ncc3cccc1c23)[C@@H]1CN2CCC1CC2 |r,wD:14.16,(5.44,-9.73,;6.92,-10.18,;7.97,-9.03,;9.51,-9.15,;10.38,-10.42,;9.94,-11.9,;10.88,-13.24,;9.97,-14.5,;8.49,-14.02,;7.16,-14.79,;5.83,-14.02,;5.83,-12.48,;7.16,-11.71,;8.5,-12.47,;7.41,-7.6,;5.88,-7.38,;5.32,-5.94,;6.28,-4.73,;7.8,-4.97,;8.36,-6.4,;7.41,-5.36,;6.65,-6.7,)| Show InChI InChI=1S/C17H20N4O/c22-17-14-3-1-2-13-10-18-21(16(13)14)9-8-20(17)15-11-19-6-4-12(15)5-7-19/h1-3,10,12,15H,4-9,11H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014554
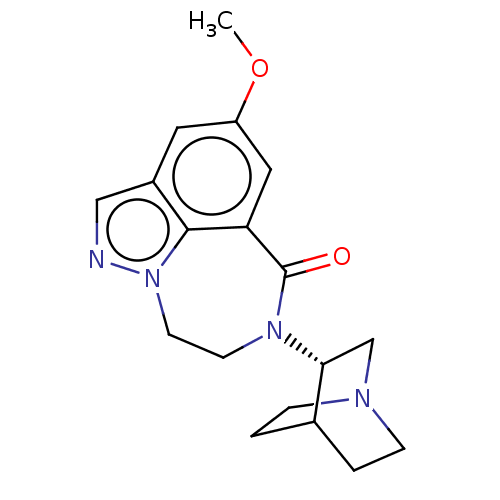
(CHEMBL3261485)Show SMILES COc1cc2C(=O)N(CCn3ncc(c1)c23)[C@@H]1CN2CCC1CC2 |r,wD:16.18,(1.85,-13.55,;3.19,-14.32,;4.52,-13.55,;4.52,-12.01,;5.85,-11.24,;5.61,-9.71,;4.14,-9.26,;6.67,-8.57,;8.2,-8.69,;9.08,-9.96,;8.63,-11.43,;9.57,-12.78,;8.66,-14.03,;7.19,-13.55,;5.85,-14.32,;7.19,-12.01,;6.1,-7.13,;4.57,-6.91,;4.01,-5.47,;4.98,-4.27,;6.5,-4.5,;7.06,-5.93,;6.1,-4.9,;5.34,-6.23,)| Show InChI InChI=1S/C18H22N4O2/c1-24-14-8-13-10-19-22-7-6-21(18(23)15(9-14)17(13)22)16-11-20-4-2-12(16)3-5-20/h8-10,12,16H,2-7,11H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334450
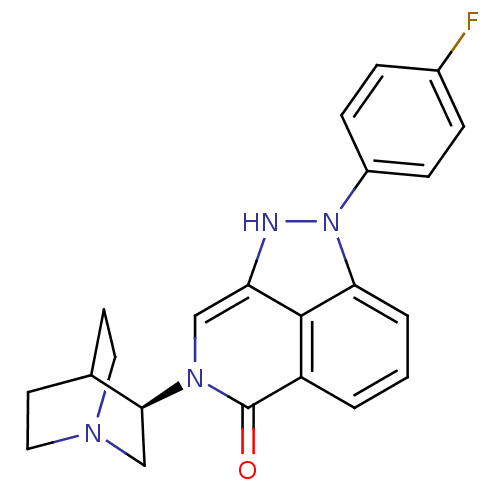
((R)-2-(4-fluorophenyl)-7-(quinuclidin-3-yl)-7,8-di...)Show SMILES Fc1ccc(cc1)-n1[nH]c2cn([C@H]3CN4CCC3CC4)c(=O)c3cccc1c23 |r,wU:12.12,(36.32,-23.94,;35.77,-22.51,;34.25,-22.26,;33.7,-20.83,;34.67,-19.63,;36.19,-19.87,;36.74,-21.31,;34.13,-18.2,;35.16,-16.45,;34,-15.41,;34.01,-13.87,;32.67,-13.1,;32.66,-11.56,;31.33,-10.8,;31.33,-9.25,;32.67,-8.48,;34,-9.26,;34,-10.79,;32.49,-10.29,;32.81,-9.65,;31.34,-13.87,;29.99,-13.08,;31.34,-15.41,;30,-16.18,;30,-17.73,;31.34,-18.5,;32.67,-17.73,;32.67,-16.18,)| Show InChI InChI=1S/C22H21FN4O/c23-15-4-6-16(7-5-15)27-19-3-1-2-17-21(19)18(24-27)12-26(22(17)28)20-13-25-10-8-14(20)9-11-25/h1-7,12,14,20,24H,8-11,13H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014547
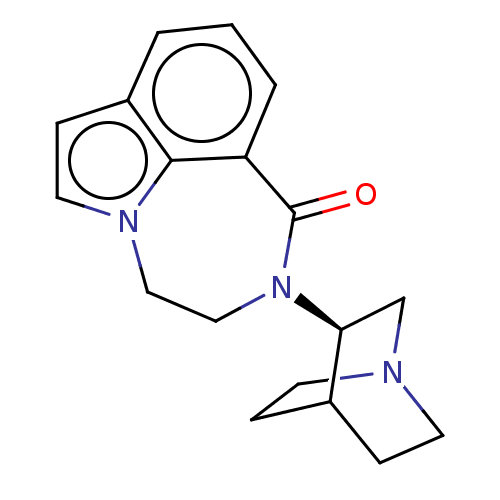
(CHEMBL3261478 | US9045501, 1)Show SMILES O=C1N(CCn2ccc3cccc1c23)[C@H]1CN2CCC1CC2 |r,wU:14.16,(3.92,-9.95,;5.4,-10.4,;6.45,-9.26,;7.99,-9.38,;8.86,-10.65,;8.41,-12.12,;9.35,-13.46,;8.44,-14.72,;6.97,-14.24,;5.64,-15.01,;4.3,-14.24,;4.31,-12.7,;5.63,-11.93,;6.97,-12.69,;5.89,-7.83,;4.36,-7.6,;3.8,-6.16,;4.76,-4.96,;6.28,-5.2,;6.84,-6.62,;5.89,-5.59,;5.13,-6.93,)| Show InChI InChI=1S/C18H21N3O/c22-18-15-3-1-2-14-6-9-20(17(14)15)10-11-21(18)16-12-19-7-4-13(16)5-8-19/h1-3,6,9,13,16H,4-5,7-8,10-12H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50014557
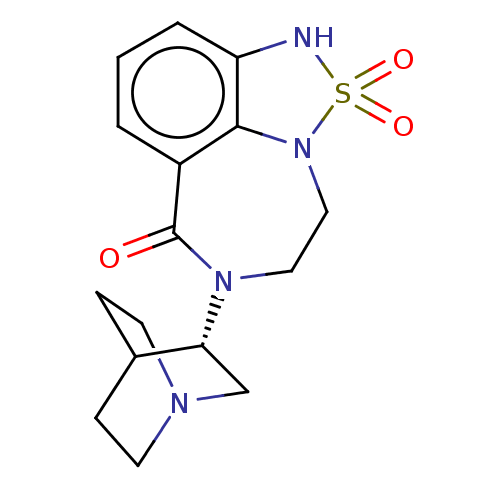
(CHEMBL3261487 | US9045501, 26)Show SMILES O=C1N(CCN2c3c(NS2(=O)=O)cccc13)[C@@H]1CN2CCC1CC2 |r,wD:16.18,(16.89,-9.61,;18.36,-10.06,;19.41,-8.91,;20.95,-9.03,;21.82,-10.31,;21.38,-11.78,;19.94,-12.35,;19.93,-13.9,;21.41,-14.38,;22.32,-13.13,;23.64,-13.91,;23.66,-12.37,;18.6,-14.67,;17.27,-13.9,;17.27,-12.36,;18.6,-11.59,;18.85,-7.48,;17.32,-7.26,;16.76,-5.82,;17.72,-4.62,;19.24,-4.85,;19.8,-6.28,;18.85,-5.25,;18.09,-6.58,)| Show InChI InChI=1S/C16H20N4O3S/c21-16-12-2-1-3-13-15(12)20(24(22,23)17-13)9-8-19(16)14-10-18-6-4-11(14)5-7-18/h1-3,11,14,17H,4-10H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 701 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assay |
Bioorg Med Chem Lett 24: 2578-81 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.074
BindingDB Entry DOI: 10.7270/Q2DZ09VX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057398

(CHEMBL3322775)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(N(C)C)c(Cl)c3)c3cccn-23)c1 Show InChI InChI=1S/C25H27ClN4O2/c1-28(2)21-8-6-17(15-19(21)26)24(31)29-13-10-25(11-14-29)23-5-4-12-30(23)22-9-7-18(32-3)16-20(22)27-25/h4-9,12,15-16,27H,10-11,13-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057402
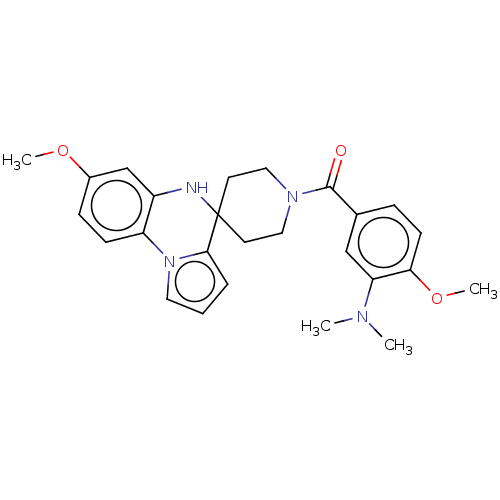
(CHEMBL3322777)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(OC)c(c3)N(C)C)c3cccn-23)c1 Show InChI InChI=1S/C26H30N4O3/c1-28(2)22-16-18(7-10-23(22)33-4)25(31)29-14-11-26(12-15-29)24-6-5-13-30(24)21-9-8-19(32-3)17-20(21)27-26/h5-10,13,16-17,27H,11-12,14-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057365

(CHEMBL3322762)Show SMILES COc1ccc-2c(NC3(CCN(Cc4ccccc4)CC3)c3cccn-23)c1 Show InChI InChI=1S/C23H25N3O/c1-27-19-9-10-21-20(16-19)24-23(22-8-5-13-26(21)22)11-14-25(15-12-23)17-18-6-3-2-4-7-18/h2-10,13,16,24H,11-12,14-15,17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057401

(CHEMBL3322776)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(OC)c(Cl)c3)c3cccn-23)c1 Show InChI InChI=1S/C24H24ClN3O3/c1-30-17-6-7-20-19(15-17)26-24(22-4-3-11-28(20)22)9-12-27(13-10-24)23(29)16-5-8-21(31-2)18(25)14-16/h3-8,11,14-15,26H,9-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057356
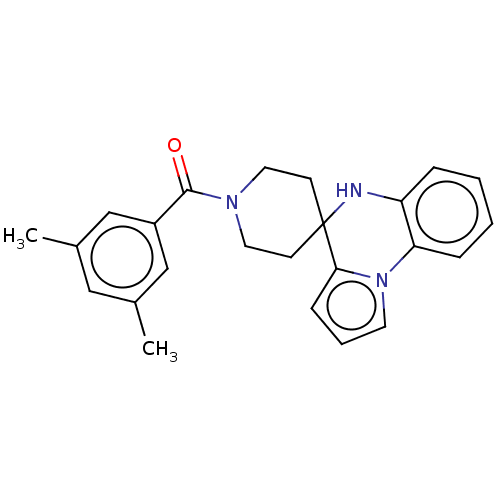
(CHEMBL3322666)Show SMILES Cc1cc(C)cc(c1)C(=O)N1CCC2(CC1)Nc1ccccc1-n1cccc21 Show InChI InChI=1S/C24H25N3O/c1-17-14-18(2)16-19(15-17)23(28)26-12-9-24(10-13-26)22-8-5-11-27(22)21-7-4-3-6-20(21)25-24/h3-8,11,14-16,25H,9-10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057374

(CHEMBL3322759)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3cccc(Cl)c3)c3cccn-23)c1 Show InChI InChI=1S/C23H22ClN3O2/c1-29-18-7-8-20-19(15-18)25-23(21-6-3-11-27(20)21)9-12-26(13-10-23)22(28)16-4-2-5-17(24)14-16/h2-8,11,14-15,25H,9-10,12-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057394
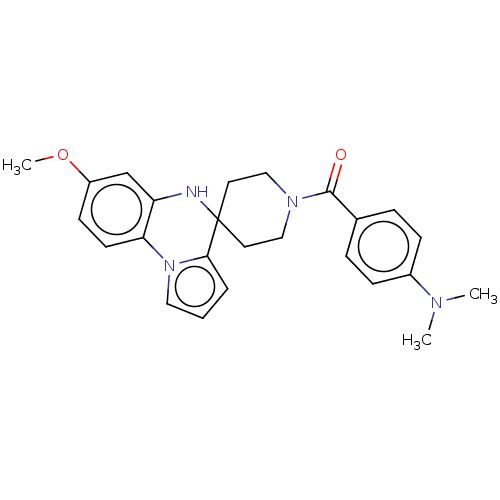
(CHEMBL3322773)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(cc3)N(C)C)c3cccn-23)c1 Show InChI InChI=1S/C25H28N4O2/c1-27(2)19-8-6-18(7-9-19)24(30)28-15-12-25(13-16-28)23-5-4-14-29(23)22-11-10-20(31-3)17-21(22)26-25/h4-11,14,17,26H,12-13,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057358
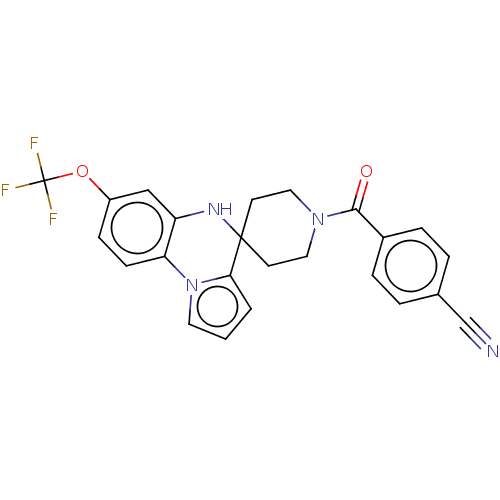
(CHEMBL3322668)Show SMILES FC(F)(F)Oc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(cc3)C#N)c3cccn-23)c1 Show InChI InChI=1S/C24H19F3N4O2/c25-24(26,27)33-18-7-8-20-19(14-18)29-23(21-2-1-11-31(20)21)9-12-30(13-10-23)22(32)17-5-3-16(15-28)4-6-17/h1-8,11,14,29H,9-10,12-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057404

(CHEMBL3322778)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(N(C)C)c(OC)c3)c3cccn-23)c1 Show InChI InChI=1S/C26H30N4O3/c1-28(2)22-9-7-18(16-23(22)33-4)25(31)29-14-11-26(12-15-29)24-6-5-13-30(24)21-10-8-19(32-3)17-20(21)27-26/h5-10,13,16-17,27H,11-12,14-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057378

(CHEMBL3322772)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(Cl)cc3)c3cccn-23)c1 Show InChI InChI=1S/C23H22ClN3O2/c1-29-18-8-9-20-19(15-18)25-23(21-3-2-12-27(20)21)10-13-26(14-11-23)22(28)16-4-6-17(24)7-5-16/h2-9,12,15,25H,10-11,13-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057405
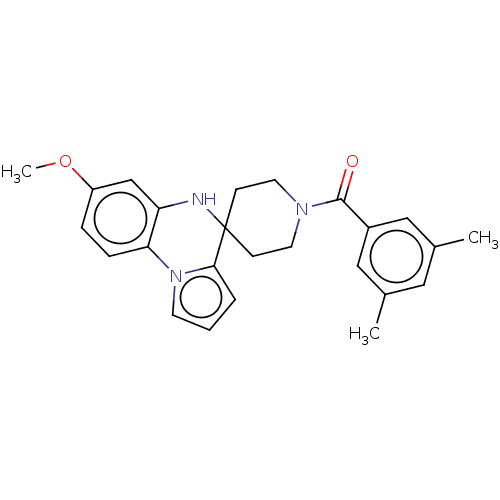
(CHEMBL3322779)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3cc(C)cc(C)c3)c3cccn-23)c1 Show InChI InChI=1S/C25H27N3O2/c1-17-13-18(2)15-19(14-17)24(29)27-11-8-25(9-12-27)23-5-4-10-28(23)22-7-6-20(30-3)16-21(22)26-25/h4-7,10,13-16,26H,8-9,11-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057375
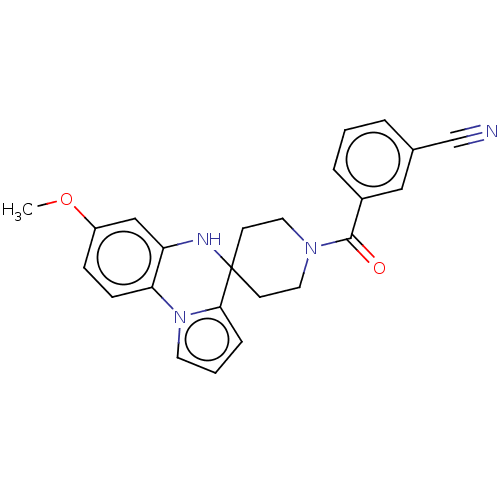
(CHEMBL3322760)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3cccc(c3)C#N)c3cccn-23)c1 Show InChI InChI=1S/C24H22N4O2/c1-30-19-7-8-21-20(15-19)26-24(22-6-3-11-28(21)22)9-12-27(13-10-24)23(29)18-5-2-4-17(14-18)16-25/h2-8,11,14-15,26H,9-10,12-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057372
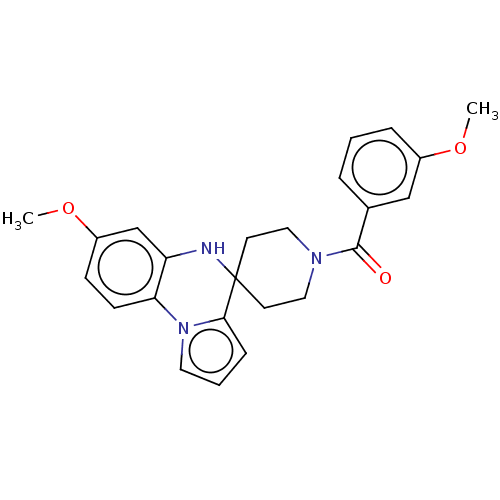
(CHEMBL3322769)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3cccc(OC)c3)c3cccn-23)c1 Show InChI InChI=1S/C24H25N3O3/c1-29-18-6-3-5-17(15-18)23(28)26-13-10-24(11-14-26)22-7-4-12-27(22)21-9-8-19(30-2)16-20(21)25-24/h3-9,12,15-16,25H,10-11,13-14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
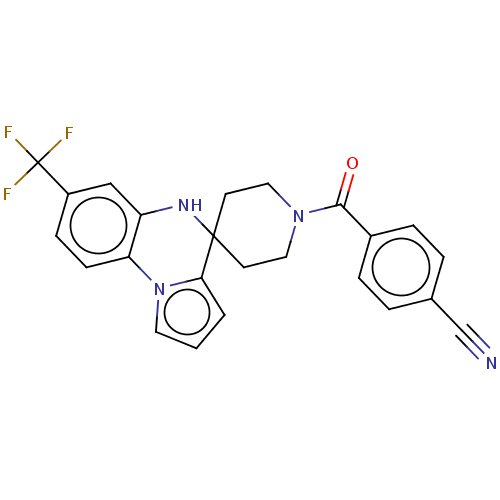
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057359
(CHEMBL3322669)Show SMILES FC(F)(F)c1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(cc3)C#N)c3cccn-23)c1 Show InChI InChI=1S/C24H19F3N4O/c25-24(26,27)18-7-8-20-19(14-18)29-23(21-2-1-11-31(20)21)9-12-30(13-10-23)22(32)17-5-3-16(15-28)4-6-17/h1-8,11,14,29H,9-10,12-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
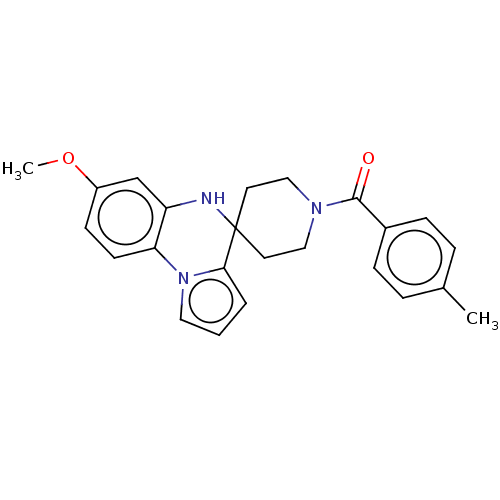
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057377
(CHEMBL3322771)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(C)cc3)c3cccn-23)c1 Show InChI InChI=1S/C24H25N3O2/c1-17-5-7-18(8-6-17)23(28)26-14-11-24(12-15-26)22-4-3-13-27(22)21-10-9-19(29-2)16-20(21)25-24/h3-10,13,16,25H,11-12,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
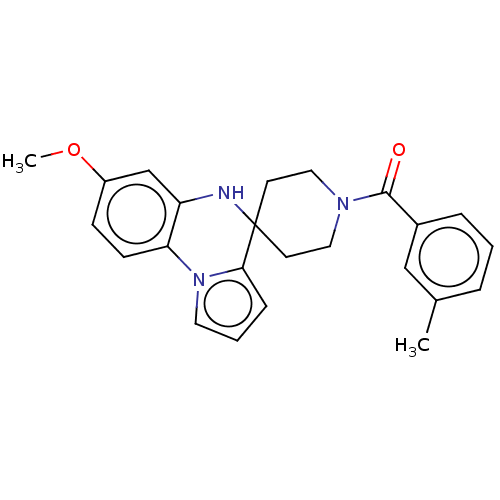
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057373
(CHEMBL3322758)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3cccc(C)c3)c3cccn-23)c1 Show InChI InChI=1S/C24H25N3O2/c1-17-5-3-6-18(15-17)23(28)26-13-10-24(11-14-26)22-7-4-12-27(22)21-9-8-19(29-2)16-20(21)25-24/h3-9,12,15-16,25H,10-11,13-14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057402

(CHEMBL3322777)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(OC)c(c3)N(C)C)c3cccn-23)c1 Show InChI InChI=1S/C26H30N4O3/c1-28(2)22-16-18(7-10-23(22)33-4)25(31)29-14-11-26(12-15-29)24-6-5-13-30(24)21-9-8-19(32-3)17-20(21)27-26/h5-10,13,16-17,27H,11-12,14-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells by whole cell voltage clamp technique |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057363
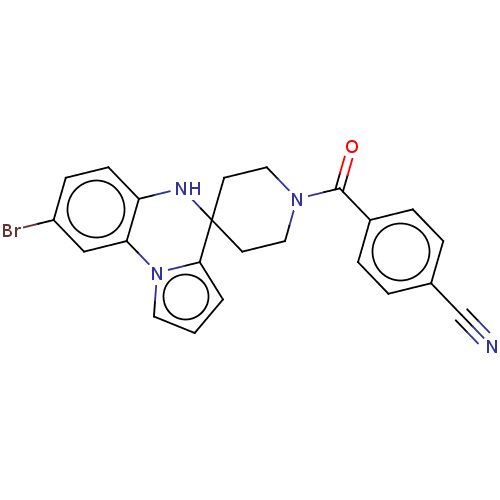
(CHEMBL3322673)Show SMILES Brc1ccc2NC3(CCN(CC3)C(=O)c3ccc(cc3)C#N)c3cccn3-c2c1 Show InChI InChI=1S/C23H19BrN4O/c24-18-7-8-19-20(14-18)28-11-1-2-21(28)23(26-19)9-12-27(13-10-23)22(29)17-5-3-16(15-25)4-6-17/h1-8,11,14,26H,9-10,12-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50057402

(CHEMBL3322777)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(OC)c(c3)N(C)C)c3cccn-23)c1 Show InChI InChI=1S/C26H30N4O3/c1-28(2)22-16-18(7-10-23(22)33-4)25(31)29-14-11-26(12-15-29)24-6-5-13-30(24)21-9-8-19(32-3)17-20(21)27-26/h5-10,13,16-17,27H,11-12,14-15H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.5 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057355
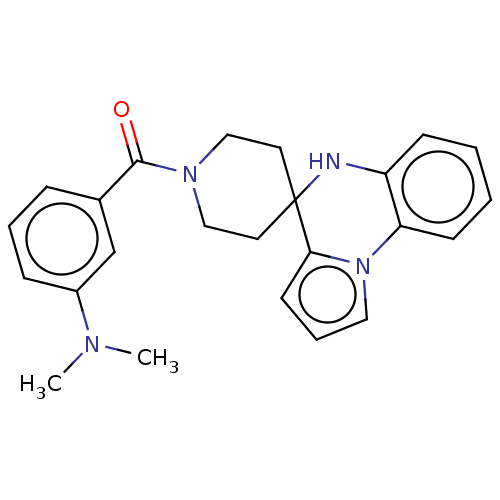
(CHEMBL3322665)Show SMILES CN(C)c1cccc(c1)C(=O)N1CCC2(CC1)Nc1ccccc1-n1cccc21 Show InChI InChI=1S/C24H26N4O/c1-26(2)19-8-5-7-18(17-19)23(29)27-15-12-24(13-16-27)22-11-6-14-28(22)21-10-4-3-9-20(21)25-24/h3-11,14,17,25H,12-13,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50057360
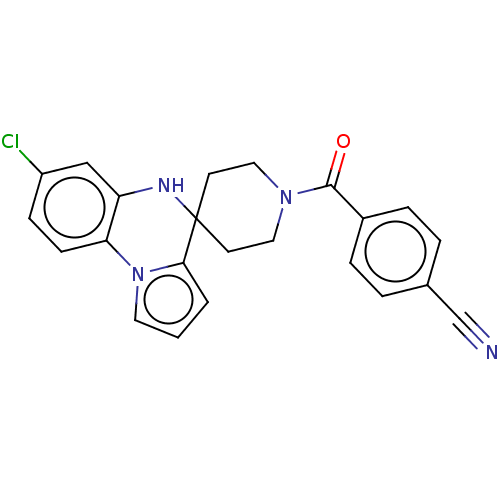
(CHEMBL3322670)Show SMILES Clc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccc(cc3)C#N)c3cccn-23)c1 Show InChI InChI=1S/C23H19ClN4O/c24-18-7-8-20-19(14-18)26-23(21-2-1-11-28(20)21)9-12-27(13-10-23)22(29)17-5-3-16(15-25)4-6-17/h1-8,11,14,26H,9-10,12-13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
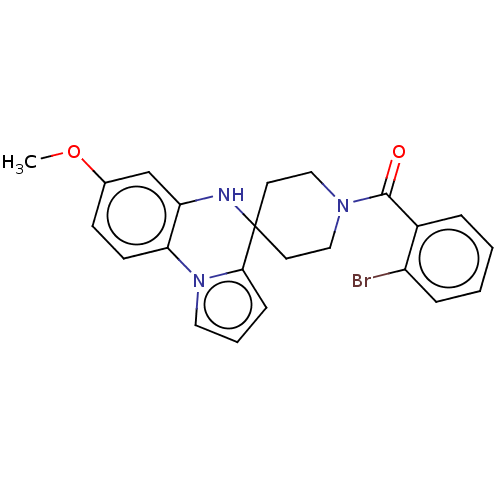
(Homo sapiens (Human)) | BDBM50057371
(CHEMBL3322768)Show SMILES COc1ccc-2c(NC3(CCN(CC3)C(=O)c3ccccc3Br)c3cccn-23)c1 Show InChI InChI=1S/C23H22BrN3O2/c1-29-16-8-9-20-19(15-16)25-23(21-7-4-12-27(20)21)10-13-26(14-11-23)22(28)17-5-2-3-6-18(17)24/h2-9,12,15,25H,10-11,13-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 414 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 channel expressed in HEK293 cells assessed as membrane potential after 60 mins by FLIPR assay |
Bioorg Med Chem Lett 24: 4110-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.060
BindingDB Entry DOI: 10.7270/Q24M9656 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data