

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human Angiotensin receptor 1 | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human Angiotensin receptor 2 | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382321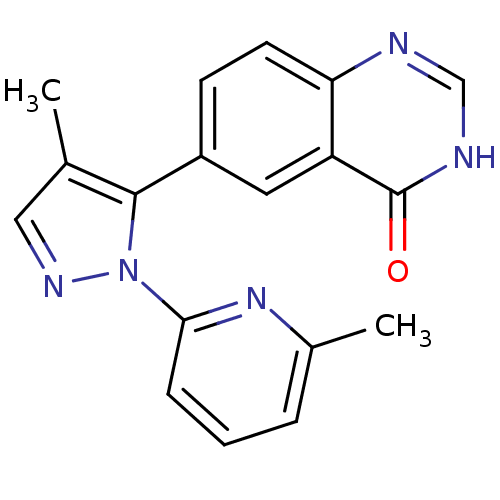 (CHEMBL2024688) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382322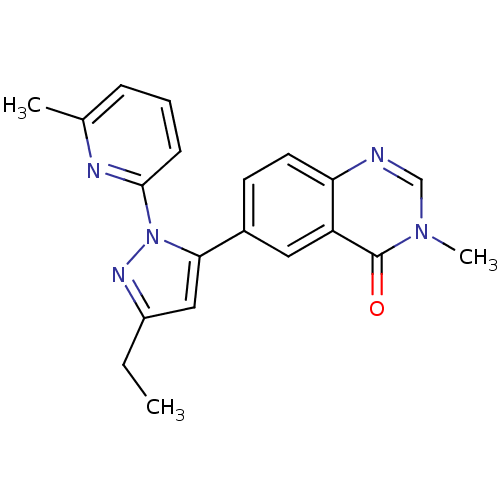 (CHEMBL2024689) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50212698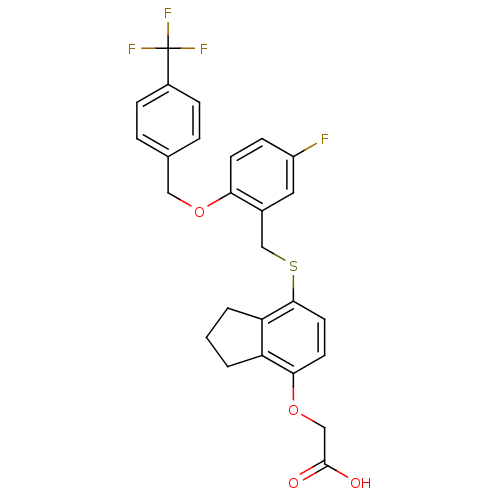 (2-(7-(2-(4-(trifluoromethyl)benzyloxy)-5-fluoroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assay | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50212693 (2-(4-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assay | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382324 (CHEMBL2024691) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661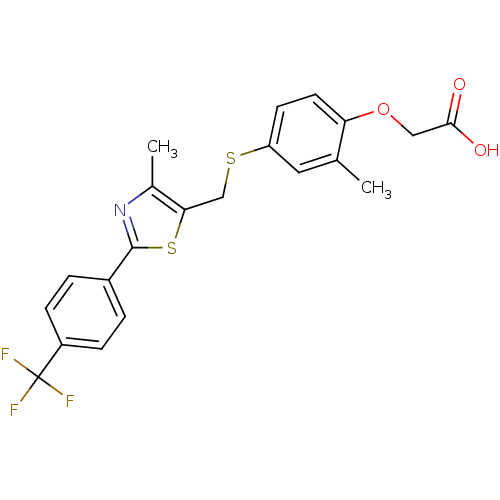 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50009718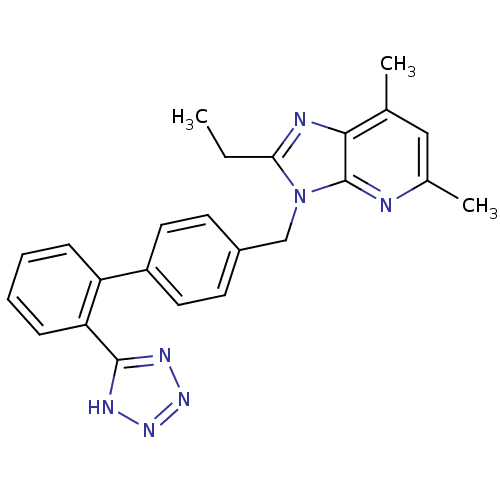 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213331 (2-(5-((4-methyl-2-(trifluoromethyl)thiazol-5-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382320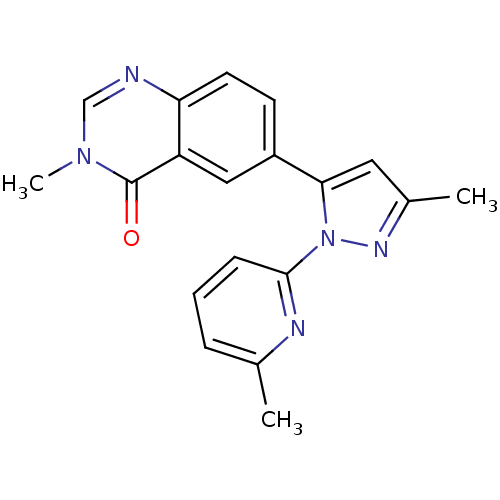 (CHEMBL2024687) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213326 (2-(6-((4-methyl-2-(4-(trifluoromethyl)phenyl)thiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347564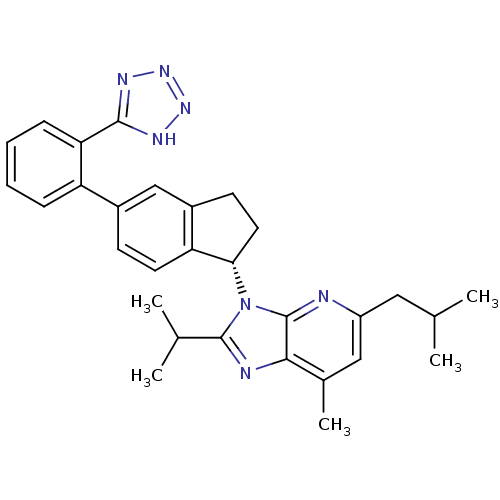 (CHEMBL1801741) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213330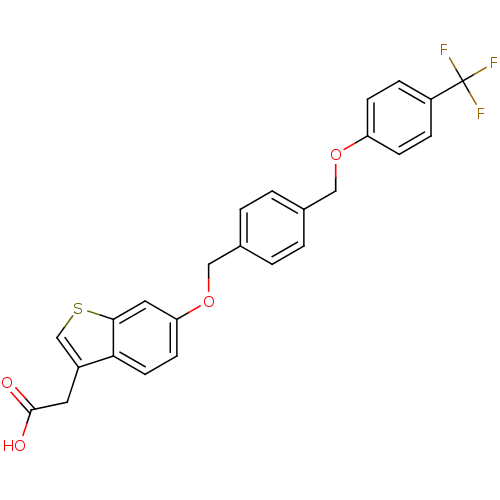 (2-(6-(4-((4-(trifluoromethyl)phenoxy)methyl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382318 (CHEMBL2024685) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382319 (CHEMBL2024686) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50212693 (2-(4-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(2-(3-(2,4-difluorophenyl)-1-heptylureido)ethyl)phenoxy)-2-methylbutanoic acid from human PPARalpha after 30 mins by SPA ass... | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382325 (CHEMBL2024693) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347565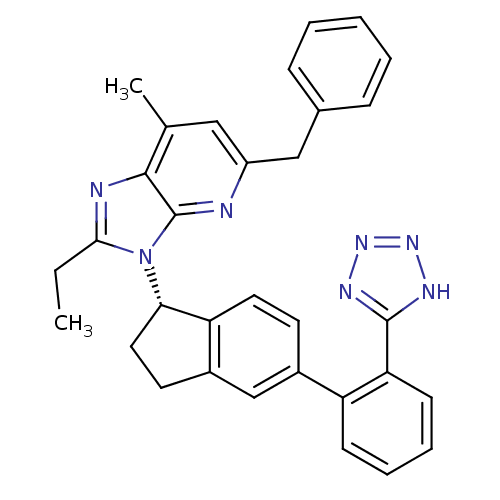 (CHEMBL1801743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382323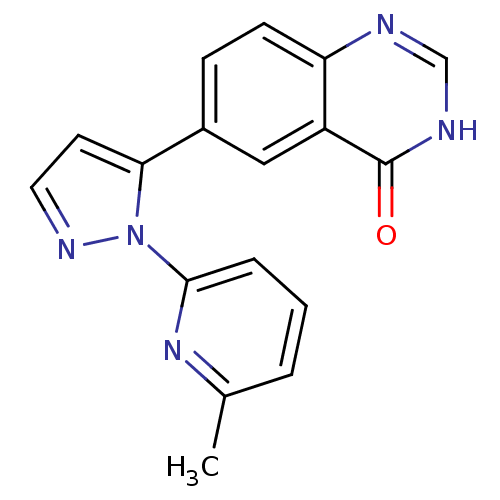 (CHEMBL2024690) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213313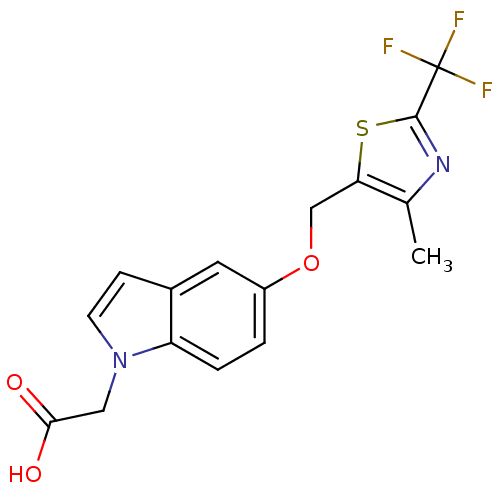 (2-(5-((4-methyl-2-(trifluoromethyl)thiazol-5-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347566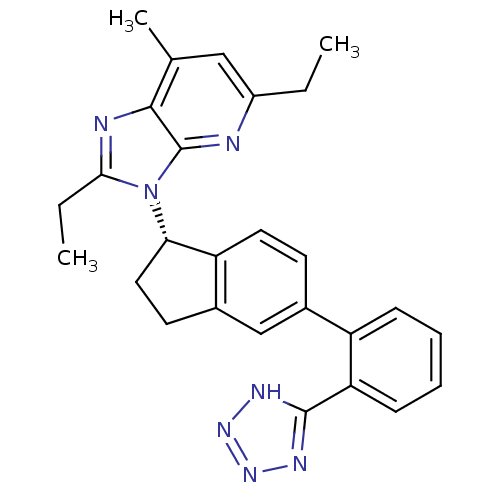 (CHEMBL1801738) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347567 (CHEMBL1801712) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213321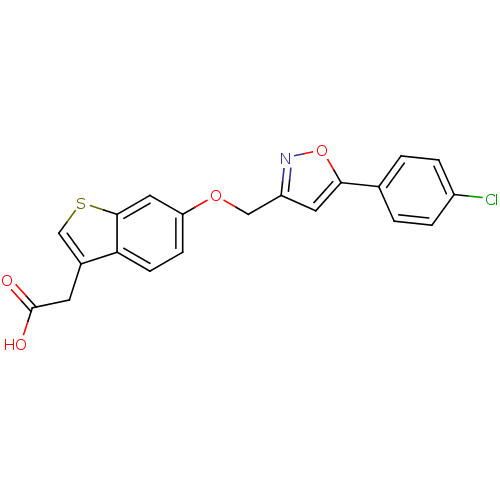 (2-(6-((5-(4-chlorophenyl)isoxazol-3-yl)methoxy)ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347568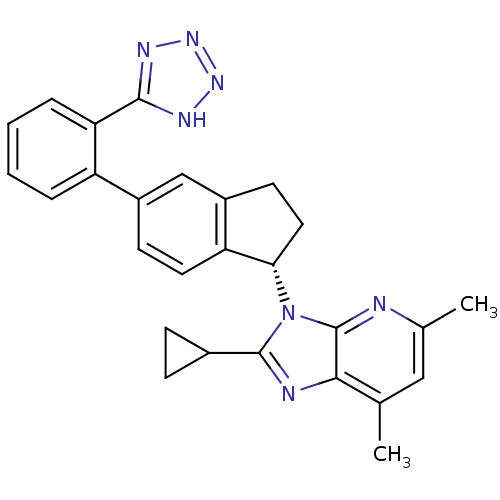 (CHEMBL1801735) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50212708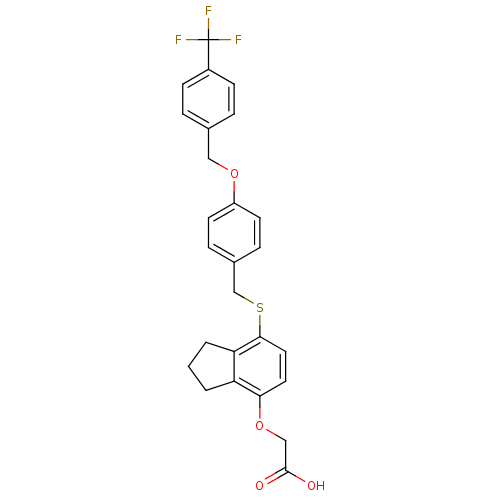 (2-(7-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assay | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347569 (CHEMBL1801734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50212696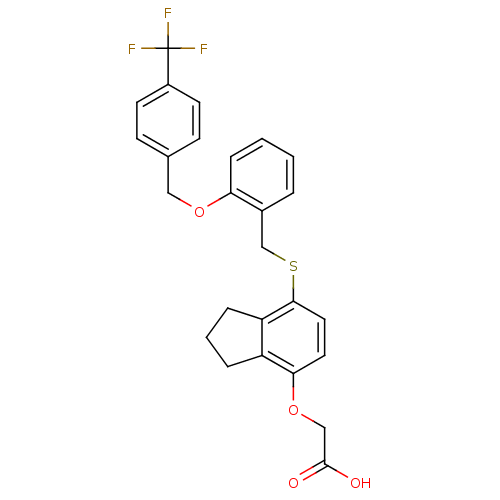 (2-(7-(2-(4-(trifluoromethyl)benzyloxy)benzylthio)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assay | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347570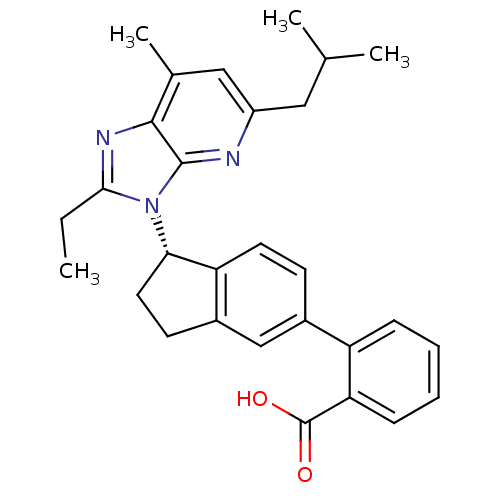 (CHEMBL1801744) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213315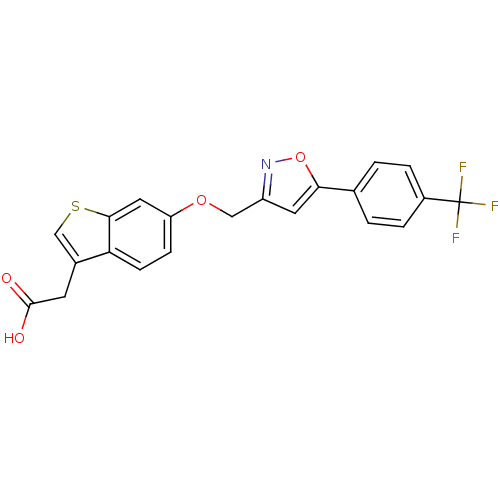 (2-(6-((5-(4-(trifluoromethyl)phenyl)isoxazol-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213316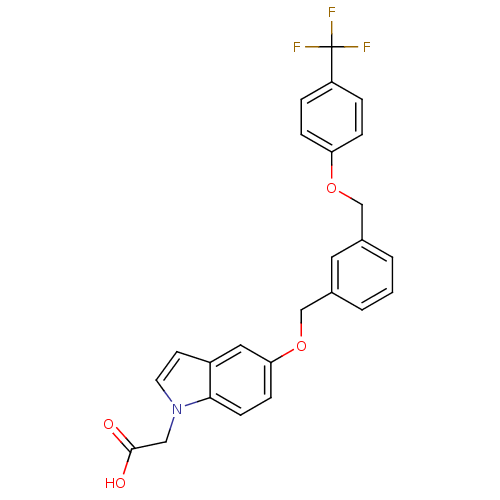 (2-(5-(3-((4-(trifluoromethyl)phenoxy)methyl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382317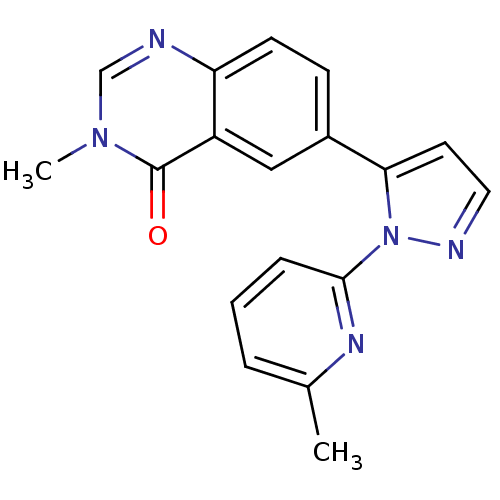 (CHEMBL2024684) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347571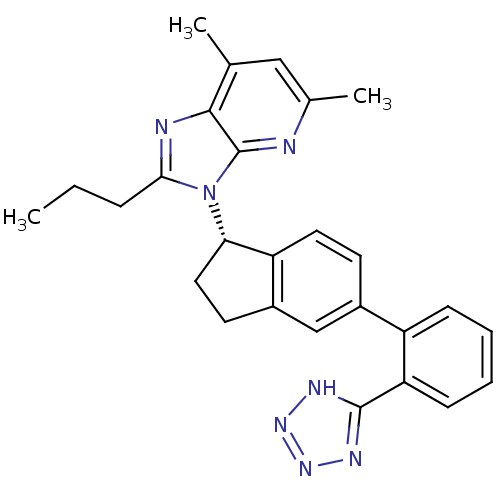 (CHEMBL1801714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213324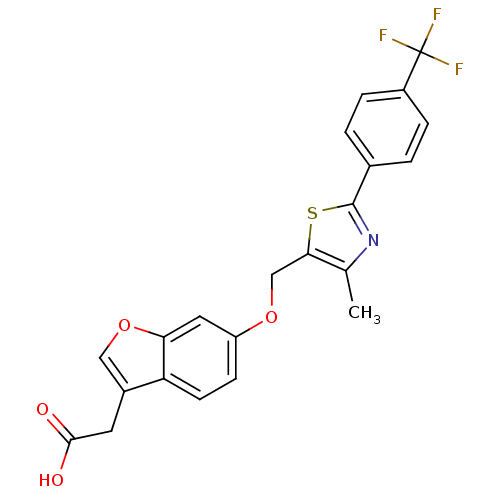 (2-(6-((4-methyl-2-(4-(trifluoromethyl)phenyl)thiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213311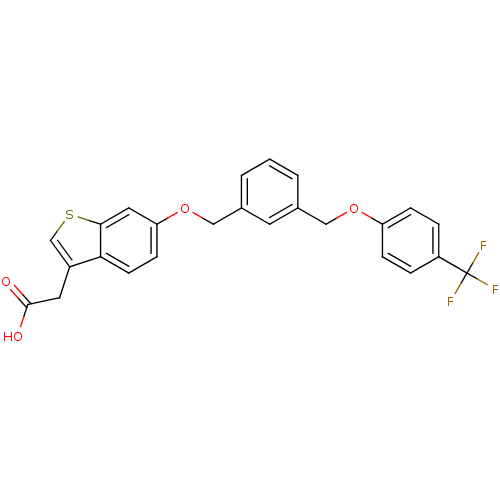 (2-(6-(3-((4-(trifluoromethyl)phenoxy)methyl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347572 (CHEMBL1801737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347573 (CHEMBL1801739) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation counting | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50212707 (2-(4-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assay | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50212701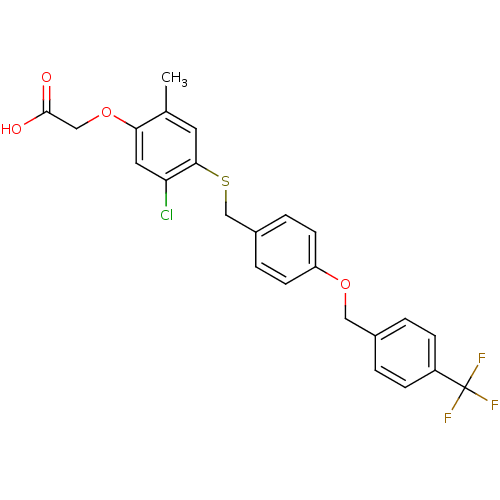 (2-(4-(4-(4-(trifluoromethyl)benzyloxy)benzylthio)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assay | Bioorg Med Chem Lett 17: 3624-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.046 BindingDB Entry DOI: 10.7270/Q2S1826N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213309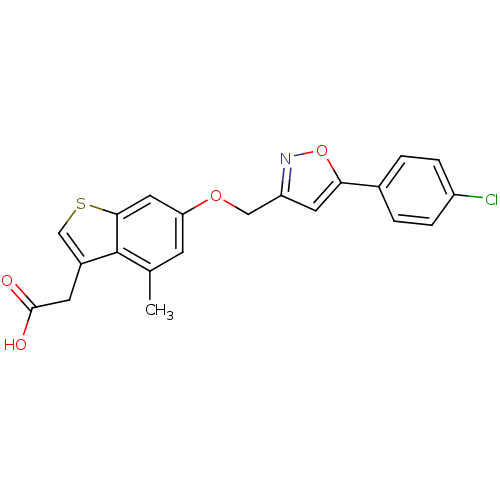 (2-(6-((5-(4-chlorophenyl)isoxazol-3-yl)methoxy)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213317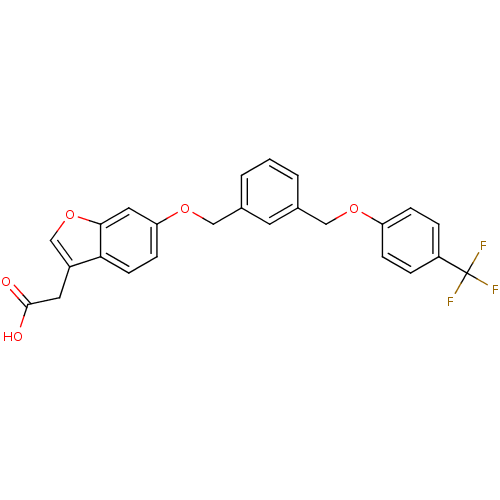 (2-(6-(3-((4-(trifluoromethyl)phenoxy)methyl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213325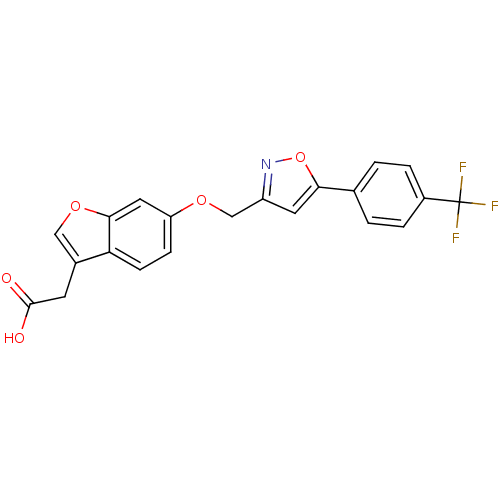 (2-(6-((5-(4-(trifluoromethyl)phenyl)isoxazol-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213320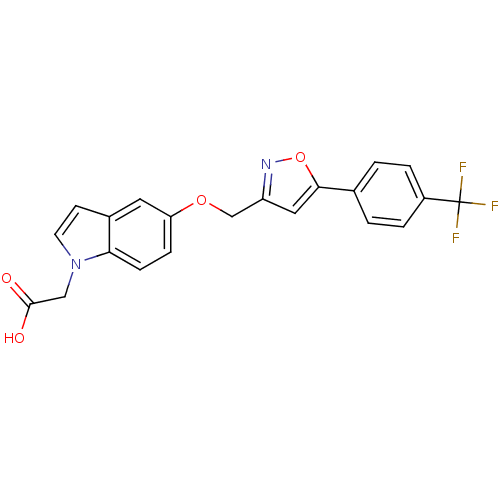 (2-(5-((5-(4-(trifluoromethyl)phenyl)isoxazol-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213318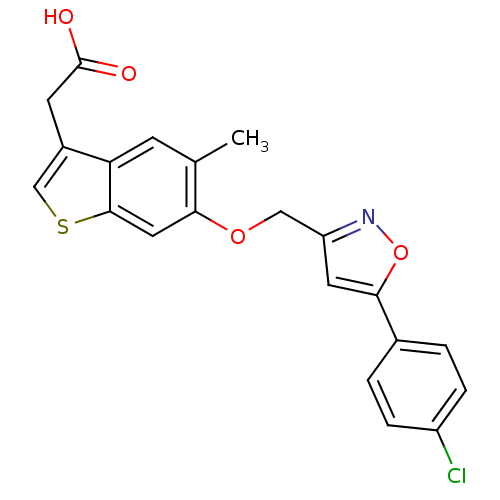 (2-(6-((5-(4-chlorophenyl)isoxazol-3-yl)methoxy)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213328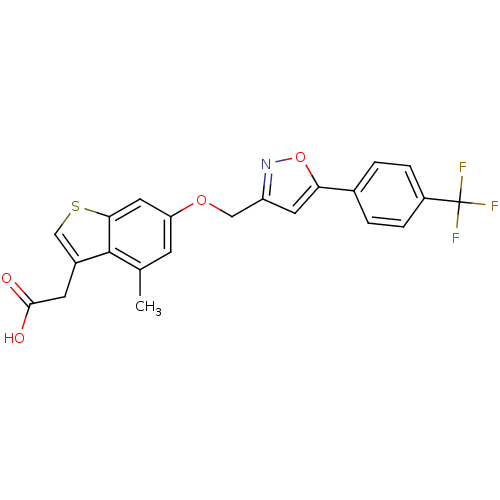 (2-(4-methyl-6-((5-(4-(trifluoromethyl)phenyl)isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213310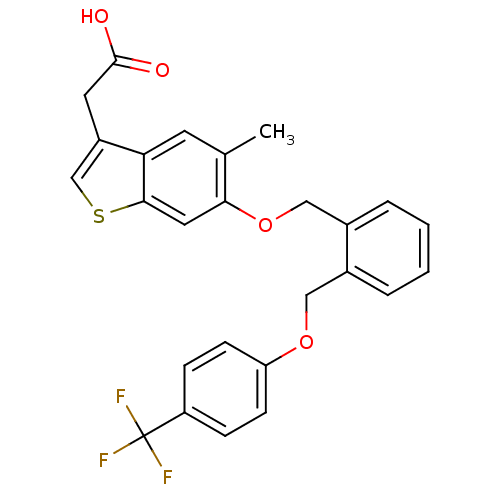 (2-(6-(2-((4-(trifluoromethyl)phenoxy)methyl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Mus musculus) | BDBM50382318 (CHEMBL2024685) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Mus musculus) | BDBM50382325 (CHEMBL2024693) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 231 total ) | Next | Last >> |