

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50392801 (CHEMBL2151247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50370067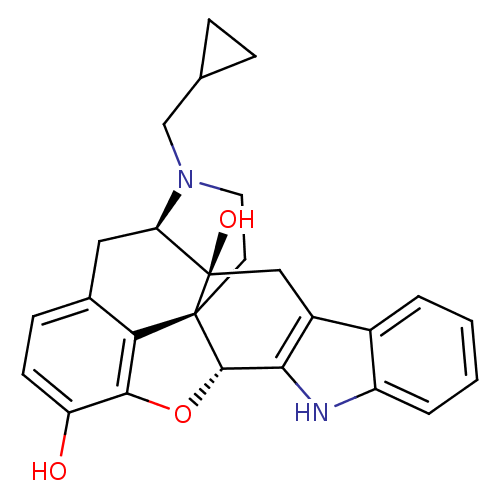 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50001707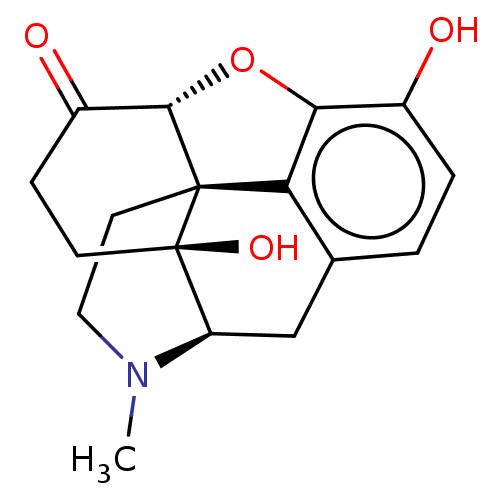 (10,17-dihydroxy-4-methyl-(13R,17S)-12-oxa-4-azapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50392797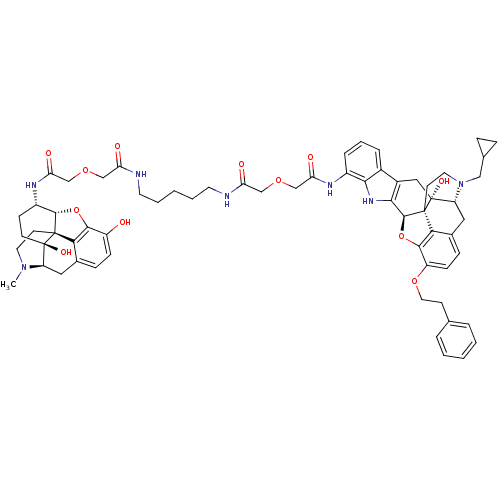 (CHEMBL2151327) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50370067 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50392799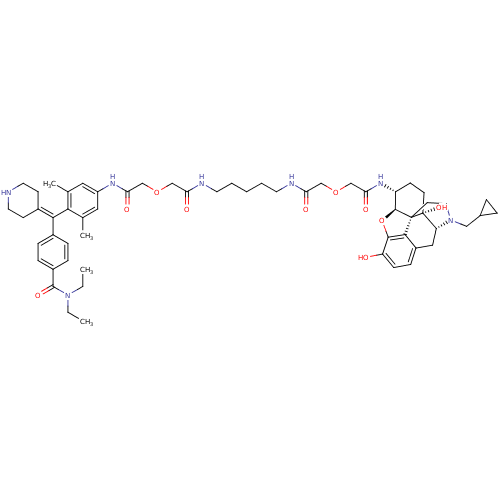 (CHEMBL2151245) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50392802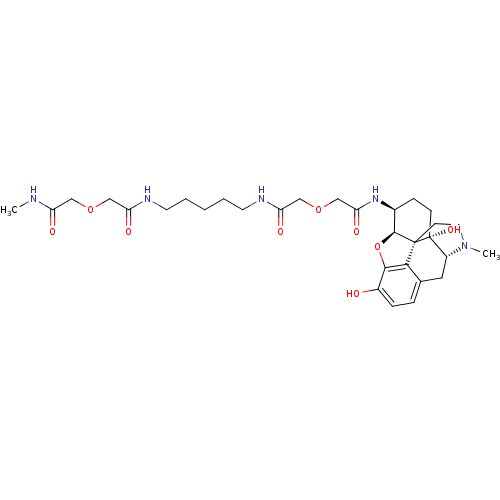 (CHEMBL2151248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50392801 (CHEMBL2151247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50392798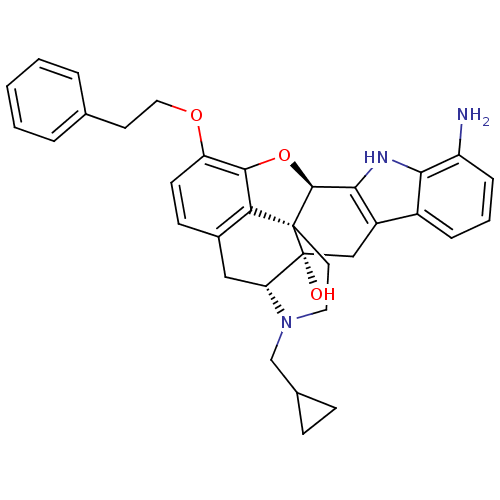 (CHEMBL2151328) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50392798 (CHEMBL2151328) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50194110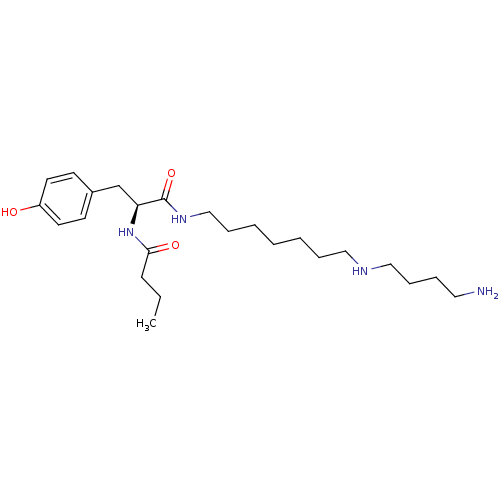 ((S)-N-(1-(7-(4-aminobutylamino)heptylamino)-3-(4-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant GluA1 receptor flip isoform expressed in Xenopus oocytes assessed as inhibition of 100 uM glutamate-induced current | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50392797 (CHEMBL2151327) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50392800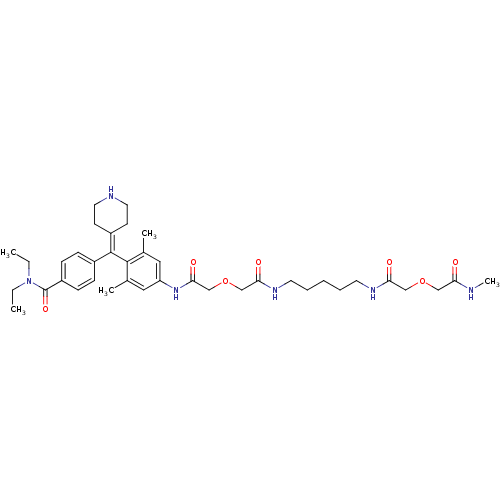 (CHEMBL2151246) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from mouse MOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50001707 (10,17-dihydroxy-4-methyl-(13R,17S)-12-oxa-4-azapen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50392800 (CHEMBL2151246) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from mouse DOR expressed in HEK293 cells | ACS Med Chem Lett 3: 640-644 (2012) Article DOI: 10.1021/ml300083p BindingDB Entry DOI: 10.7270/Q2BR8T8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895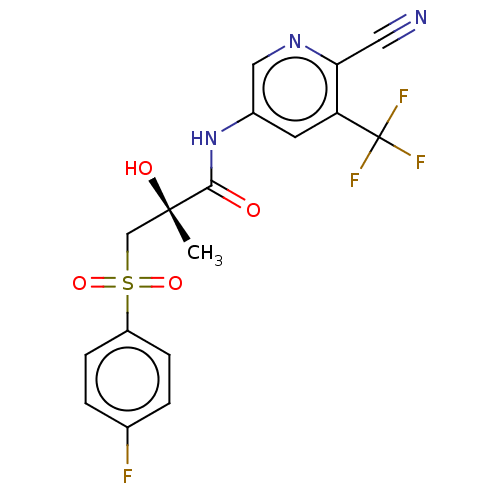 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM50105843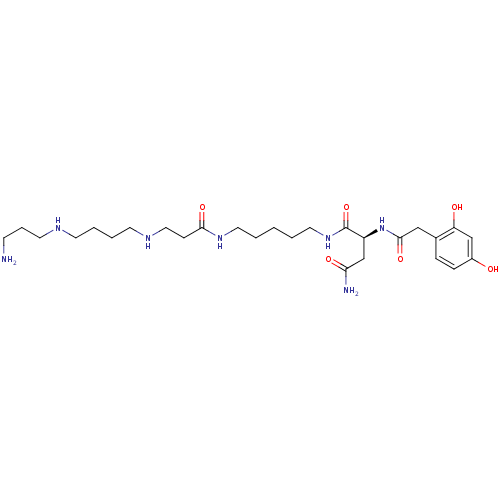 (2-Amino-N-{3-[4-(3-amino-propylamino)-butylamino]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant GluA3 receptor flop isoform expressed in Xenopus oocytes assessed as inhibition of 300 uM kainate-induced current by patch ... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50105843 (2-Amino-N-{3-[4-(3-amino-propylamino)-butylamino]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant GluA1 receptor flop isoform expressed in Xenopus oocytes assessed as inhibition of 300 uM kainate-induced current by patch ... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50316374 (CHEMBL1096289 | N-(4-hydroxyphenylpropanyl)-spermi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of rat recombinant GluA3 receptor expressed in Xenopus oocytes assessed as inhibition of 300 uM kainate-induced current | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant GluA3 receptor flip isoform expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current by patch ... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GluA3 receptor expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current by patch clamp electrophysiologica... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Rattus norvegicus) | BDBM50316374 (CHEMBL1096289 | N-(4-hydroxyphenylpropanyl)-spermi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of rat recombinant GluA4 receptor expressed in Xenopus oocytes assessed as inhibition of 300 uM kainate-induced current | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GluA1 receptor expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current by patch clamp electrophysiologica... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of recombinant GluA1 receptor flip isoform expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current by patch ... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Homo sapiens (Human)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of GluA4 receptor expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current by patch clamp electrophysiologica... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50316374 (CHEMBL1096289 | N-(4-hydroxyphenylpropanyl)-spermi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of rat recombinant GluA1 receptor expressed in Xenopus oocytes assessed as inhibition of 300 uM kainate-induced current | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239191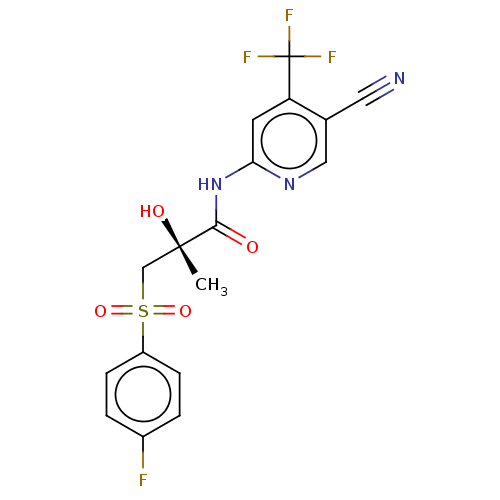 (US10053433, FC 3.077) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242618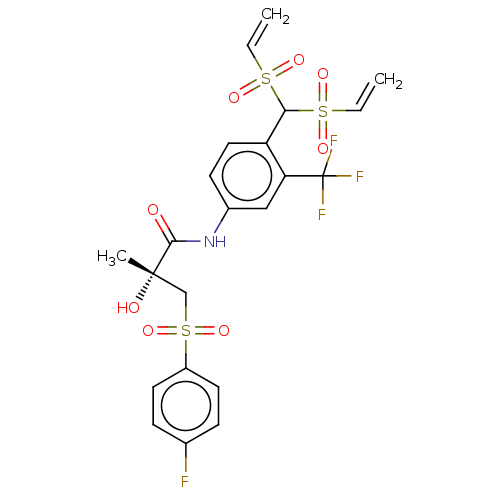 (US10053433, FC 4.025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242588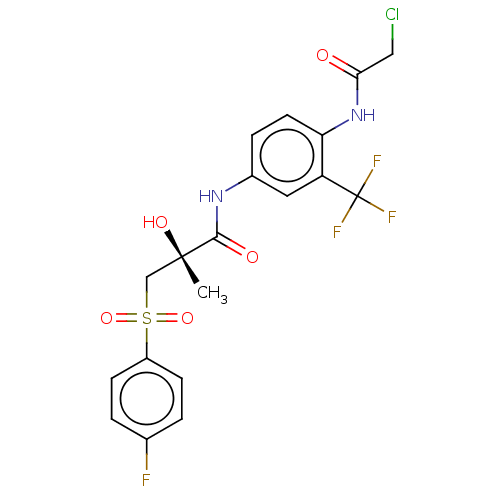 (US10053433, FC 4.039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 750 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50253632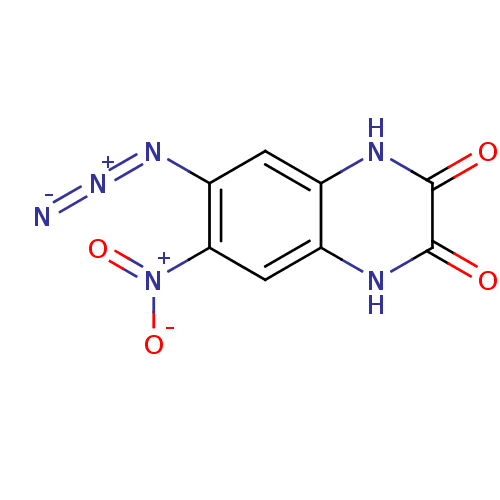 (6-Azido-7-nitro-1,4-dihydroquinoxaline-2,3-dione |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of GluR2 receptor (unknown origin) | J Med Chem 51: 5856-60 (2008) Article DOI: 10.1021/jm701517b BindingDB Entry DOI: 10.7270/Q2W095R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM238895 (US10053433, Casodex (CDX) | US10053433, FC 4.037 (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242618 (US10053433, FC 4.025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239363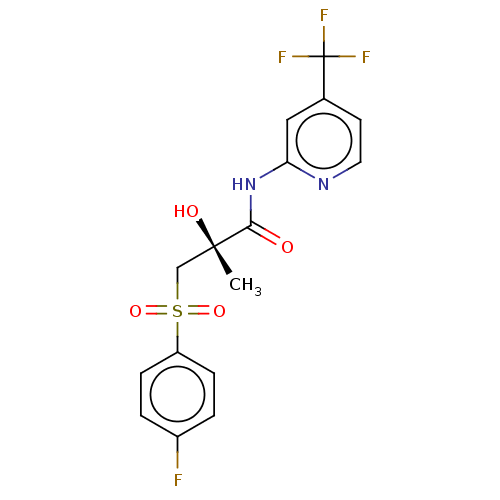 (US10053433, FC 4.126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50425732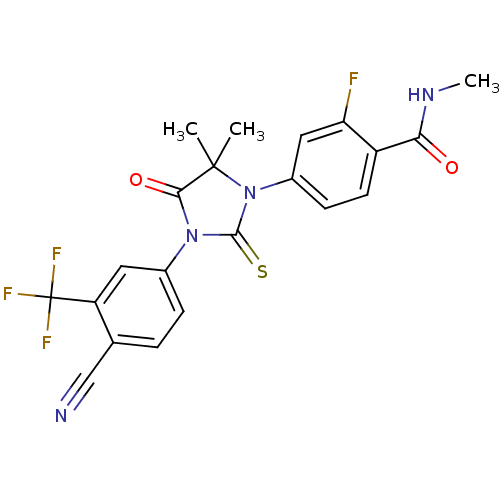 (ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239316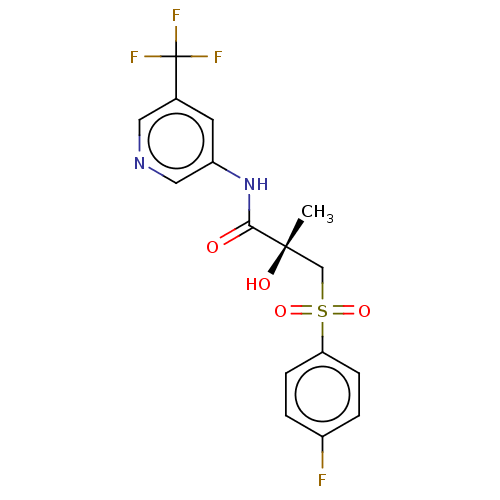 (US10053433, FC 4.116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50094298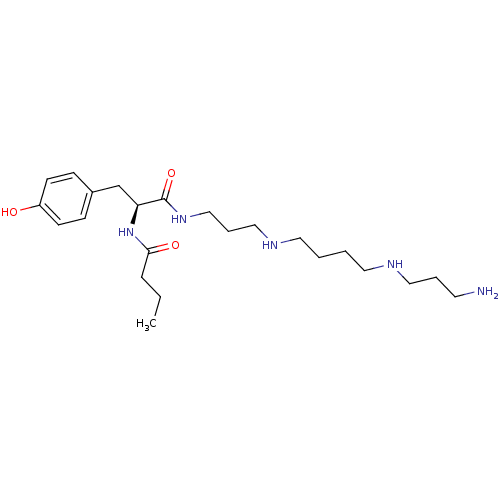 (CHEMBL16117 | N-[(S)-1-{3-[4-(3-Amino-propylamino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of rat recombinant GluA1 receptor flop isoform expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Activity at recombinant GluA1 receptor flop isoform expressed in Xenopus oocytes assessed as effect of 100 uM kainate-induced current by patch clamp ... | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50316373 (Argiotoxin-636 | CHEMBL1098240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of rat recombinant GluA1 receptor flop isoform expressed in Xenopus oocytes assessed as inhibition of 100 uM kainate-induced current | Bioorg Med Chem 18: 1381-7 (2010) Article DOI: 10.1016/j.bmc.2009.12.072 BindingDB Entry DOI: 10.7270/Q27W6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242578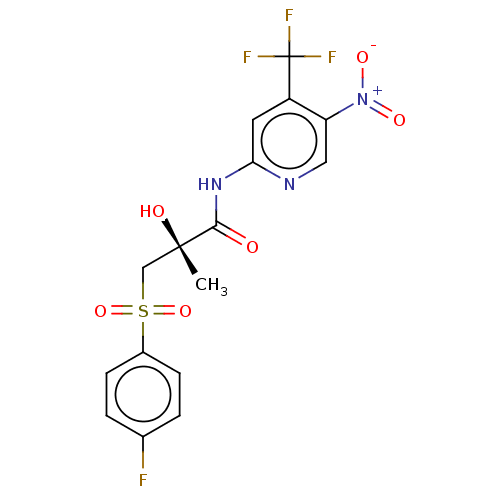 (US10053433, FC 4.127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.26E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Briefly, the AR LBD is expressed as a fusion with the Gal4 transcription factor, which binds to the Gal4 reporter DNA. Upon activation with agonist h... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50253650 (CHEMBL462490 | [1,2,5]oxadiazolo[3,4-g]quinoxaline...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of GluR2 receptor (unknown origin) | J Med Chem 51: 5856-60 (2008) Article DOI: 10.1021/jm701517b BindingDB Entry DOI: 10.7270/Q2W095R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50425732 (ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 8.61E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM242578 (US10053433, FC 4.127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.74E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM239363 (US10053433, FC 4.126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50094975 (956104-40-8 | ARN-509 | JNJ-56021927 | US10053433,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description An AR-response element is contained within the mmTV sequence and drives the expression of luciferase. The effect of antiandrogens (competitive antago... | US Patent US10053433 (2018) BindingDB Entry DOI: 10.7270/Q2R78H77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |