 Found 369 hits with Last Name = 'faller' and Initial = 'a'
Found 369 hits with Last Name = 'faller' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287729

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CN(CCOc1ccc(CC(Oc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C25H24N2O5/c1-27(25-26-21-9-5-6-10-22(21)32-25)15-16-30-19-13-11-18(12-14-19)17-23(24(28)29)31-20-7-3-2-4-8-20/h2-14,23H,15-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085043
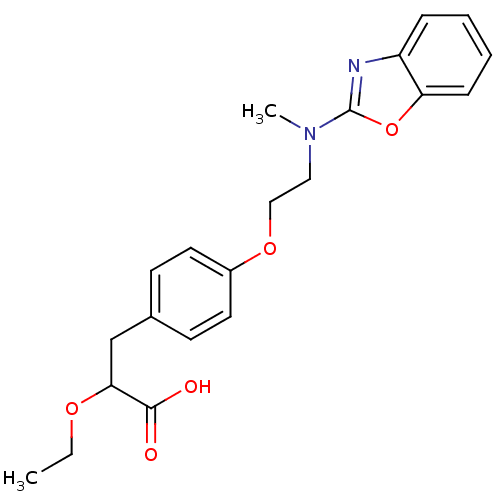
(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CCOC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C21H24N2O5/c1-3-26-19(20(24)25)14-15-8-10-16(11-9-15)27-13-12-23(2)21-22-17-6-4-5-7-18(17)28-21/h4-11,19H,3,12-14H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085043

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CCOC(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)C(O)=O Show InChI InChI=1S/C21H24N2O5/c1-3-26-19(20(24)25)14-15-8-10-16(11-9-15)27-13-12-23(2)21-22-17-6-4-5-7-18(17)28-21/h4-11,19H,3,12-14H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287732

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show InChI InChI=1S/C20H22N2O5/c1-22(20-21-16-5-3-4-6-17(16)27-20)11-12-26-15-9-7-14(8-10-15)13-18(25-2)19(23)24/h3-10,18H,11-13H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049244
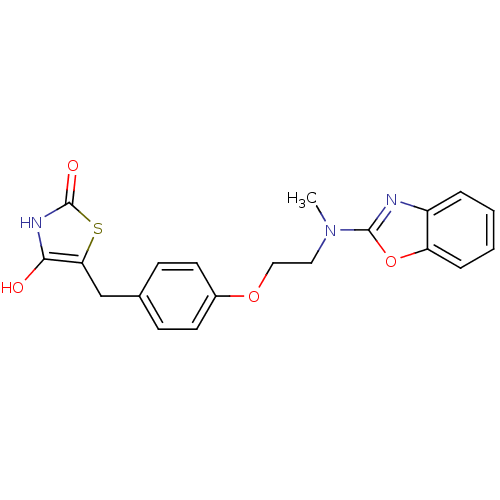
(5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C20H19N3O4S/c1-23(19-21-15-4-2-3-5-16(15)27-19)10-11-26-14-8-6-13(7-9-14)12-17-18(24)22-20(25)28-17/h2-9,24H,10-12H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50049244

(5-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(Cc2sc(=O)[nH]c2O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C20H19N3O4S/c1-23(19-21-15-4-2-3-5-16(15)27-19)10-11-26-14-8-6-13(7-9-14)12-17-18(24)22-20(25)28-17/h2-9,24H,10-12H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287733

(2-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-be...)Show SMILES CN(CCOc1ccc(CC(CCCc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C28H30N2O4/c1-30(28-29-25-12-5-6-13-26(25)34-28)18-19-33-24-16-14-22(15-17-24)20-23(27(31)32)11-7-10-21-8-3-2-4-9-21/h2-6,8-9,12-17,23H,7,10-11,18-20H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287727

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show InChI InChI=1S/C19H19ClN2O4/c1-22(19-21-16-4-2-3-5-17(16)26-19)10-11-25-14-8-6-13(7-9-14)12-15(20)18(23)24/h2-9,15H,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287730
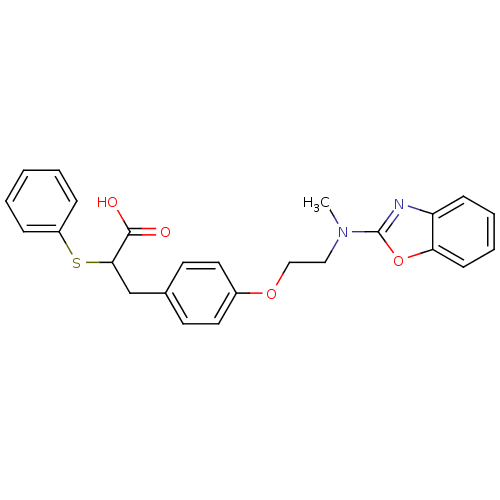
(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES CN(CCOc1ccc(CC(Sc2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C25H24N2O4S/c1-27(25-26-21-9-5-6-10-22(21)31-25)15-16-30-19-13-11-18(12-14-19)17-23(24(28)29)32-20-7-3-2-4-8-20/h2-14,23H,15-17H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287728
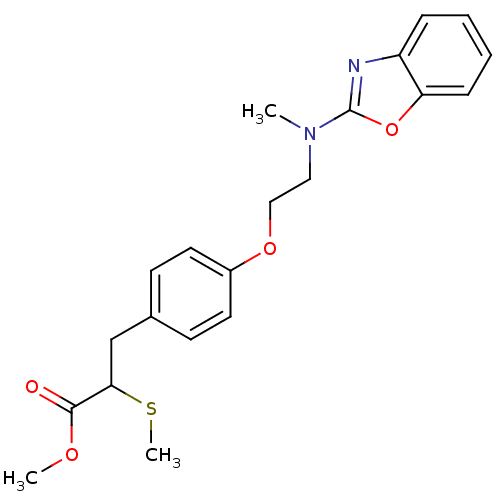
(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show SMILES COC(=O)C(Cc1ccc(OCCN(C)c2nc3ccccc3o2)cc1)SC Show InChI InChI=1S/C21H24N2O4S/c1-23(21-22-17-6-4-5-7-18(17)27-21)12-13-26-16-10-8-15(9-11-16)14-19(28-3)20(24)25-2/h4-11,19H,12-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287734
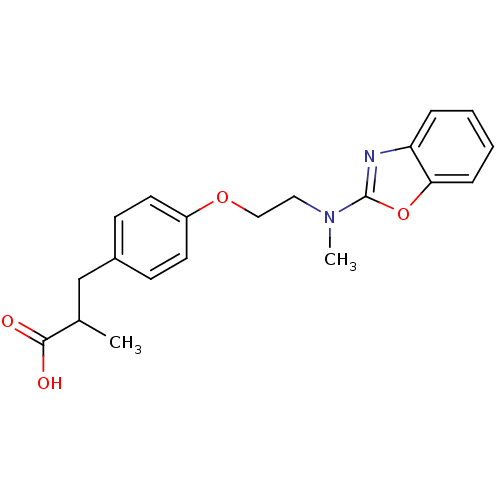
(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show InChI InChI=1S/C20H22N2O4/c1-14(19(23)24)13-15-7-9-16(10-8-15)25-12-11-22(2)20-21-17-5-3-4-6-18(17)26-20/h3-10,14H,11-13H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2127-2130 (1996)
Article DOI: 10.1016/0960-894X(96)00382-4
BindingDB Entry DOI: 10.7270/Q2J1034Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085045
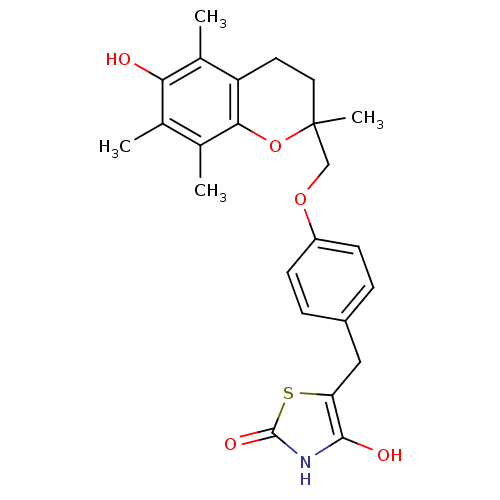
(5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...)Show SMILES Cc1c(C)c2OC(C)(COc3ccc(Cc4sc(=O)[nH]c4O)cc3)CCc2c(C)c1O Show InChI InChI=1S/C24H27NO5S/c1-13-14(2)21-18(15(3)20(13)26)9-10-24(4,30-21)12-29-17-7-5-16(6-8-17)11-19-22(27)25-23(28)31-19/h5-8,26-27H,9-12H2,1-4H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50287731

(3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-ph...)Show InChI InChI=1S/C20H22N2O4/c1-22(20-21-17-5-3-4-6-18(17)26-20)13-14-25-16-10-7-15(8-11-16)9-12-19(23)24-2/h3-8,10-11H,9,12-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [125I]-SB 236636 binding to human PPAR gamma receptor |
Bioorg Med Chem Lett 6: 2121-2126 (1996)
Article DOI: 10.1016/0960-894X(96)00383-6
BindingDB Entry DOI: 10.7270/Q2NS0TW1 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM532950

((?)-5-fluoro-6-[1-[4-(5-methyl-1,3,4-oxadiazol-2-y...)Show SMILES CC(N1CCC(CC1)c1nnc(C)o1)c1nc2OCCc2cc1F |w:1.0| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay buffer is prepared to give a final concentration of 50 mM H2NaPO3—HNa2PO3, 0.01% bovine serum albumin and 0.01% Triton™ X-100 in water, at ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1V76 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM532948
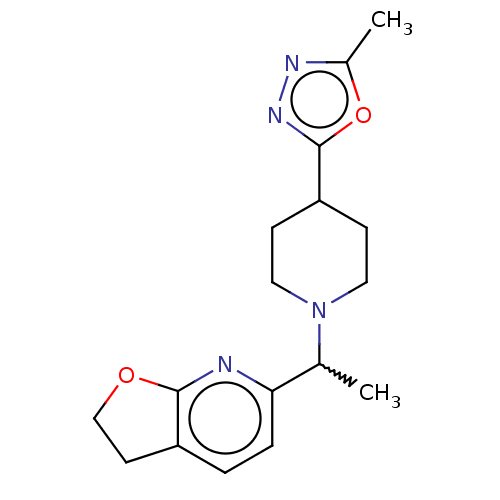
((?)-6-[1-[4-(5-methyl-1,3,4-oxadiazol-2-yl)-1-pipe...)Show SMILES CC(N1CCC(CC1)c1nnc(C)o1)c1ccc2CCOc2n1 |w:1.0| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay buffer is prepared to give a final concentration of 50 mM H2NaPO3—HNa2PO3, 0.01% bovine serum albumin and 0.01% Triton™ X-100 in water, at ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29S1V76 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26502

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O4S/c1-2-36-26-17-24(18-27(19-26)38-13-6-7-14-43(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26788
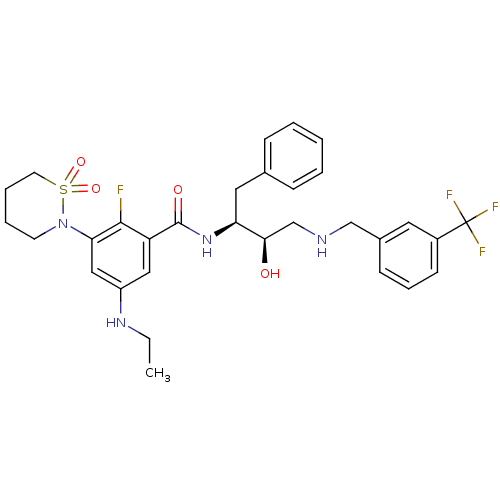
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H36F4N4O4S/c1-2-37-24-17-25(29(32)27(18-24)39-13-6-7-14-44(39,42)43)30(41)38-26(16-21-9-4-3-5-10-21)28(40)20-36-19-22-11-8-12-23(15-22)31(33,34)35/h3-5,8-12,15,17-18,26,28,36-37,40H,2,6-7,13-14,16,19-20H2,1H3,(H,38,41)/t26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26786
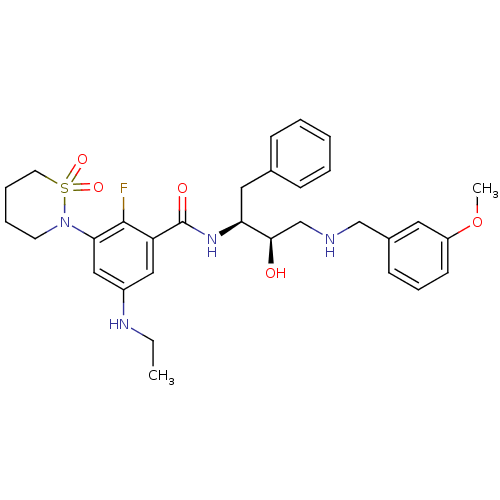
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC)c1 |r| Show InChI InChI=1S/C31H39FN4O5S/c1-3-34-24-18-26(30(32)28(19-24)36-14-7-8-15-42(36,39)40)31(38)35-27(17-22-10-5-4-6-11-22)29(37)21-33-20-23-12-9-13-25(16-23)41-2/h4-6,9-13,16,18-19,27,29,33-34,37H,3,7-8,14-15,17,20-21H2,1-2H3,(H,35,38)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26782

(N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[(3...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(OC)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H38F2N4O5S/c1-3-35-26-15-23(16-27(18-26)37-9-4-5-10-43(37,40)41)31(39)36-29(14-22-11-24(32)17-25(33)12-22)30(38)20-34-19-21-7-6-8-28(13-21)42-2/h6-8,11-13,15-18,29-30,34-35,38H,3-5,9-10,14,19-20H2,1-2H3,(H,36,39)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26776
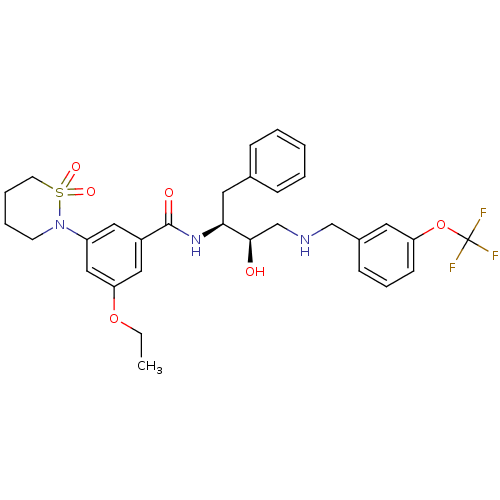
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC(F)(F)F)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H36F3N3O6S/c1-2-42-27-18-24(17-25(19-27)37-13-6-7-14-44(37,40)41)30(39)36-28(16-22-9-4-3-5-10-22)29(38)21-35-20-23-11-8-12-26(15-23)43-31(32,33)34/h3-5,8-12,15,17-19,28-29,35,38H,2,6-7,13-14,16,20-21H2,1H3,(H,36,39)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26774

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-3-hydr...)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C32H38F3N3O4S/c1-2-9-24-16-26(20-28(18-24)38-14-6-7-15-43(38,41)42)31(40)37-29(19-23-10-4-3-5-11-23)30(39)22-36-21-25-12-8-13-27(17-25)32(33,34)35/h3-5,8,10-13,16-18,20,29-30,36,39H,2,6-7,9,14-15,19,21-22H2,1H3,(H,37,40)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 23-8 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FT6 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26777
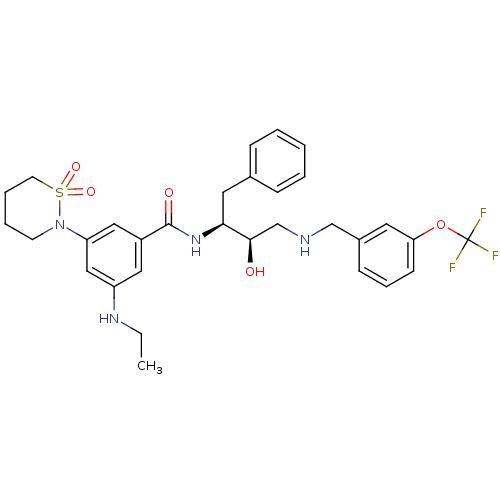
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC(F)(F)F)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O5S/c1-2-36-25-17-24(18-26(19-25)38-13-6-7-14-44(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-27(15-23)43-31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26787

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-4-{[(1...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cnn(CC)c1 |r| Show InChI InChI=1S/C29H39FN6O4S/c1-3-32-23-15-24(28(30)26(16-23)36-12-8-9-13-41(36,39)40)29(38)34-25(14-21-10-6-5-7-11-21)27(37)19-31-17-22-18-33-35(4-2)20-22/h5-7,10-11,15-16,18,20,25,27,31-32,37H,3-4,8-9,12-14,17,19H2,1-2H3,(H,34,38)/t25-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26773
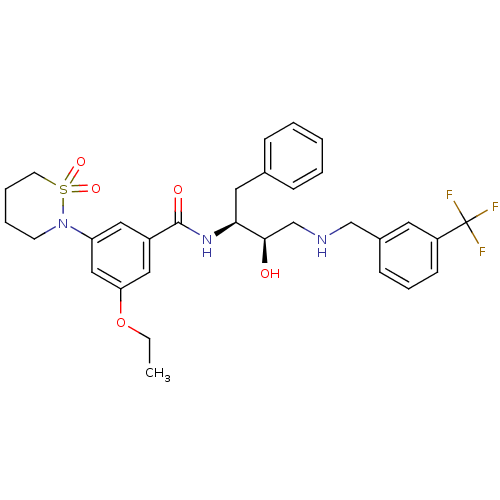
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H36F3N3O5S/c1-2-42-27-18-24(17-26(19-27)37-13-6-7-14-43(37,40)41)30(39)36-28(16-22-9-4-3-5-10-22)29(38)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35,38H,2,6-7,13-14,16,20-21H2,1H3,(H,36,39)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069610
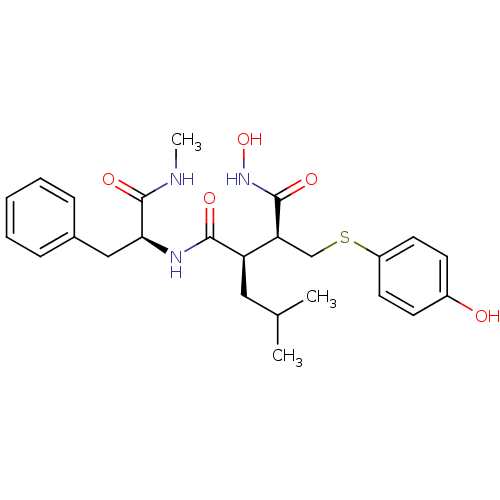
((2R,3S)-N*1*-Hydroxy-2-(4-hydroxy-phenylsulfanylme...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1ccc(O)cc1)C(=O)NO Show InChI InChI=1S/C25H33N3O5S/c1-16(2)13-20(21(24(31)28-33)15-34-19-11-9-18(29)10-12-19)23(30)27-22(25(32)26-3)14-17-7-5-4-6-8-17/h4-12,16,20-22,29,33H,13-15H2,1-3H3,(H,26,32)(H,27,30)(H,28,31)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26781
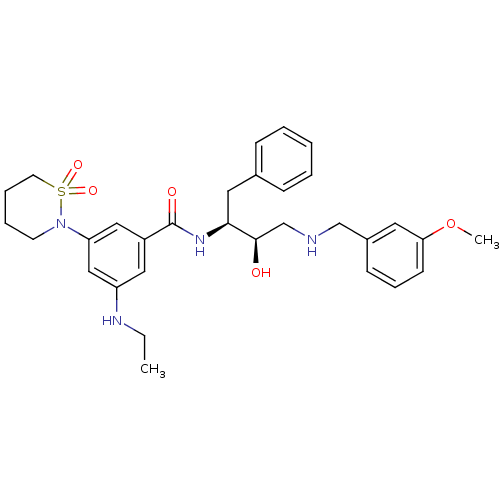
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H40N4O5S/c1-3-33-26-18-25(19-27(20-26)35-14-7-8-15-41(35,38)39)31(37)34-29(17-23-10-5-4-6-11-23)30(36)22-32-21-24-12-9-13-28(16-24)40-2/h4-6,9-13,16,18-20,29-30,32-33,36H,3,7-8,14-15,17,21-22H2,1-2H3,(H,34,37)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50231695
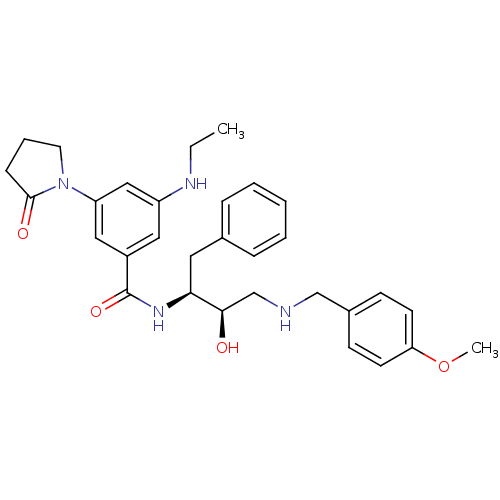
(CHEMBL404500 | N-((2S,3R)-4-(4-methoxybenzylamino)...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1ccc(OC)cc1)N1CCCC1=O Show InChI InChI=1S/C31H38N4O4/c1-3-33-25-17-24(18-26(19-25)35-15-7-10-30(35)37)31(38)34-28(16-22-8-5-4-6-9-22)29(36)21-32-20-23-11-13-27(39-2)14-12-23/h4-6,8-9,11-14,17-19,28-29,32-33,36H,3,7,10,15-16,20-21H2,1-2H3,(H,34,38)/t28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1017-21 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.019
BindingDB Entry DOI: 10.7270/Q2J67GP2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26775
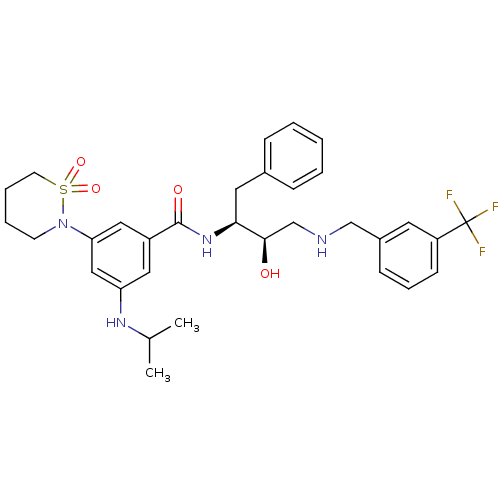
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-3-hydr...)Show SMILES CC(C)Nc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C32H39F3N4O4S/c1-22(2)37-27-17-25(18-28(19-27)39-13-6-7-14-44(39,42)43)31(41)38-29(16-23-9-4-3-5-10-23)30(40)21-36-20-24-11-8-12-26(15-24)32(33,34)35/h3-5,8-12,15,17-19,22,29-30,36-37,40H,6-7,13-14,16,20-21H2,1-2H3,(H,38,41)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069616
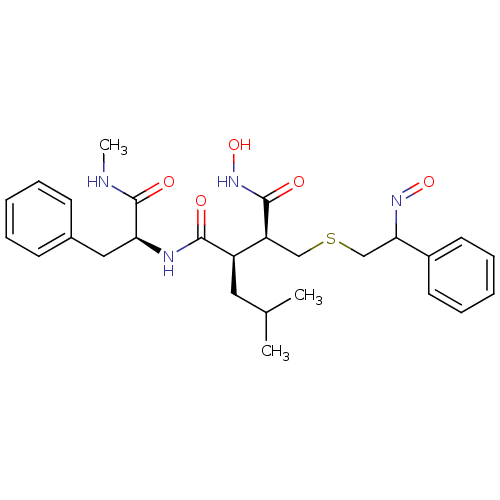
((2R,3S)-N*1*-Hydroxy-2-{2-[(E)-hydroxyimino]-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSCC(N=O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H36N4O5S/c1-18(2)14-21(25(32)29-23(27(34)28-3)15-19-10-6-4-7-11-19)22(26(33)31-36)16-37-17-24(30-35)20-12-8-5-9-13-20/h4-13,18,21-24,36H,14-17H2,1-3H3,(H,28,34)(H,29,32)(H,31,33)/t21-,22+,23+,24?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26780
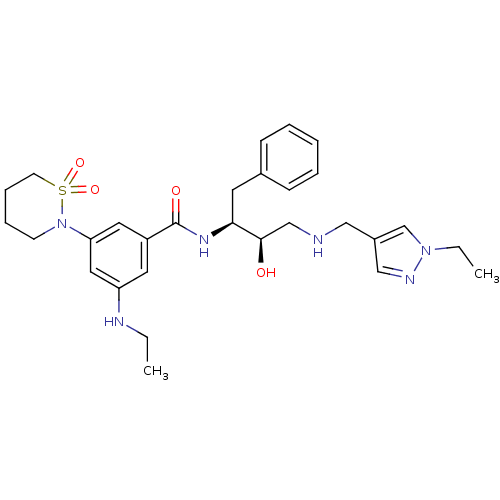
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-4-{[(1...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cnn(CC)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C29H40N6O4S/c1-3-31-25-15-24(16-26(17-25)35-12-8-9-13-40(35,38)39)29(37)33-27(14-22-10-6-5-7-11-22)28(36)20-30-18-23-19-32-34(4-2)21-23/h5-7,10-11,15-17,19,21,27-28,30-31,36H,3-4,8-9,12-14,18,20H2,1-2H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231682
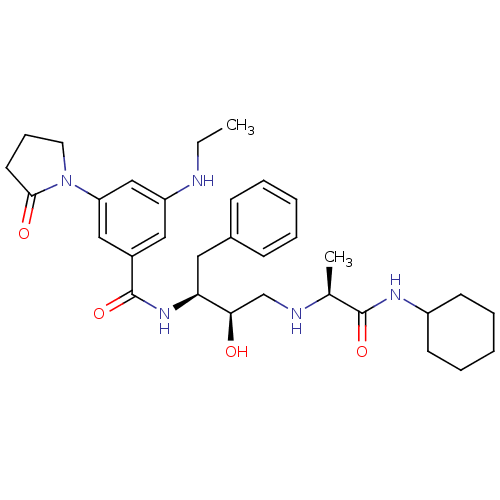
(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1017-21 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.019
BindingDB Entry DOI: 10.7270/Q2J67GP2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231682

(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069604
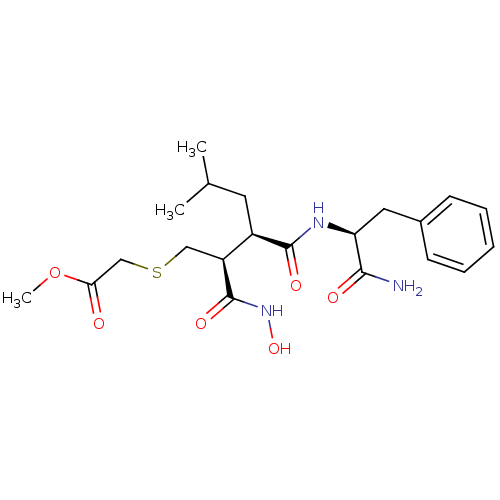
(CHEMBL118054 | [(2S,3R)-3-((S)-1-Carbamoyl-2-pheny...)Show SMILES COC(=O)CSC[C@@H]([C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(=O)NO Show InChI InChI=1S/C21H31N3O6S/c1-13(2)9-15(16(21(28)24-29)11-31-12-18(25)30-3)20(27)23-17(19(22)26)10-14-7-5-4-6-8-14/h4-8,13,15-17,29H,9-12H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231676
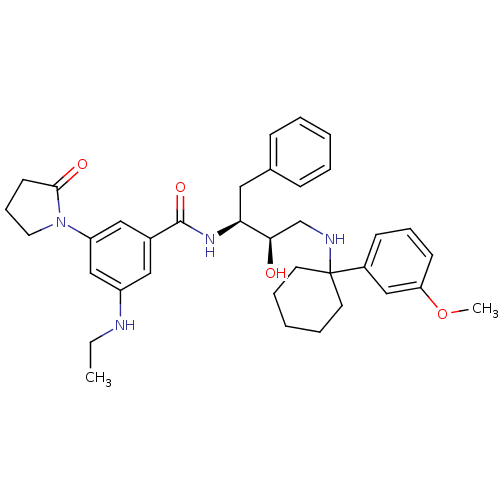
(3-(ethylamino)-N-((2S,3R)-3-hydroxy-4-(1-(3-methox...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1(CCCCC1)c1cccc(OC)c1)N1CCCC1=O Show InChI InChI=1S/C36H46N4O4/c1-3-37-29-21-27(22-30(24-29)40-19-11-16-34(40)42)35(43)39-32(20-26-12-6-4-7-13-26)33(41)25-38-36(17-8-5-9-18-36)28-14-10-15-31(23-28)44-2/h4,6-7,10,12-15,21-24,32-33,37-38,41H,3,5,8-9,11,16-20,25H2,1-2H3,(H,39,43)/t32-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1017-21 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.019
BindingDB Entry DOI: 10.7270/Q2J67GP2 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069601
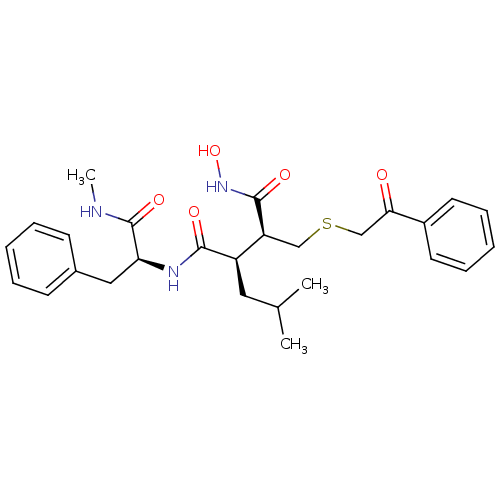
((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSCC(=O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C27H35N3O5S/c1-18(2)14-21(25(32)29-23(27(34)28-3)15-19-10-6-4-7-11-19)22(26(33)30-35)16-36-17-24(31)20-12-8-5-9-13-20/h4-13,18,21-23,35H,14-17H2,1-3H3,(H,28,34)(H,29,32)(H,30,33)/t21-,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231685
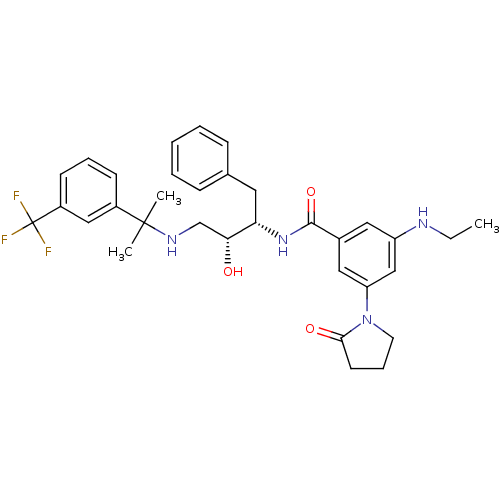
(3-(ethylamino)-N-((2S,3R)-3-hydroxy-1-phenyl-4-(2-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC(C)(C)c1cccc(c1)C(F)(F)F)N1CCCC1=O Show InChI InChI=1S/C33H39F3N4O3/c1-4-37-26-17-23(18-27(20-26)40-15-9-14-30(40)42)31(43)39-28(16-22-10-6-5-7-11-22)29(41)21-38-32(2,3)24-12-8-13-25(19-24)33(34,35)36/h5-8,10-13,17-20,28-29,37-38,41H,4,9,14-16,21H2,1-3H3,(H,39,43)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1017-21 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.019
BindingDB Entry DOI: 10.7270/Q2J67GP2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26779

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CCc2ccc(OC)cc12)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C33H42N4O5S/c1-3-34-26-18-25(19-27(20-26)37-15-7-8-16-43(37,40)41)33(39)36-31(17-23-9-5-4-6-10-23)32(38)22-35-30-14-12-24-11-13-28(42-2)21-29(24)30/h4-6,9-11,13,18-21,30-32,34-35,38H,3,7-8,12,14-17,22H2,1-2H3,(H,36,39)/t30?,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082225
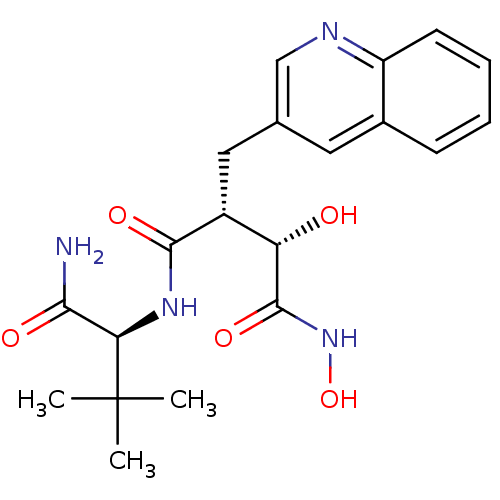
((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1cnc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C20H26N4O5/c1-20(2,3)16(17(21)26)23-18(27)13(15(25)19(28)24-29)9-11-8-12-6-4-5-7-14(12)22-10-11/h4-8,10,13,15-16,25,29H,9H2,1-3H3,(H2,21,26)(H,23,27)(H,24,28)/t13-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069583

((R)-N*1*-((S)-1-Benzylcarbamoyl-2-phenyl-ethyl)-2-...)Show SMILES ONC(=O)C[C@@H](CC1CCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C26H33N3O4/c30-24(29-33)17-22(15-19-11-7-8-12-19)25(31)28-23(16-20-9-3-1-4-10-20)26(32)27-18-21-13-5-2-6-14-21/h1-6,9-10,13-14,19,22-23,33H,7-8,11-12,15-18H2,(H,27,32)(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 23-8 (1999)
BindingDB Entry DOI: 10.7270/Q2ZK5FT6 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082218
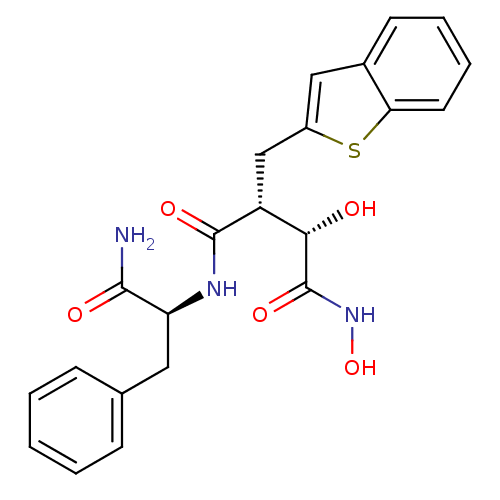
((2R,3S)-2-Benzo[b]thiophen-2-ylmethyl-N*1*-((S)-1-...)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc2ccccc2s1)[C@H](O)C(=O)NO Show InChI InChI=1S/C22H23N3O5S/c23-20(27)17(10-13-6-2-1-3-7-13)24-21(28)16(19(26)22(29)25-30)12-15-11-14-8-4-5-9-18(14)31-15/h1-9,11,16-17,19,26,30H,10,12H2,(H2,23,27)(H,24,28)(H,25,29)/t16-,17+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50082226
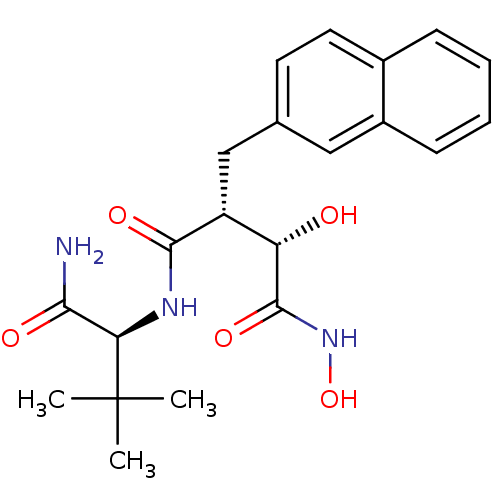
((2R,3S)-N*4*-((S)-1-Carbamoyl-2,2-dimethyl-propyl)...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)[C@H](O)C(=O)NO)C(N)=O Show InChI InChI=1S/C21H27N3O5/c1-21(2,3)17(18(22)26)23-19(27)15(16(25)20(28)24-29)11-12-8-9-13-6-4-5-7-14(13)10-12/h4-10,15-17,25,29H,11H2,1-3H3,(H2,22,26)(H,23,27)(H,24,28)/t15-,16+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against IgE receptor |
Bioorg Med Chem Lett 9: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C37 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069608
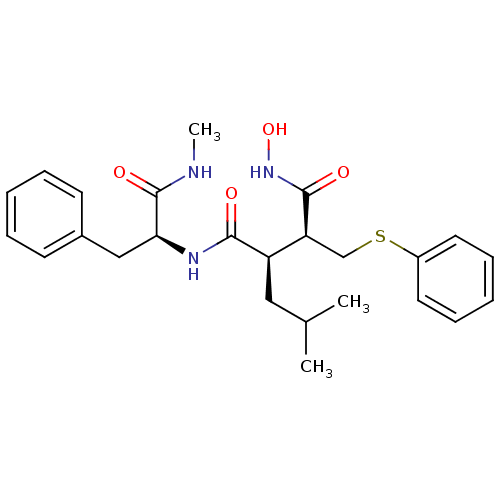
((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1ccccc1)C(=O)NO Show InChI InChI=1S/C25H33N3O4S/c1-17(2)14-20(21(24(30)28-32)16-33-19-12-8-5-9-13-19)23(29)27-22(25(31)26-3)15-18-10-6-4-7-11-18/h4-13,17,20-22,32H,14-16H2,1-3H3,(H,26,31)(H,27,29)(H,28,30)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231694
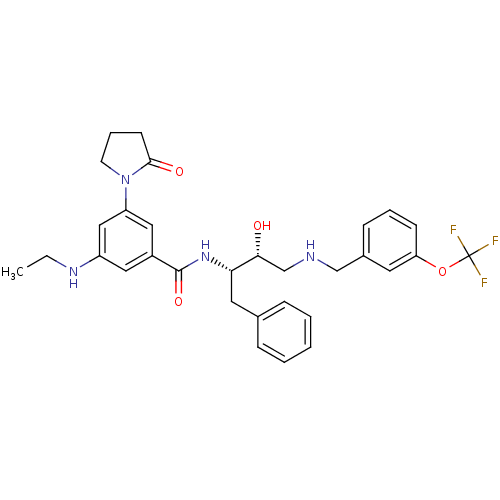
(CHEMBL402348 | N-((2S,3R)-4-(3-(trifluoromethoxy)b...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC(F)(F)F)c1)N1CCCC1=O Show InChI InChI=1S/C31H35F3N4O4/c1-2-36-24-16-23(17-25(18-24)38-13-7-12-29(38)40)30(41)37-27(15-21-8-4-3-5-9-21)28(39)20-35-19-22-10-6-11-26(14-22)42-31(32,33)34/h3-6,8-11,14,16-18,27-28,35-36,39H,2,7,12-13,15,19-20H2,1H3,(H,37,41)/t27-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1017-21 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.019
BindingDB Entry DOI: 10.7270/Q2J67GP2 |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069620
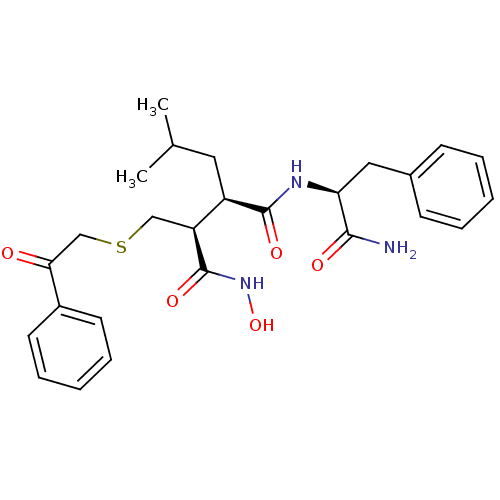
((2R,3S)-N*1*-((S)-1-Carbamoyl-2-phenyl-ethyl)-N*4*...)Show SMILES CC(C)C[C@H]([C@H](CSCC(=O)c1ccccc1)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C26H33N3O5S/c1-17(2)13-20(25(32)28-22(24(27)31)14-18-9-5-3-6-10-18)21(26(33)29-34)15-35-16-23(30)19-11-7-4-8-12-19/h3-12,17,20-22,34H,13-16H2,1-2H3,(H2,27,31)(H,28,32)(H,29,33)/t20-,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069612
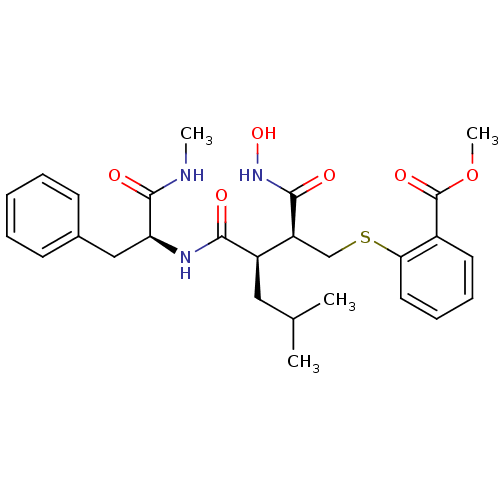
(2-[(2S,3R)-2-Hydroxycarbamoyl-5-methyl-3-((S)-1-me...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1ccccc1C(=O)OC)C(=O)NO Show InChI InChI=1S/C27H35N3O6S/c1-17(2)14-20(24(31)29-22(26(33)28-3)15-18-10-6-5-7-11-18)21(25(32)30-35)16-37-23-13-9-8-12-19(23)27(34)36-4/h5-13,17,20-22,35H,14-16H2,1-4H3,(H,28,33)(H,29,31)(H,30,32)/t20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069606

((2R,3S)-2-Benzylsulfanylmethyl-N*1*-hydroxy-3-isob...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C26H35N3O4S/c1-18(2)14-21(22(25(31)29-33)17-34-16-20-12-8-5-9-13-20)24(30)28-23(26(32)27-3)15-19-10-6-4-7-11-19/h4-13,18,21-23,33H,14-17H2,1-3H3,(H,27,32)(H,28,30)(H,29,31)/t21-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069582
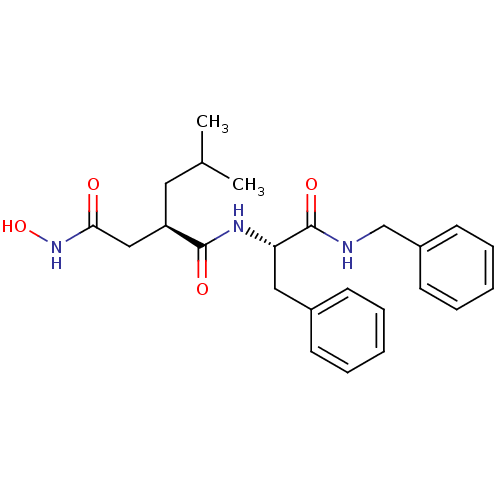
((R)-N*1*-((S)-1-Benzylcarbamoyl-2-phenyl-ethyl)-N*...)Show SMILES CC(C)C[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H31N3O4/c1-17(2)13-20(15-22(28)27-31)23(29)26-21(14-18-9-5-3-6-10-18)24(30)25-16-19-11-7-4-8-12-19/h3-12,17,20-21,31H,13-16H2,1-2H3,(H,25,30)(H,26,29)(H,27,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound towards human recombinant fibroblast collagenase |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |
Low affinity immunoglobulin epsilon Fc receptor
(Homo sapiens (Human)) | BDBM50069606

((2R,3S)-2-Benzylsulfanylmethyl-N*1*-hydroxy-3-isob...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C26H35N3O4S/c1-18(2)14-21(22(25(31)29-33)17-34-16-20-12-8-5-9-13-20)24(30)28-23(26(32)27-3)15-19-10-6-4-7-11-19/h4-13,18,21-23,33H,14-17H2,1-3H3,(H,27,32)(H,28,30)(H,29,31)/t21-,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Tested for inhibition against CD23 (IgE receptor) proteolysis in membranes derived from RPM18866 cells ( a human B-cell line); value ranges from 30-1... |
Bioorg Med Chem Lett 8: 29-34 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q3W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data