 Found 32 hits with Last Name = 'fisher' and Initial = 'jf'
Found 32 hits with Last Name = 'fisher' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82122

(Vinylsulfone, 7)Show InChI InChI=1S/C15H14O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-10,12,19H,11H2/b12-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368890

(CHEMBL1790792)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1SCCCCC[C@@H]1SCC2NC(=O)NC12)C(=O)NCCc1scnc1C Show InChI InChI=1S/C44H68N6O6S3/c1-6-27(4)37(43(55)45-21-20-33-28(5)46-25-59-33)49-42(54)36(26(2)3)40(52)39(51)31(23-29-15-9-7-10-16-29)47-41(53)30-17-12-13-18-34(30)57-22-14-8-11-19-35-38-32(24-58-35)48-44(56)50-38/h12-13,17-18,25-27,29,31-32,35-40,51-52H,6-11,14-16,19-24H2,1-5H3,(H,45,55)(H,47,53)(H,49,54)(H2,48,50,56)/t27-,31+,32?,35+,36-,37+,38?,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368891

(CHEMBL1790796)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1OCCCCC[C@@H]1SC[C@H]2NC(=O)N[C@@H]12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C44H66N6O7S/c1-5-28(4)37(43(55)46-25-30-18-13-14-22-45-30)49-42(54)36(27(2)3)40(52)39(51)32(24-29-16-8-6-9-17-29)47-41(53)31-19-11-12-20-34(31)57-23-15-7-10-21-35-38-33(26-58-35)48-44(56)50-38/h11-14,18-20,22,27-29,32-33,35-40,51-52H,5-10,15-17,21,23-26H2,1-4H3,(H,46,55)(H,47,53)(H,49,54)(H2,48,50,56)/t28-,32+,33-,35+,36-,37+,38-,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368893
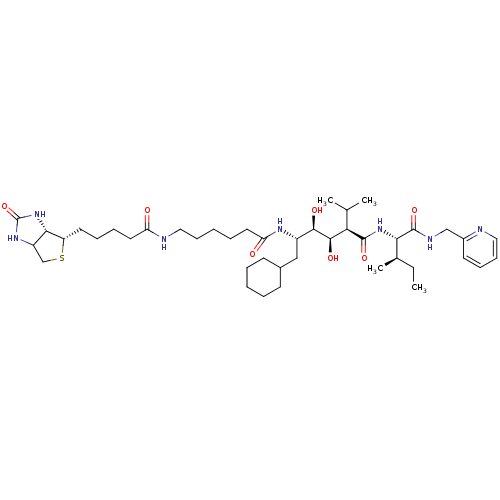
(CHEMBL1790794)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCCCCNC(=O)CCCC[C@@H]1SCC2NC(=O)N[C@H]12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C43H71N7O7S/c1-5-28(4)37(42(56)46-25-30-18-13-15-22-44-30)49-41(55)36(27(2)3)40(54)39(53)31(24-29-16-8-6-9-17-29)47-35(52)21-10-7-14-23-45-34(51)20-12-11-19-33-38-32(26-58-33)48-43(57)50-38/h13,15,18,22,27-29,31-33,36-40,53-54H,5-12,14,16-17,19-21,23-26H2,1-4H3,(H,45,51)(H,46,56)(H,47,52)(H,49,55)(H2,48,50,57)/t28-,31+,32?,33+,36-,37+,38+,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368889
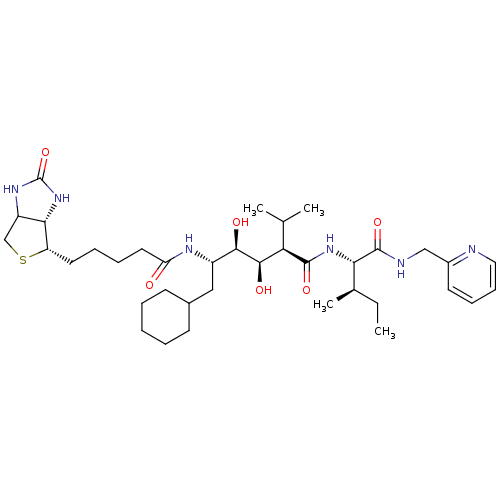
(CHEMBL1790790)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCCC[C@@H]1SCC2NC(=O)N[C@H]12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C37H60N6O6S/c1-5-23(4)31(36(48)39-20-25-15-11-12-18-38-25)42-35(47)30(22(2)3)34(46)33(45)26(19-24-13-7-6-8-14-24)40-29(44)17-10-9-16-28-32-27(21-50-28)41-37(49)43-32/h11-12,15,18,22-24,26-28,30-34,45-46H,5-10,13-14,16-17,19-21H2,1-4H3,(H,39,48)(H,40,44)(H,42,47)(H2,41,43,49)/t23-,26+,27?,28+,30-,31+,32+,33-,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368892

(CHEMBL1790797)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1SCCCCC[C@@H]1SCC2NC(=O)NC12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C44H66N6O6S2/c1-5-28(4)37(43(55)46-25-30-18-13-14-22-45-30)49-42(54)36(27(2)3)40(52)39(51)32(24-29-16-8-6-9-17-29)47-41(53)31-19-11-12-20-34(31)57-23-15-7-10-21-35-38-33(26-58-35)48-44(56)50-38/h11-14,18-20,22,27-29,32-33,35-40,51-52H,5-10,15-17,21,23-26H2,1-4H3,(H,46,55)(H,47,53)(H,49,54)(H2,48,50,56)/t28-,32+,33?,35+,36-,37+,38?,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368887
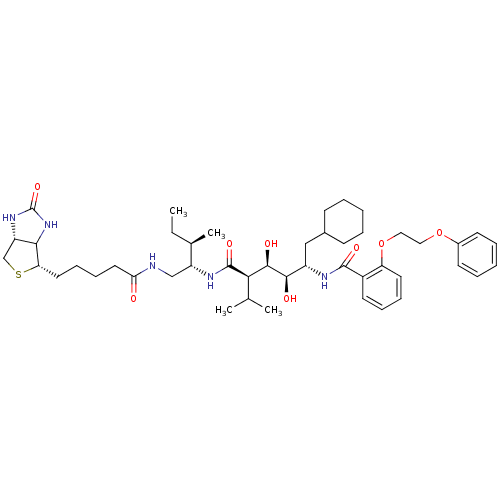
(CHEMBL1790791)Show SMILES CC[C@@H](C)[C@@H](CNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)NC12)NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1OCCOc1ccccc1 Show InChI InChI=1S/C46H69N5O8S/c1-5-30(4)35(27-47-39(52)23-15-14-22-38-41-36(28-60-38)50-46(57)51-41)49-45(56)40(29(2)3)43(54)42(53)34(26-31-16-8-6-9-17-31)48-44(55)33-20-12-13-21-37(33)59-25-24-58-32-18-10-7-11-19-32/h7,10-13,18-21,29-31,34-36,38,40-43,53-54H,5-6,8-9,14-17,22-28H2,1-4H3,(H,47,52)(H,48,55)(H,49,56)(H2,50,51,57)/t30-,34+,35-,36+,38+,40-,41?,42-,43-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368888
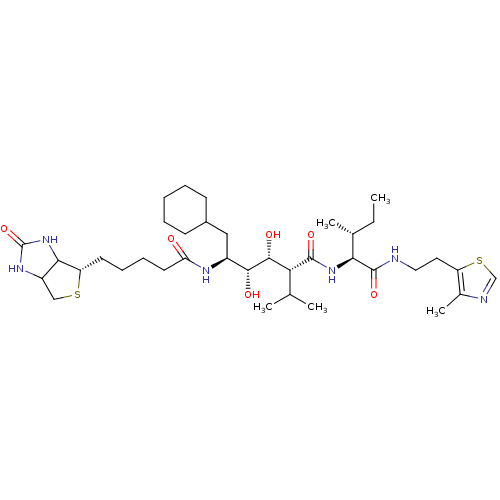
(CHEMBL1790793)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)CCCC[C@@H]1SCC2NC(=O)NC12)C(=O)NCCc1scnc1C Show InChI InChI=1S/C37H62N6O6S2/c1-6-22(4)31(36(48)38-17-16-27-23(5)39-20-51-27)42-35(47)30(21(2)3)34(46)33(45)25(18-24-12-8-7-9-13-24)40-29(44)15-11-10-14-28-32-26(19-50-28)41-37(49)43-32/h20-22,24-26,28,30-34,45-46H,6-19H2,1-5H3,(H,38,48)(H,40,44)(H,42,47)(H2,41,43,49)/t22-,25+,26?,28+,30-,31+,32?,33-,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Pseudomonas aeruginosa) | BDBM50386412
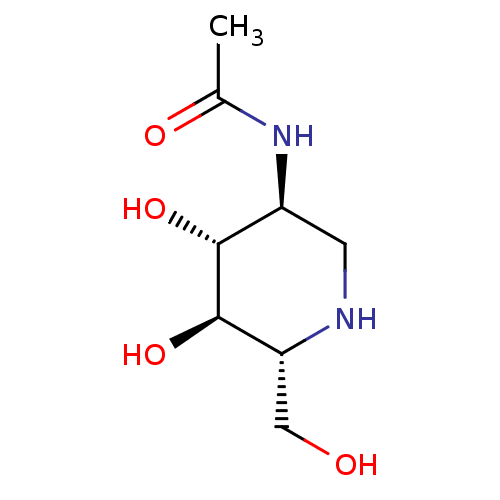
(CHEMBL2047302)Show SMILES CC(=O)N[C@H]1CN[C@H](CO)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H16N2O4/c1-4(12)10-5-2-9-6(3-11)8(14)7(5)13/h5-9,11,13-14H,2-3H2,1H3,(H,10,12)/t5-,6+,7+,8+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pseudomonas aeruginosa PAO1 NagZ using PNP-GlcNAc as substrate assessed as release of 4-nitrophenolate ion after 4 mins by ... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82121

(Gama, gama-dimethyl derivative, 6)Show InChI InChI=1S/C17H18O3S2/c1-17(2)16(21-17)12-22(18,19)15-10-8-14(9-11-15)20-13-6-4-3-5-7-13/h3-11,16H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82119

(Sulfoxide, 4)Show InChI InChI=1S/C15H14O2S2/c16-19(11-14-10-18-14)15-8-6-13(7-9-15)17-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Pseudomonas aeruginosa) | BDBM50386415
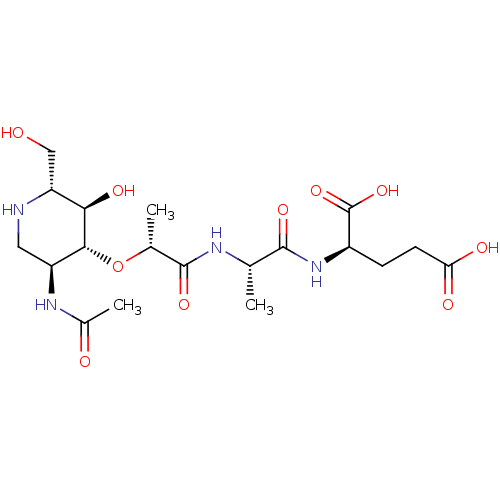
(CHEMBL2047305)Show SMILES C[C@H](NC(=O)[C@@H](C)O[C@@H]1[C@H](CN[C@H](CO)[C@H]1O)NC(C)=O)C(=O)N[C@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H32N4O10/c1-8(17(29)23-11(19(31)32)4-5-14(26)27)21-18(30)9(2)33-16-12(22-10(3)25)6-20-13(7-24)15(16)28/h8-9,11-13,15-16,20,24,28H,4-7H2,1-3H3,(H,21,30)(H,22,25)(H,23,29)(H,26,27)(H,31,32)/t8-,9+,11+,12-,13+,15+,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pseudomonas aeruginosa PAO1 NagZ using PNP-GlcNAc as substrate assessed as release of 4-nitrophenolate ion after 4 mins by ... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Pseudomonas aeruginosa) | BDBM50386414

(CHEMBL2047304)Show SMILES C[C@H](NC(=O)[C@@H](C)O[C@@H]1[C@H](CN[C@H](CO)[C@H]1O)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C14H25N3O7/c1-6(14(22)23)16-13(21)7(2)24-12-9(17-8(3)19)4-15-10(5-18)11(12)20/h6-7,9-12,15,18,20H,4-5H2,1-3H3,(H,16,21)(H,17,19)(H,22,23)/t6-,7+,9-,10+,11+,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pseudomonas aeruginosa PAO1 NagZ using PNP-GlcNAc as substrate assessed as release of 4-nitrophenolate ion after 4 mins by ... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82120

(Alpha, alpha-dimethyl derivative, 5)Show InChI InChI=1S/C17H18O3S2/c1-17(2,16-12-21-16)22(18,19)15-10-8-14(9-11-15)20-13-6-4-3-5-7-13/h3-11,16H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase
(Pseudomonas aeruginosa) | BDBM50386413

(CHEMBL2047303)Show SMILES C[C@@H](O[C@@H]1[C@H](CN[C@H](CO)[C@H]1O)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C11H20N2O6/c1-5(11(17)18)19-10-7(13-6(2)15)3-12-8(4-14)9(10)16/h5,7-10,12,14,16H,3-4H2,1-2H3,(H,13,15)(H,17,18)/t5-,7+,8-,9-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pseudomonas aeruginosa PAO1 NagZ using PNP-GlcNAc as substrate assessed as release of 4-nitrophenolate ion after 4 mins by ... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 2
(Staphylococcus aureus) | BDBM50482776
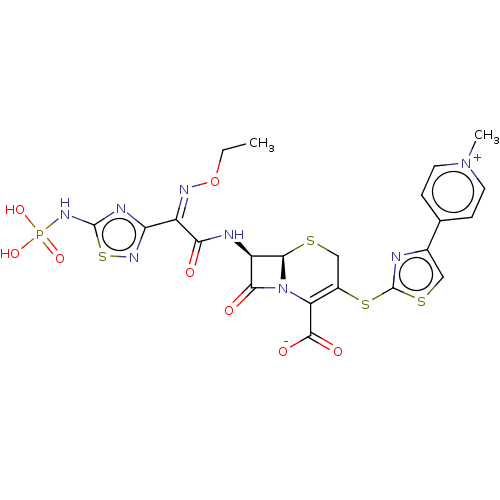
(CHEBI:70718 | Ceftaroline Fosamil | PPI-0903 | TAK...)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(NP(O)(O)=O)n1)C([O-])=O |r,c:19| Show InChI InChI=1S/C22H21N8O8PS4/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37)/b26-13-/t14-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Binding affinity to methicillin-susceptible Staphylococcus aureus PBP 2 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein, transpeptidase domain protein
(Staphylococcus aureus) | BDBM50482776

(CHEBI:70718 | Ceftaroline Fosamil | PPI-0903 | TAK...)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(NP(O)(O)=O)n1)C([O-])=O |r,c:19| Show InChI InChI=1S/C22H21N8O8PS4/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37)/b26-13-/t14-,19-/m1/s1 | PDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Binding affinity to methicillin-susceptible Staphylococcus aureus PBP 3 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein, transpeptidase domain protein
(Staphylococcus aureus) | BDBM50483327
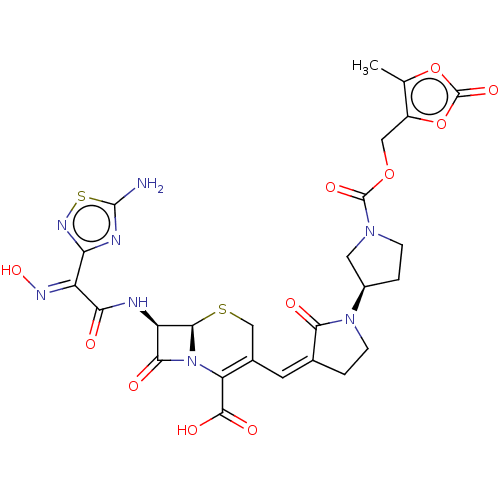
(CEFTOBIPROLE MEDOCARIL)Show SMILES [H][C@]12SCC(\C=C3/CCN([C@@H]4CCN(C4)C(=O)OCc4oc(=O)oc4C)C3=O)=C(N1C(=O)[C@H]2NC(=O)C(=N\O)\c1nsc(N)n1)C(O)=O |r,c:30| Show InChI InChI=1S/C26H26N8O11S2/c1-10-14(45-26(41)44-10)8-43-25(40)32-4-3-13(7-32)33-5-2-11(20(33)36)6-12-9-46-22-16(21(37)34(22)17(12)23(38)39)28-19(35)15(30-42)18-29-24(27)47-31-18/h6,13,16,22,42H,2-5,7-9H2,1H3,(H,28,35)(H,38,39)(H2,27,29,31)/b11-6+,30-15+/t13-,16-,22-/m1/s1 | PDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of methicillin-susceptible Staphylococcus aureus ATCC 29213 PBP 3 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Putative penicillin-binding protein 2B
(Staphylococcus aureus) | BDBM50483327

(CEFTOBIPROLE MEDOCARIL)Show SMILES [H][C@]12SCC(\C=C3/CCN([C@@H]4CCN(C4)C(=O)OCc4oc(=O)oc4C)C3=O)=C(N1C(=O)[C@H]2NC(=O)C(=N\O)\c1nsc(N)n1)C(O)=O |r,c:30| Show InChI InChI=1S/C26H26N8O11S2/c1-10-14(45-26(41)44-10)8-43-25(40)32-4-3-13(7-32)33-5-2-11(20(33)36)6-12-9-46-22-16(21(37)34(22)17(12)23(38)39)28-19(35)15(30-42)18-29-24(27)47-31-18/h6,13,16,22,42H,2-5,7-9H2,1H3,(H,28,35)(H,38,39)(H2,27,29,31)/b11-6+,30-15+/t13-,16-,22-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of methicillin-susceptible Staphylococcus aureus ATCC 29213 PBP 1 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Putative penicillin-binding protein 2B
(Staphylococcus aureus) | BDBM50482776

(CHEBI:70718 | Ceftaroline Fosamil | PPI-0903 | TAK...)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(NP(O)(O)=O)n1)C([O-])=O |r,c:19| Show InChI InChI=1S/C22H21N8O8PS4/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37)/b26-13-/t14-,19-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Binding affinity to methicillin-susceptible Staphylococcus aureus PBP 1 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Peptidase
(Staphylococcus aureus) | BDBM50482776

(CHEBI:70718 | Ceftaroline Fosamil | PPI-0903 | TAK...)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(NP(O)(O)=O)n1)C([O-])=O |r,c:19| Show InChI InChI=1S/C22H21N8O8PS4/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37)/b26-13-/t14-,19-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of methicillin-resistant Staphylococcus aureus PBP 2a |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Peptidase
(Staphylococcus aureus) | BDBM50483328
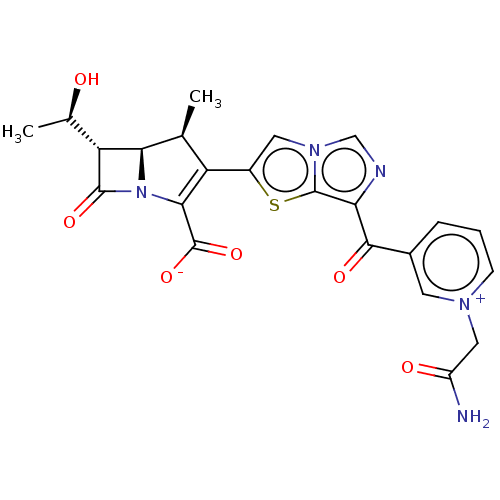
(CHEMBL387640 | ME1036)Show SMILES [H][C@]12[C@@H](C)C(=C(N1C(=O)[C@]2([H])[C@@H](C)O)C([O-])=O)c1cn2cnc(C(=O)c3ccc[n+](CC(N)=O)c3)c2s1 |c:4| Show InChI InChI=1S/C23H21N5O6S/c1-10-15(19(23(33)34)28-18(10)16(11(2)29)21(28)32)13-7-27-9-25-17(22(27)35-13)20(31)12-4-3-5-26(6-12)8-14(24)30/h3-7,9-11,16,18,29H,8H2,1-2H3,(H2-,24,30,33,34)/t10-,11+,16+,18+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Binding affinity to methicillin-resistant Staphylococcus aureus MF535HR PBP 2a by competitive binding assay |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 2
(Staphylococcus aureus) | BDBM50483327

(CEFTOBIPROLE MEDOCARIL)Show SMILES [H][C@]12SCC(\C=C3/CCN([C@@H]4CCN(C4)C(=O)OCc4oc(=O)oc4C)C3=O)=C(N1C(=O)[C@H]2NC(=O)C(=N\O)\c1nsc(N)n1)C(O)=O |r,c:30| Show InChI InChI=1S/C26H26N8O11S2/c1-10-14(45-26(41)44-10)8-43-25(40)32-4-3-13(7-32)33-5-2-11(20(33)36)6-12-9-46-22-16(21(37)34(22)17(12)23(38)39)28-19(35)15(30-42)18-29-24(27)47-31-18/h6,13,16,22,42H,2-5,7-9H2,1H3,(H,28,35)(H,38,39)(H2,27,29,31)/b11-6+,30-15+/t13-,16-,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of methicillin-susceptible Staphylococcus aureus ATCC 29213 PBP 2 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Peptidase
(Staphylococcus aureus) | BDBM50483327

(CEFTOBIPROLE MEDOCARIL)Show SMILES [H][C@]12SCC(\C=C3/CCN([C@@H]4CCN(C4)C(=O)OCc4oc(=O)oc4C)C3=O)=C(N1C(=O)[C@H]2NC(=O)C(=N\O)\c1nsc(N)n1)C(O)=O |r,c:30| Show InChI InChI=1S/C26H26N8O11S2/c1-10-14(45-26(41)44-10)8-43-25(40)32-4-3-13(7-32)33-5-2-11(20(33)36)6-12-9-46-22-16(21(37)34(22)17(12)23(38)39)28-19(35)15(30-42)18-29-24(27)47-31-18/h6,13,16,22,42H,2-5,7-9H2,1H3,(H,28,35)(H,38,39)(H2,27,29,31)/b11-6+,30-15+/t13-,16-,22-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of methicillin-resistant Staphylococcus aureus OC 3726 PBP 2a |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 4
(Staphylococcus aureus) | BDBM50483327

(CEFTOBIPROLE MEDOCARIL)Show SMILES [H][C@]12SCC(\C=C3/CCN([C@@H]4CCN(C4)C(=O)OCc4oc(=O)oc4C)C3=O)=C(N1C(=O)[C@H]2NC(=O)C(=N\O)\c1nsc(N)n1)C(O)=O |r,c:30| Show InChI InChI=1S/C26H26N8O11S2/c1-10-14(45-26(41)44-10)8-43-25(40)32-4-3-13(7-32)33-5-2-11(20(33)36)6-12-9-46-22-16(21(37)34(22)17(12)23(38)39)28-19(35)15(30-42)18-29-24(27)47-31-18/h6,13,16,22,42H,2-5,7-9H2,1H3,(H,28,35)(H,38,39)(H2,27,29,31)/b11-6+,30-15+/t13-,16-,22-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of methicillin-susceptible Staphylococcus aureus ATCC 29213 PBP 4 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Penicillin-binding protein 4
(Staphylococcus aureus) | BDBM50482776

(CHEBI:70718 | Ceftaroline Fosamil | PPI-0903 | TAK...)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(NP(O)(O)=O)n1)C([O-])=O |r,c:19| Show InChI InChI=1S/C22H21N8O8PS4/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37)/b26-13-/t14-,19-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Binding affinity to methicillin-susceptible Staphylococcus aureus PBP 4 |
Antimicrob Agents Chemother 53: 4051-63 (2009)
Article DOI: 10.1128/AAC.00084-09
BindingDB Entry DOI: 10.7270/Q2R21479 |
More data for this
Ligand-Target Pair | |
Membrane-bound lytic murein transglycosylase B
(Escherichia coli (strain K12)) | BDBM50386415

(CHEMBL2047305)Show SMILES C[C@H](NC(=O)[C@@H](C)O[C@@H]1[C@H](CN[C@H](CO)[C@H]1O)NC(C)=O)C(=O)N[C@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H32N4O10/c1-8(17(29)23-11(19(31)32)4-5-14(26)27)21-18(30)9(2)33-16-12(22-10(3)25)6-20-13(7-24)15(16)28/h8-9,11-13,15-16,20,24,28H,4-7H2,1-3H3,(H,21,30)(H,22,25)(H,23,29)(H,26,27)(H,31,32)/t8-,9+,11+,12-,13+,15+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.01E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to Escherichia coli K12 MG1655 His6x-tagged MltB lytic transglycosylase assessed as intrinsic fluorescence of tryptophan and/or tyro... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |
Membrane-bound lytic murein transglycosylase B
(Escherichia coli (strain K12)) | BDBM50386413

(CHEMBL2047303)Show SMILES C[C@@H](O[C@@H]1[C@H](CN[C@H](CO)[C@H]1O)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C11H20N2O6/c1-5(11(17)18)19-10-7(13-6(2)15)3-12-8(4-14)9(10)16/h5,7-10,12,14,16H,3-4H2,1-2H3,(H,13,15)(H,17,18)/t5-,7+,8-,9-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to Escherichia coli K12 MG1655 His6x-tagged MltB lytic transglycosylase assessed as intrinsic fluorescence of tryptophan and/or tyro... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |
Membrane-bound lytic murein transglycosylase B
(Escherichia coli (strain K12)) | BDBM50386414

(CHEMBL2047304)Show SMILES C[C@H](NC(=O)[C@@H](C)O[C@@H]1[C@H](CN[C@H](CO)[C@H]1O)NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C14H25N3O7/c1-6(14(22)23)16-13(21)7(2)24-12-9(17-8(3)19)4-15-10(5-18)11(12)20/h6-7,9-12,15,18,20H,4-5H2,1-3H3,(H,16,21)(H,17,19)(H,22,23)/t6-,7+,9-,10+,11+,12+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.89E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to Escherichia coli K12 MG1655 His6x-tagged MltB lytic transglycosylase assessed as intrinsic fluorescence of tryptophan and/or tyro... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |
Membrane-bound lytic murein transglycosylase B
(Escherichia coli (strain K12)) | BDBM50386412

(CHEMBL2047302)Show SMILES CC(=O)N[C@H]1CN[C@H](CO)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H16N2O4/c1-4(12)10-5-2-9-6(3-11)8(14)7(5)13/h5-9,11,13-14H,2-3H2,1H3,(H,10,12)/t5-,6+,7+,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.74E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to Escherichia coli K12 MG1655 His6x-tagged MltB lytic transglycosylase assessed as intrinsic fluorescence of tryptophan and/or tyro... |
ACS Med Chem Lett 3: 238-242 (2012)
Article DOI: 10.1021/ml2002746
BindingDB Entry DOI: 10.7270/Q2KD1ZZJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data