

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023323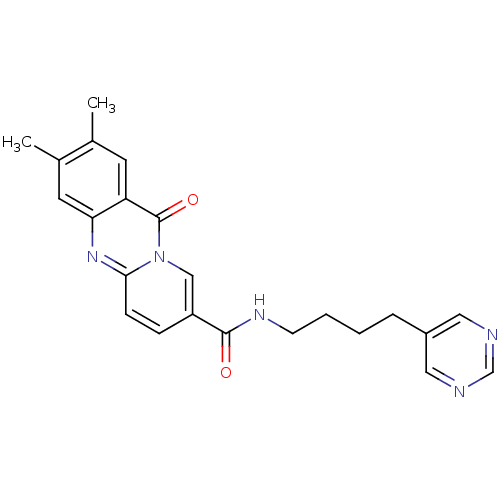 (2,3-Dimethyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50021516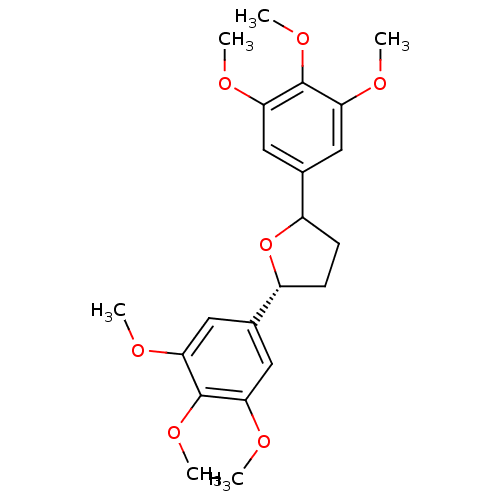 (2,5-Bis-(3,4,5-trimethoxy-phenyl)-tetrahydro-furan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023325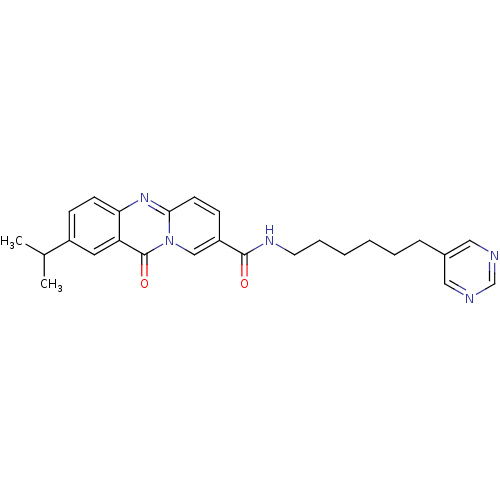 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019687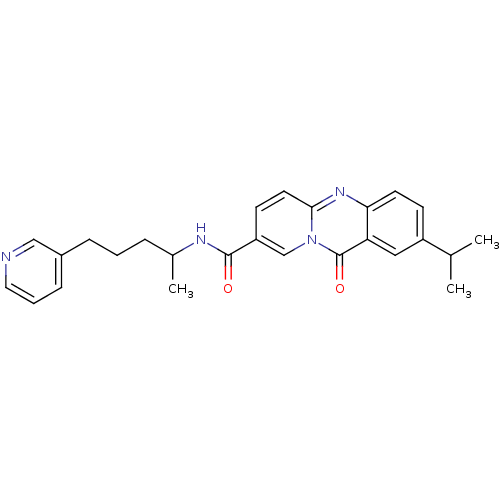 (CHEMBL1202755 | CHEMBL57825) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019665 (2,3-Dimethyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019669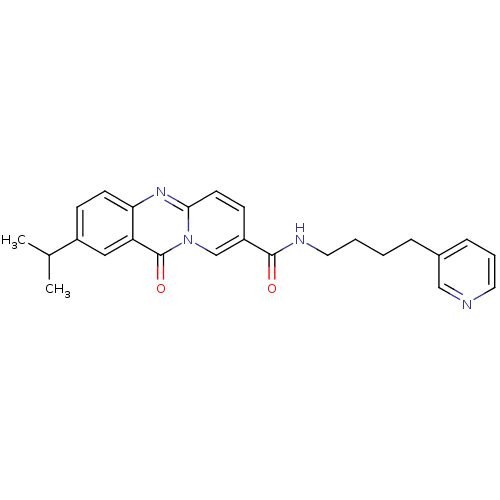 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019678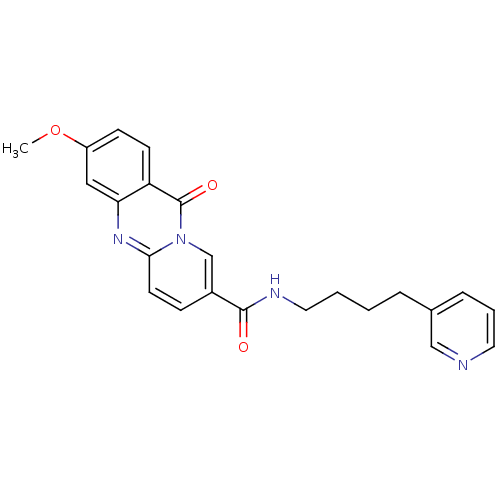 (3-Methoxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019684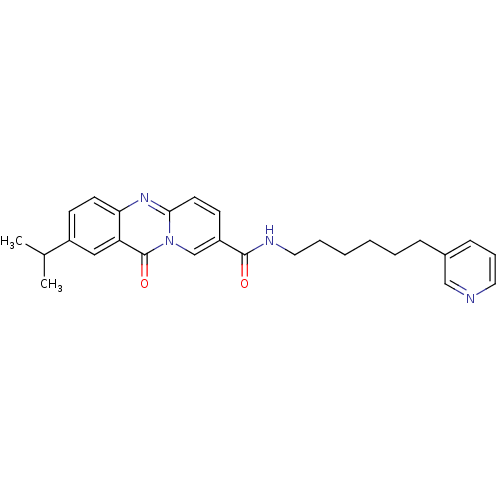 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023315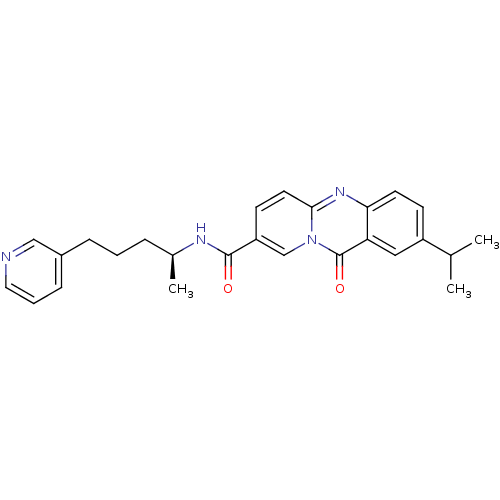 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019686 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019676 (2-Methyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023324 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019671 (2-Isopropoxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50006048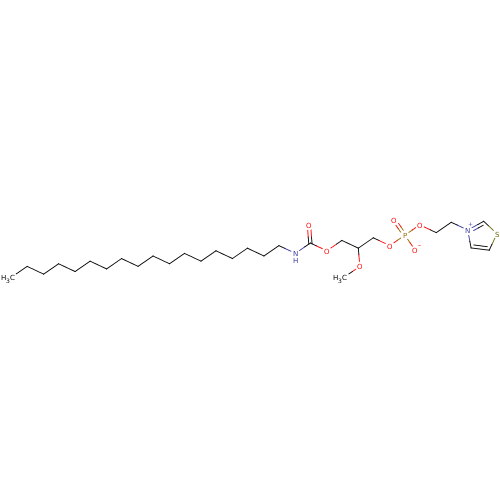 (2-methoxy-3-(octadecylcarbamoyloxy)propyl 2-(thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019688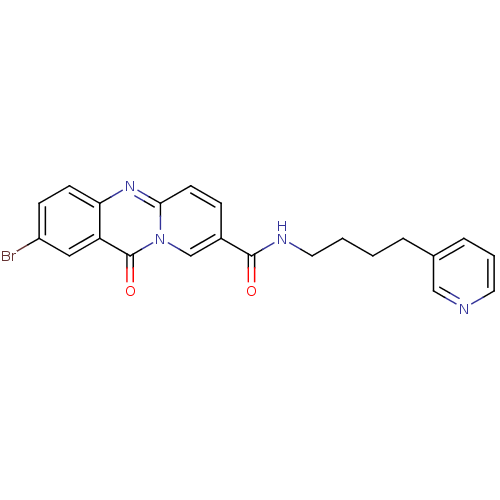 (2-Bromo-11-oxo-11H-pyrido[2,1-b]quinazoline-8-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019680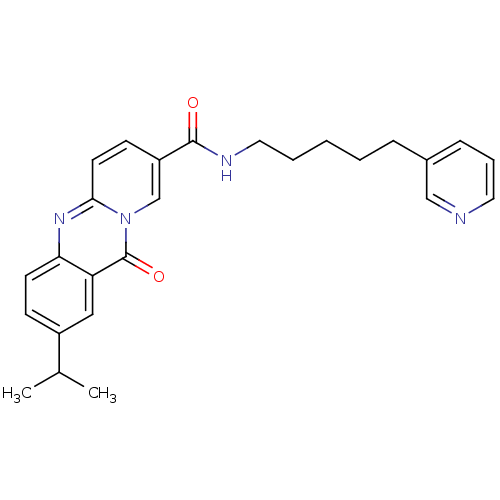 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019675 (2-Methoxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023321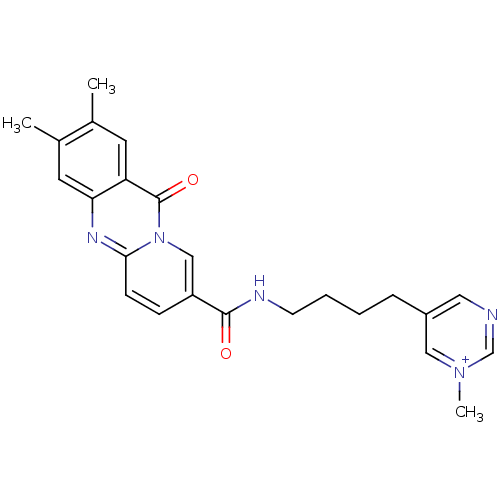 (5-{4-[(2,3-Dimethyl-11-oxo-11H-pyrido[2,1-b]quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019673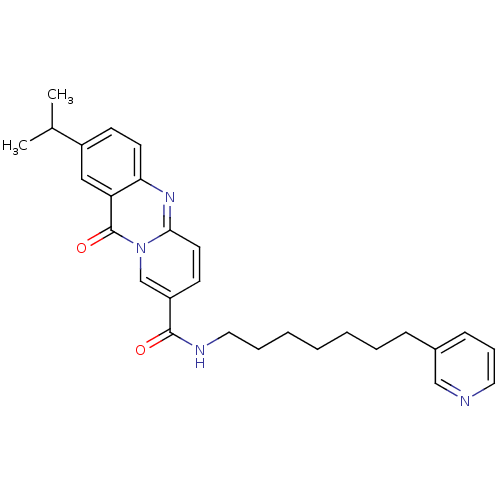 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023322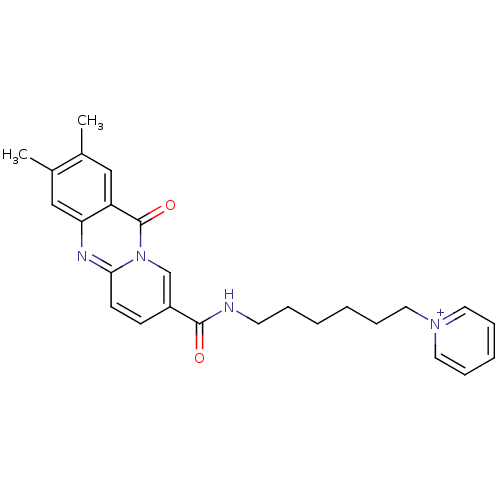 (1-{6-[(2,3-Dimethyl-11-oxo-11H-pyrido[2,1-b]quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023319 (2-Isopropyl-8-(6-pyridin-3-yl-hexyl)-pyrido[2,1-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019672 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023320 (3-{4-[(2,3-Dimethyl-11-oxo-11H-pyrido[2,1-b]quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023318 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023316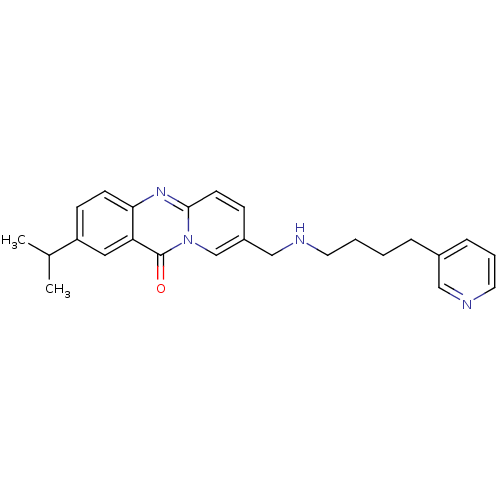 (2-Isopropyl-8-[(4-pyridin-3-yl-butylamino)-methyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50023317 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019689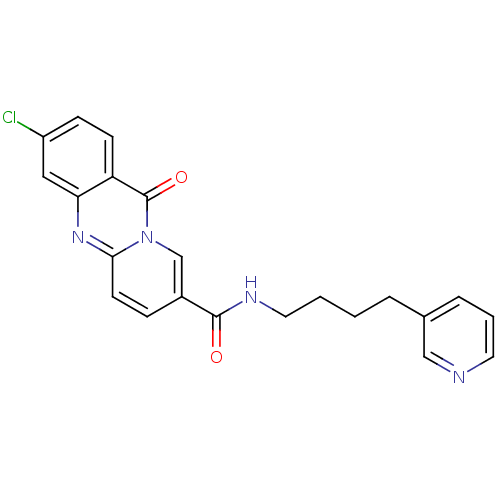 (3-Chloro-11-oxo-11H-pyrido[2,1-b]quinazoline-8-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019670 (2-Hydroxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019677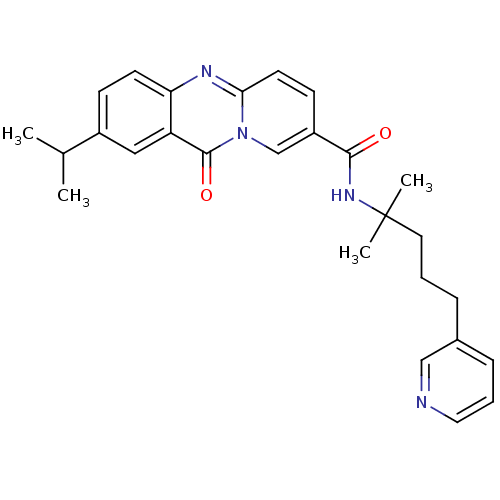 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019668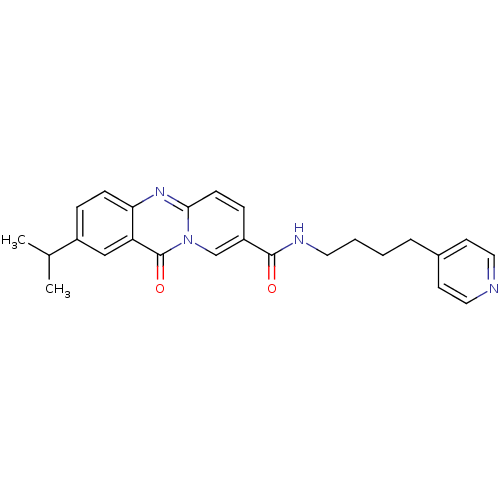 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019683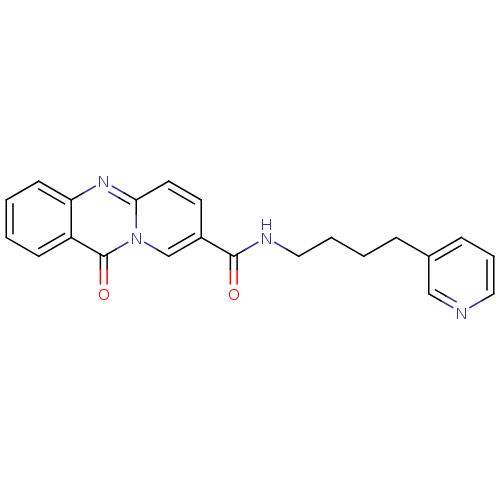 (11-Oxo-11H-pyrido[2,1-b]quinazoline-8-carboxylic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019690 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019681 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50019679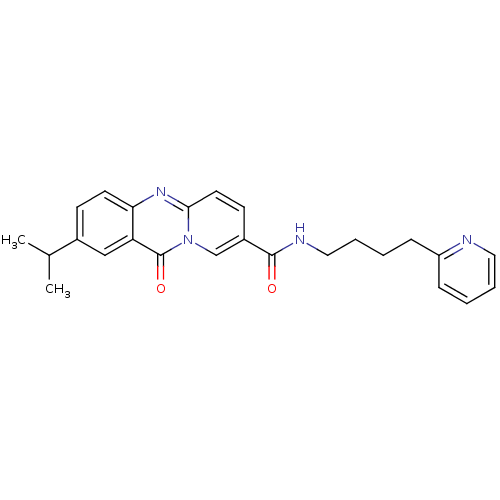 (2-Isopropyl-11-oxo-11H-pyrido[2,1-b]quinazoline-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Ability to inhibit platelet activating factor (PAF) binding to platelets by 50% was determined by using [3H]-PAF as radioligand | J Med Chem 31: 466-72 (1988) BindingDB Entry DOI: 10.7270/Q2KK99S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||