

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288587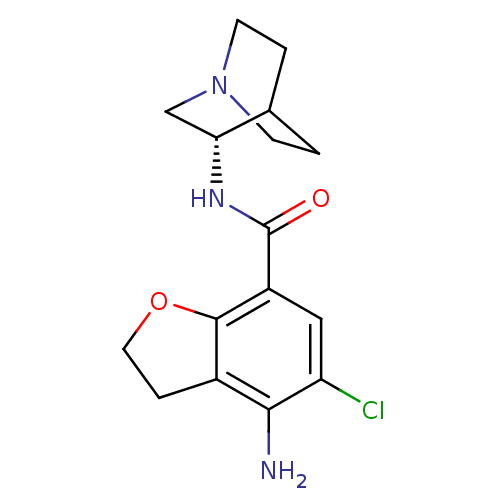 (2-(4-amino-5-chloro-2,3-dihydrobenzo[b]furan-7-ylc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50056419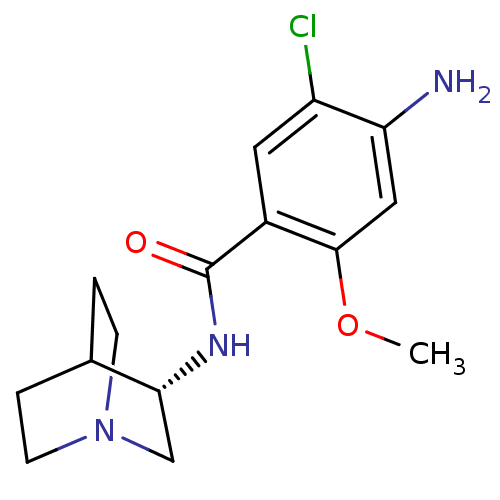 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro relaxation of carbachol pre-contracted rat oesophageal TMM. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50288595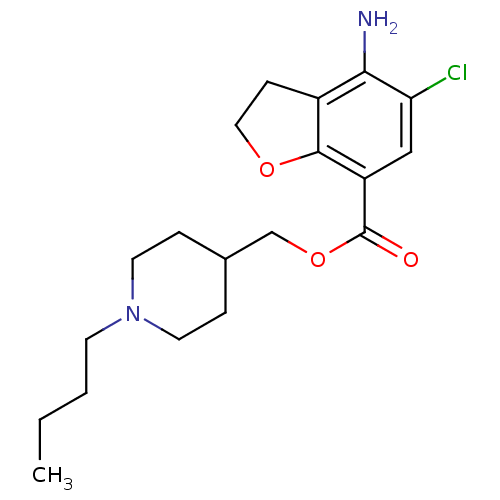 (4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 4 receptor in rat striatum by [3H]GR-113808 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288590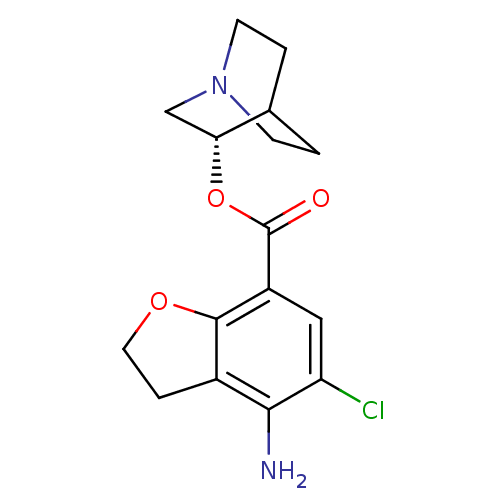 (4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The binding affinity was measured on 5-hydroxytryptamine 2 receptor in rat brain tissue | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471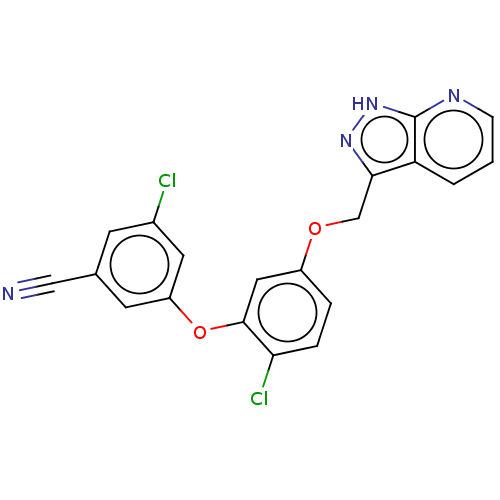 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The compound was tested for its binding affinity towards 5-hydroxytryptamine 2 receptor by displacing [3H]ketanserin radioligand in rat cerebral cort... | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039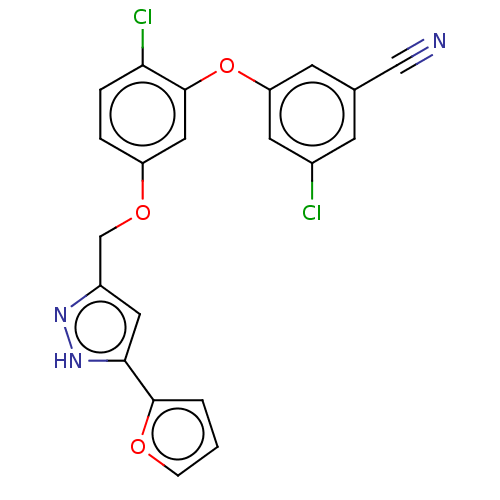 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029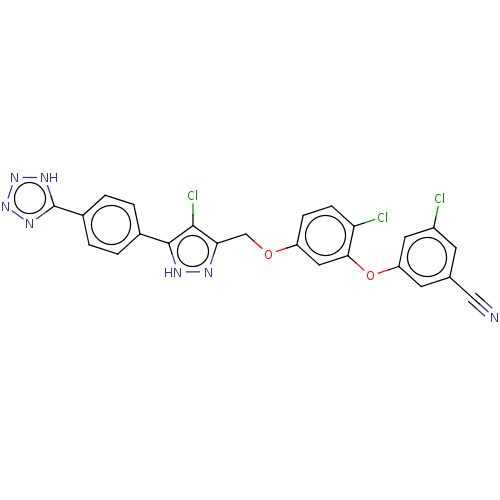 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443217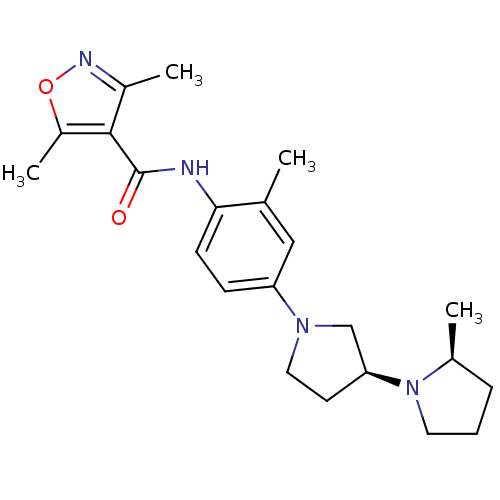 (CHEMBL3087669) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484635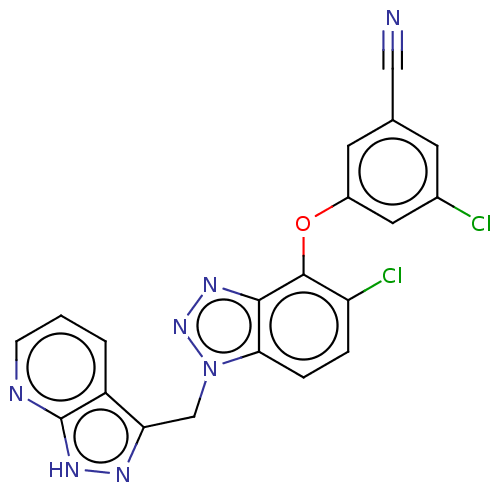 (CHEMBL1939500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001876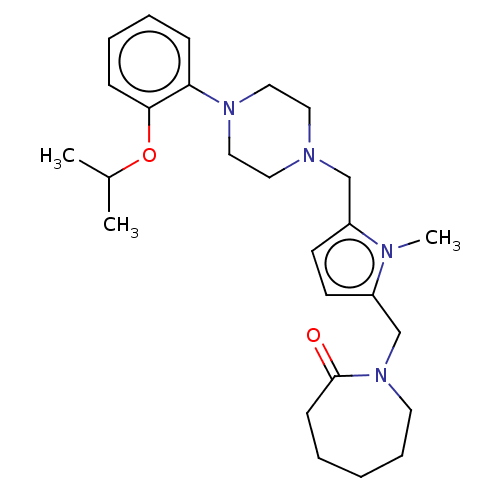 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484496 (CHEMBL1928648) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045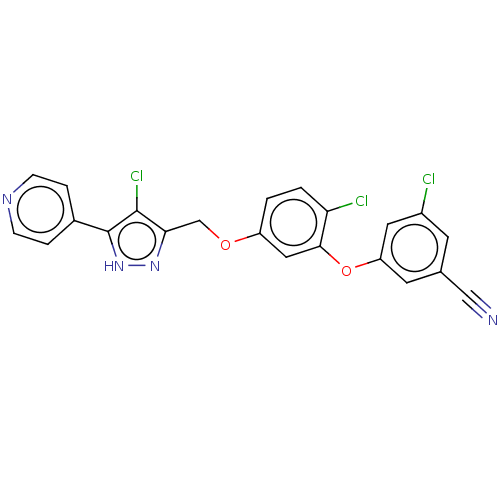 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484632 (Mk-6186 | Mk6186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001855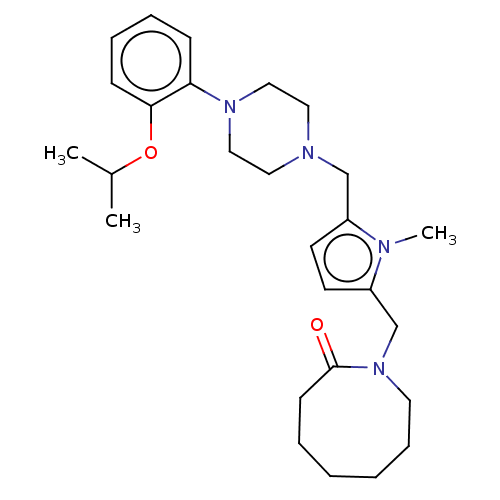 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484031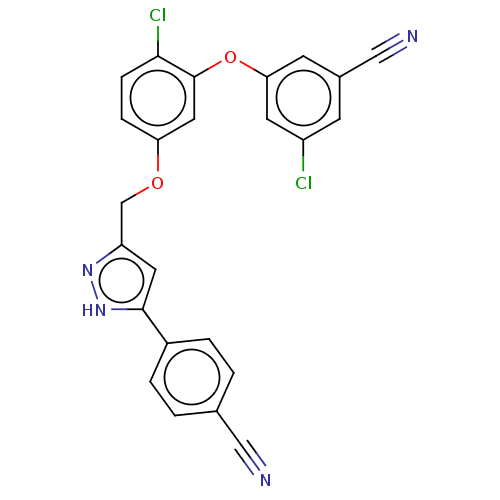 (CHEMBL1801255) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484040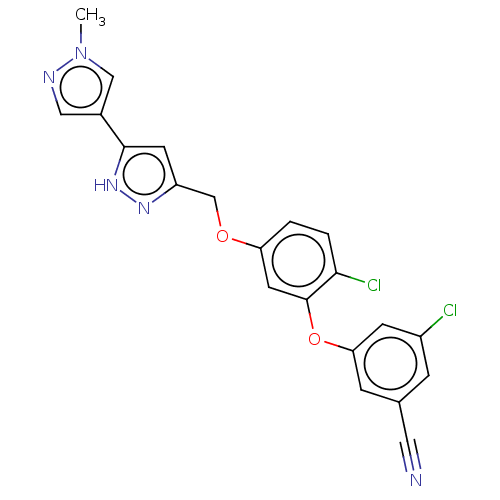 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity determined in radioreceptor binding assay by using [3H]ketanserin radioligand against 5-hydroxytryptamine 2 receptor | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484032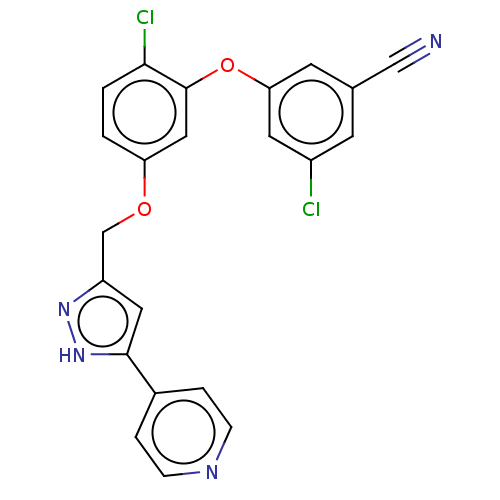 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001869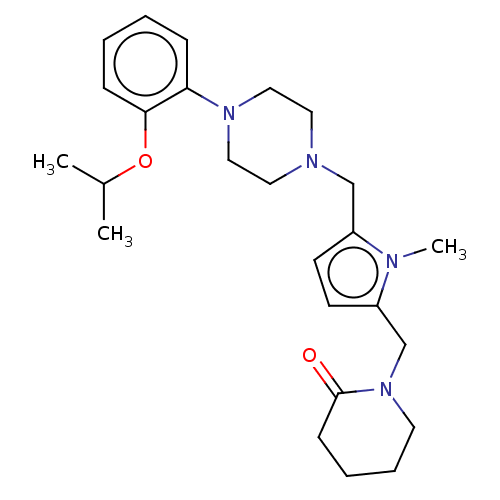 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50443217 (CHEMBL3087669) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]-methylhistamine from rat histamine H3 receptor (445 amino acid residues) transfected in human 293 cells after 1 hr by scintillat... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484022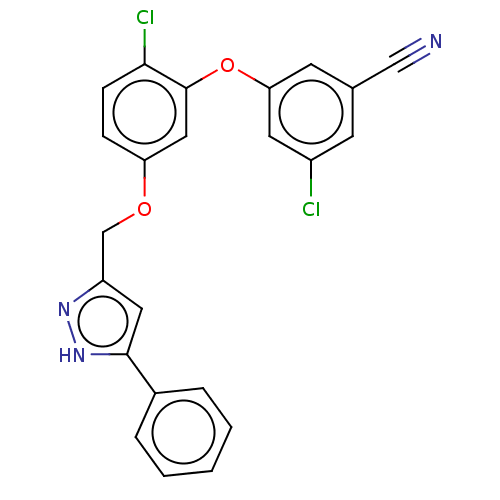 (CHEMBL1801223) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50288597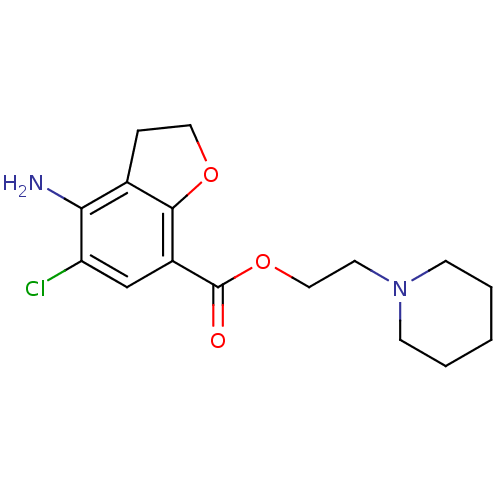 (4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 4 receptor in rat striatum by [3H]GR-113808 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484629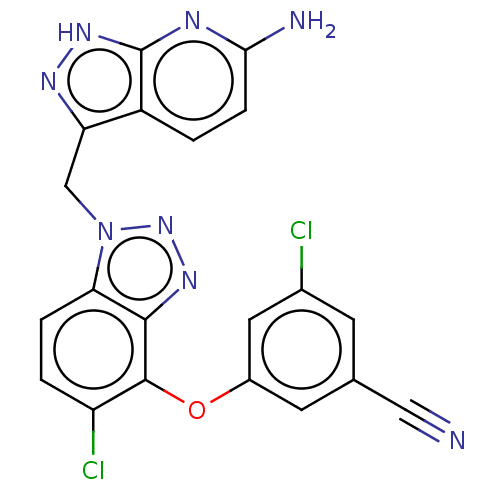 (CHEMBL1939503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50161758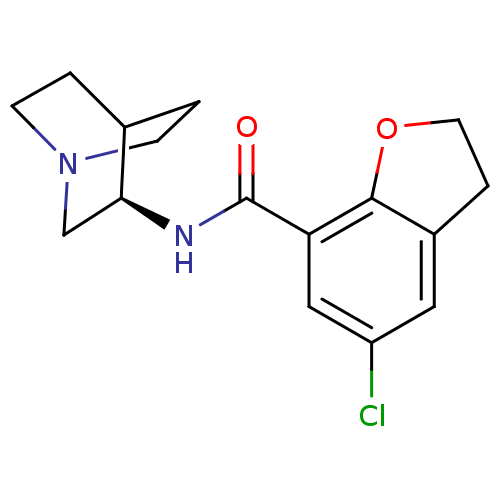 (2-(5-chloro-2,3-dihydrobenzo[b]furan-7-ylcarboxami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 4 receptor in rat striatum by [3H]GR-113808 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288581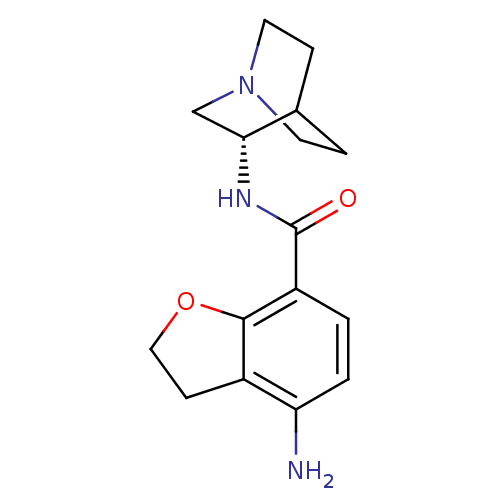 (2-(4-amino-2,3-dihydrobenzo[b]furan-7-ylcarboxamid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 4 receptor in rat striatum by [3H]GR-113808 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288583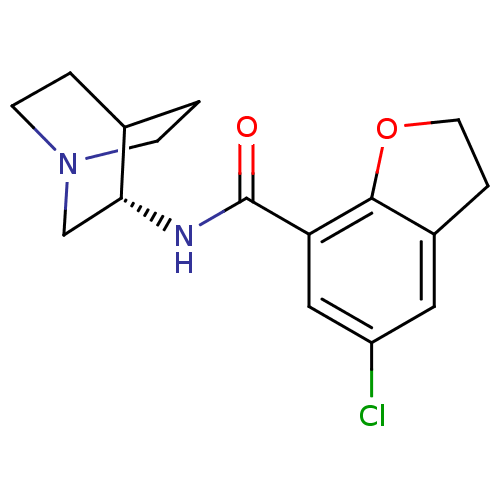 (2-(5-chloro-2,3-dihydrobenzo[b]furan-7-ylcarboxami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro relaxation of carbachol pre-contracted rat oesophageal TMM. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288586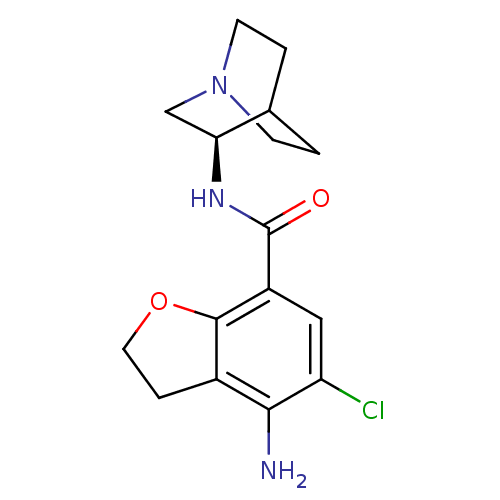 (2-(4-amino-5-chloro-2,3-dihydrobenzo[b]furan-7-ylc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288589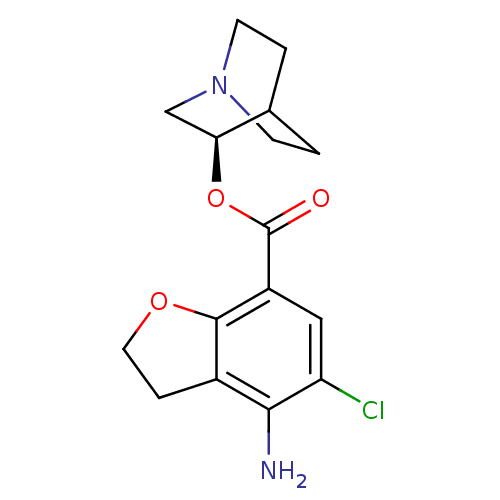 (4-Amino-5-chloro-2,3-dihydro-benzofuran-7-carboxyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001889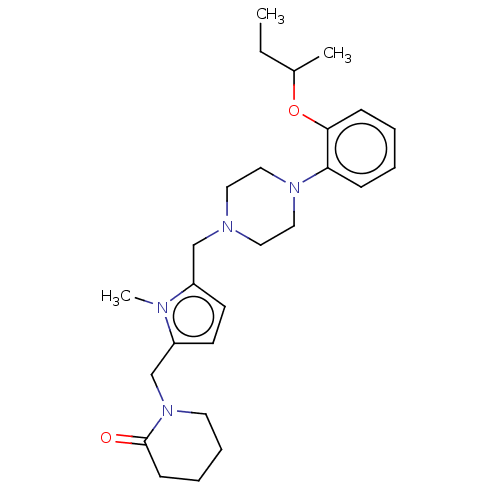 (1-{5-[4-(2-sec-Butoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484023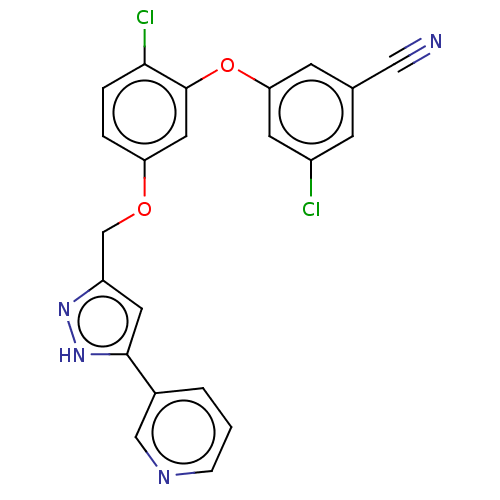 (CHEMBL1801230) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443216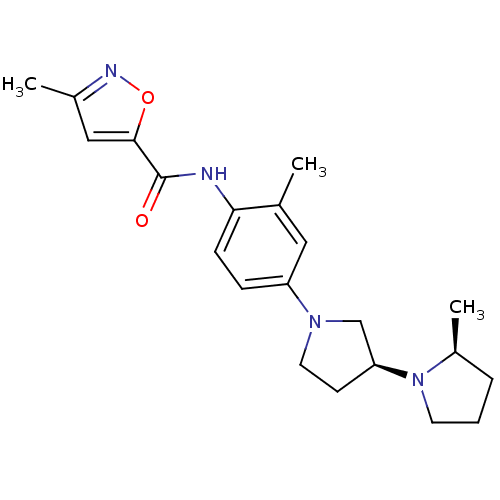 (CHEMBL3087667) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001858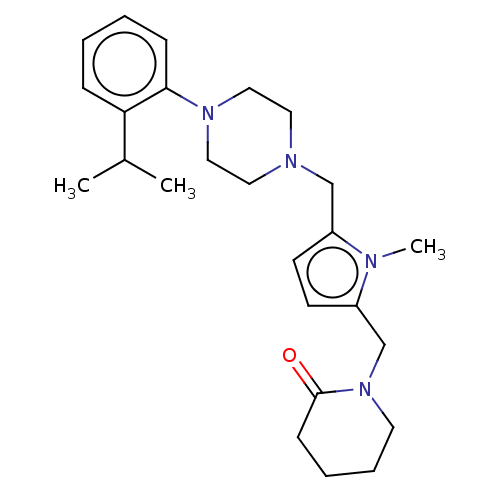 (1-{5-[4-(2-Isopropyl-phenyl)-piperazin-1-ylmethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards dopamine receptor D2 by displacing [3H]spiperone radioligand in rat striatum | J Med Chem 35: 552-8 (1992) BindingDB Entry DOI: 10.7270/Q2PR7WMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50288591 (2-(2,3-dihydrobenzo[b]furan-7-ylcarboxamido)-(2S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity at 5-hydroxytryptamine 3 receptor in rat entorhinal cortex by [3H]BRL-43694 displacement. | Bioorg Med Chem Lett 6: 263-266 (1996) Article DOI: 10.1016/0960-894X(96)00002-9 BindingDB Entry DOI: 10.7270/Q2MC90JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50443227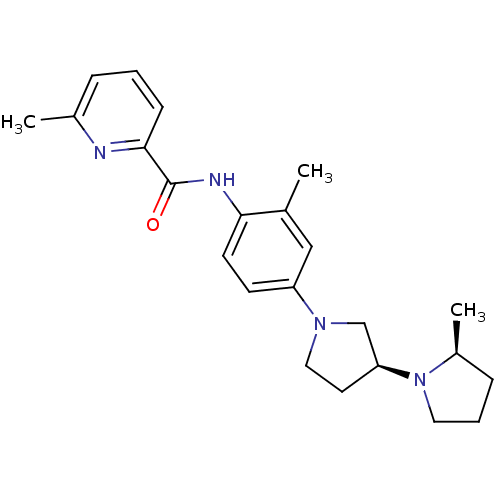 (CHEMBL3087356) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from recombinant rhesus monkey histamine H3 receptor transfected in CHO cells after 1 hr by scintillation... | Bioorg Med Chem Lett 23: 6269-73 (2013) Article DOI: 10.1016/j.bmcl.2013.09.081 BindingDB Entry DOI: 10.7270/Q27D2WK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484495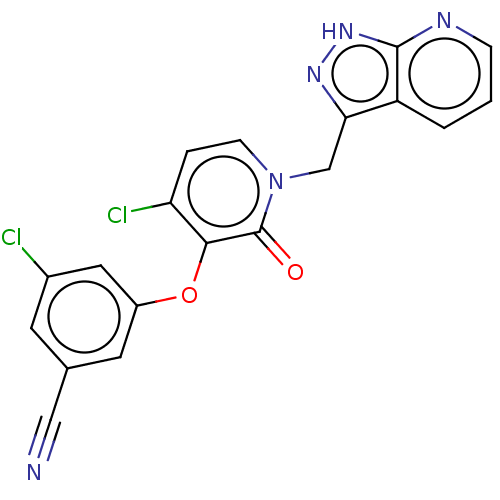 (CHEMBL1928645) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2069 total ) | Next | Last >> |