

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311944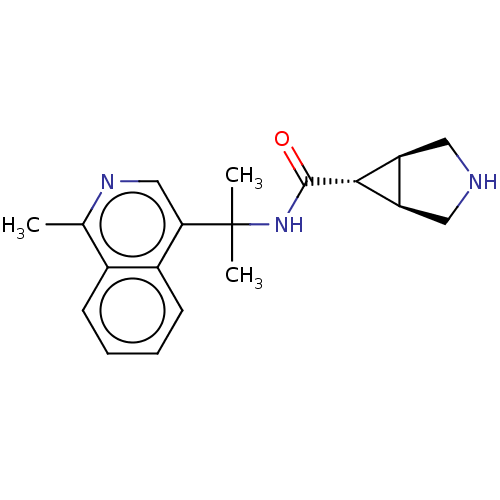 (US10166214, Example 80 | US10675268, Example 80 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311944 (US10166214, Example 80 | US10675268, Example 80 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311944 (US10166214, Example 80 | US10675268, Example 80 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311888 (US10166214, Example 24 | US10675268, Example 24 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311888 (US10166214, Example 24 | US10675268, Example 24 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311888 (US10166214, Example 24 | US10675268, Example 24 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311990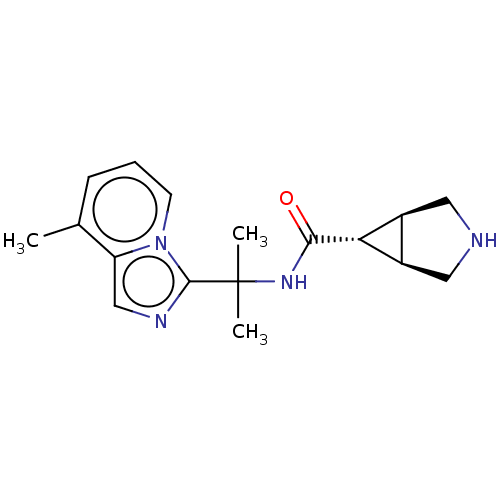 (US10166214, Example 126 | US10675268, Example 126 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311990 (US10166214, Example 126 | US10675268, Example 126 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311990 (US10166214, Example 126 | US10675268, Example 126 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311971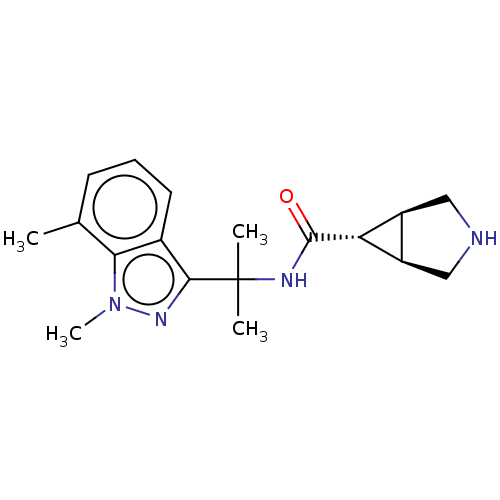 (US10166214, Example 107 | US10675268, Example 107 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311971 (US10166214, Example 107 | US10675268, Example 107 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311971 (US10166214, Example 107 | US10675268, Example 107 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311957 (US10166214, Example 93 | US10675268, Example 93 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311957 (US10166214, Example 93 | US10675268, Example 93 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311957 (US10166214, Example 93 | US10675268, Example 93 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50081058 (CHEMBL3421842 | US10166214, Example 22 | US1067526...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50081058 (CHEMBL3421842 | US10166214, Example 22 | US1067526...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50081058 (CHEMBL3421842 | US10166214, Example 22 | US1067526...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50081055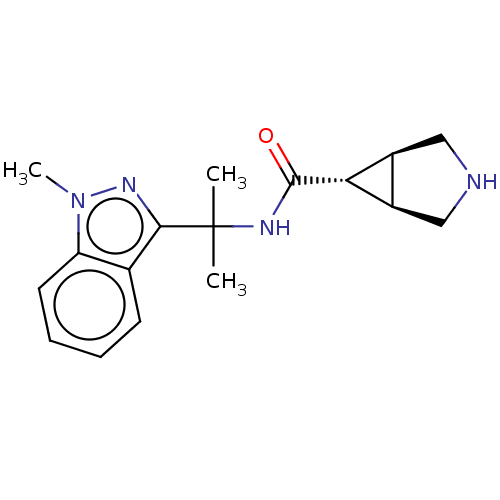 (CHEMBL3421843 | US10166214, Example 49 | US1067526...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50081055 (CHEMBL3421843 | US10166214, Example 49 | US1067526...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50081055 (CHEMBL3421843 | US10166214, Example 49 | US1067526...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311992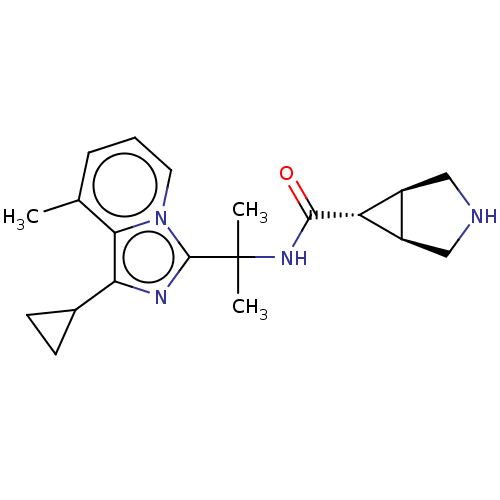 (US10166214, Example 128 | US10675268, Example 128 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311992 (US10166214, Example 128 | US10675268, Example 128 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311992 (US10166214, Example 128 | US10675268, Example 128 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311885 (US10166214, Example 21 | US10675268, Example 21 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311885 (US10166214, Example 21 | US10675268, Example 21 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311885 (US10166214, Example 21 | US10675268, Example 21 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490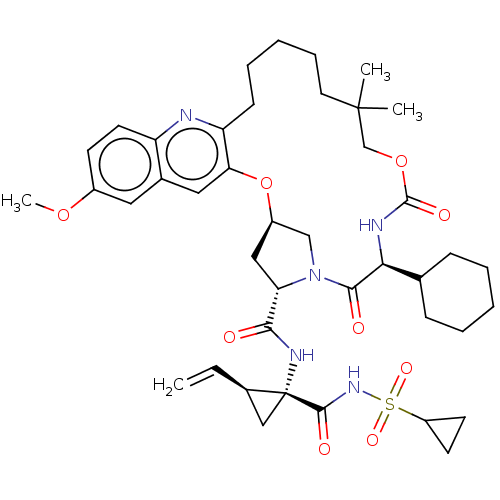 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311958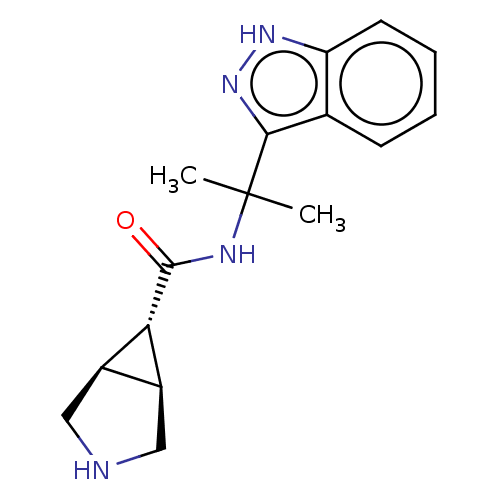 (US10166214, Example 94 | US10675268, Example 94 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311958 (US10166214, Example 94 | US10675268, Example 94 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311958 (US10166214, Example 94 | US10675268, Example 94 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM322581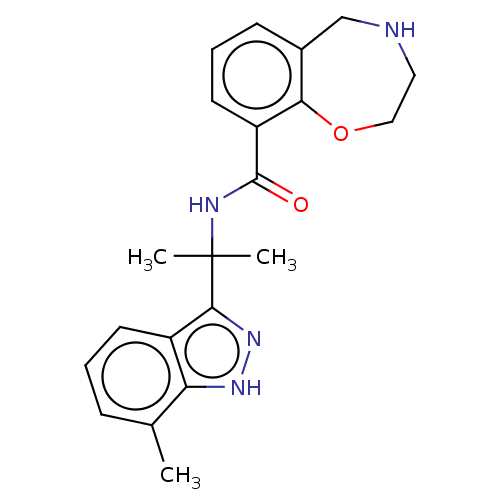 (US10183940, Example 33) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Method description for binding assays with human Somatostatin receptors by use of CHO cell membranes expressing recombinant human SSTR1 or human SSTR... | US Patent US10183940 (2019) BindingDB Entry DOI: 10.7270/Q2KW5J45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493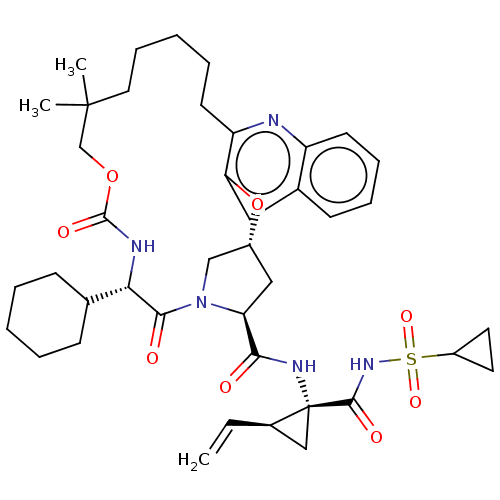 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM322587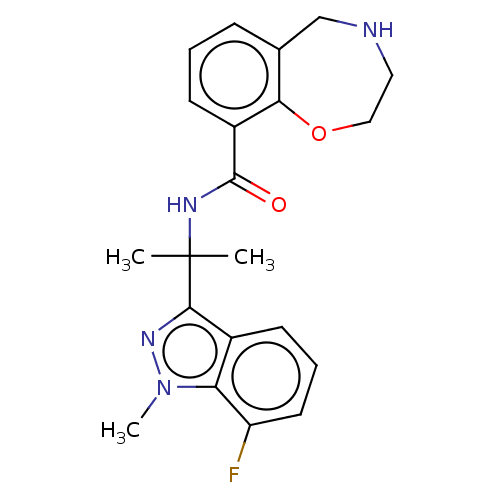 (US10183940, Example 39) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 29.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Method description for binding assays with human Somatostatin receptors by use of CHO cell membranes expressing recombinant human SSTR1 or human SSTR... | US Patent US10183940 (2019) BindingDB Entry DOI: 10.7270/Q2KW5J45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM312012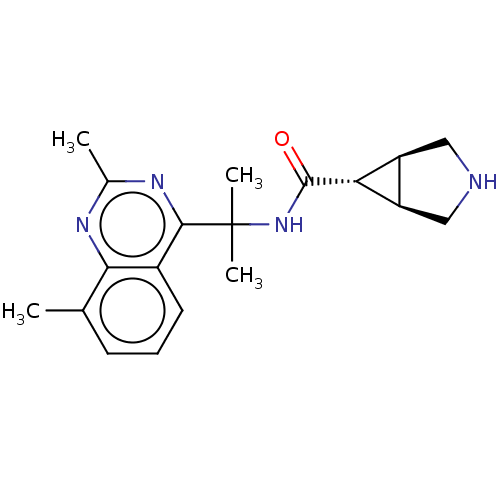 (US10166214, Example 148 | US10675268, Example 148 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10166214 (2019) BindingDB Entry DOI: 10.7270/Q2D50Q16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM312012 (US10166214, Example 148 | US10675268, Example 148 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM312012 (US10166214, Example 148 | US10675268, Example 148 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10675268 (2020) BindingDB Entry DOI: 10.7270/Q21C20XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM311904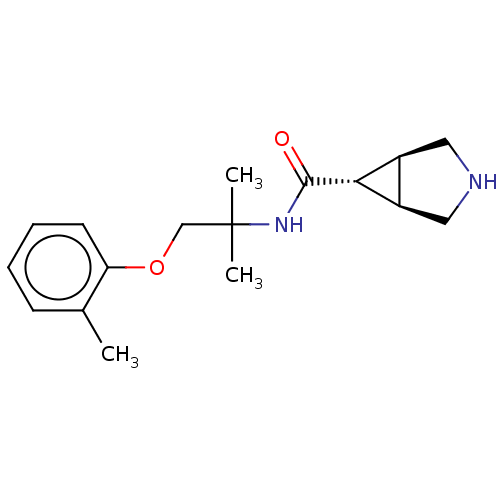 (US10166214, Example 40 | US10675268, Example 40 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US9789082 (2017) BindingDB Entry DOI: 10.7270/Q2CN760Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2287 total ) | Next | Last >> |