 Found 330 hits with Last Name = 'fisher' and Initial = 'r'
Found 330 hits with Last Name = 'fisher' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50362892
(CHEMBL1946564)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(C)=O)O\N=C\c1ccc-2c(Cc3ccccc-23)c1)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C58H75N11O18/c1-30(56(84)68-24-8-13-44(68)58(86)67-23-7-12-43(67)53(81)63-40(17-20-47(74)75)51(79)62-39(50(59)78)16-19-46(72)73)61-55(83)49(31(2)70)65-54(82)45-27-36(87-60-28-33-14-15-38-35(25-33)26-34-9-4-5-10-37(34)38)29-69(45)57(85)41(18-21-48(76)77)64-52(80)42-11-6-22-66(42)32(3)71/h4-5,9-10,14-15,25,28,30-31,36,39-45,49,70H,6-8,11-13,16-24,26-27,29H2,1-3H3,(H2,59,78)(H,61,83)(H,62,79)(H,63,81)(H,64,80)(H,65,82)(H,72,73)(H,74,75)(H,76,77)/b60-28+/t30-,31+,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... |
ACS Med Chem Lett 2: 337-341 (2011)
Article DOI: 10.1021/ml1002579
BindingDB Entry DOI: 10.7270/Q22B8ZF4 |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50362891

(CHEMBL1946260)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(C)=O)O\N=C\c1ccc(OC(=O)c2ccc3OCOc3c2)cc1)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C59H75N11O22/c1-30(56(85)69-24-6-9-42(69)58(87)68-23-5-8-41(68)53(82)64-38(16-20-47(75)76)51(80)63-37(50(60)79)15-19-46(73)74)62-55(84)49(31(2)71)66-54(83)43-26-36(28-70(43)57(86)39(17-21-48(77)78)65-52(81)40-7-4-22-67(40)32(3)72)92-61-27-33-10-13-35(14-11-33)91-59(88)34-12-18-44-45(25-34)90-29-89-44/h10-14,18,25,27,30-31,36-43,49,71H,4-9,15-17,19-24,26,28-29H2,1-3H3,(H2,60,79)(H,62,84)(H,63,80)(H,64,82)(H,65,81)(H,66,83)(H,73,74)(H,75,76)(H,77,78)/b61-27+/t30-,31+,36+,37-,38-,39-,40-,41-,42-,43-,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... |
ACS Med Chem Lett 2: 337-341 (2011)
Article DOI: 10.1021/ml1002579
BindingDB Entry DOI: 10.7270/Q22B8ZF4 |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388546
(CHEMBL213072 | CHEMBL333363)Show InChI InChI=1S/C19H16N2O2/c20-10-5-11-21-17-13-7-2-3-8-14(13)18(22)16(17)12-6-1-4-9-15(12)19(21)23/h1-4,6-9H,5,10-11,20H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant Tdp1 after 1 hr by FRET assay |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50362890
(CHEMBL1946259)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(C)=O)O\N=C\c1ccc(o1)-c1nc2ccccc2s1)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C56H72N12O19S/c1-28(54(83)67-24-8-12-39(67)56(85)66-23-7-11-38(66)50(80)61-35(16-20-44(73)74)48(78)60-34(47(57)77)15-19-43(71)72)59-52(82)46(29(2)69)64-51(81)40-25-32(87-58-26-31-14-18-41(86-31)53-63-33-9-4-5-13-42(33)88-53)27-68(40)55(84)36(17-21-45(75)76)62-49(79)37-10-6-22-65(37)30(3)70/h4-5,9,13-14,18,26,28-29,32,34-40,46,69H,6-8,10-12,15-17,19-25,27H2,1-3H3,(H2,57,77)(H,59,82)(H,60,78)(H,61,80)(H,62,79)(H,64,81)(H,71,72)(H,73,74)(H,75,76)/b58-26+/t28-,29+,32+,34-,35-,36-,37-,38-,39-,40-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... |
ACS Med Chem Lett 2: 337-341 (2011)
Article DOI: 10.1021/ml1002579
BindingDB Entry DOI: 10.7270/Q22B8ZF4 |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50362889
(CHEMBL1946129)Show SMILES COc1ccc(\C=N\O[C@@H]2C[C@H](N(C2)C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]2CCCN2C(C)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N2CCC[C@H]2C(=O)N2CCC[C@H]2C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O)cc1OC |r| Show InChI InChI=1S/C53H75N11O20/c1-27(51(79)63-22-8-11-37(63)53(81)62-21-7-10-36(62)48(76)58-33(14-18-42(69)70)46(74)57-32(45(54)73)13-17-41(67)68)56-50(78)44(28(2)65)60-49(77)38-24-31(84-55-25-30-12-16-39(82-4)40(23-30)83-5)26-64(38)52(80)34(15-19-43(71)72)59-47(75)35-9-6-20-61(35)29(3)66/h12,16,23,25,27-28,31-38,44,65H,6-11,13-15,17-22,24,26H2,1-5H3,(H2,54,73)(H,56,78)(H,57,74)(H,58,76)(H,59,75)(H,60,77)(H,67,68)(H,69,70)(H,71,72)/b55-25+/t27-,28+,31+,32-,33-,34-,35-,36-,37-,38-,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... |
ACS Med Chem Lett 2: 337-341 (2011)
Article DOI: 10.1021/ml1002579
BindingDB Entry DOI: 10.7270/Q22B8ZF4 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50139763
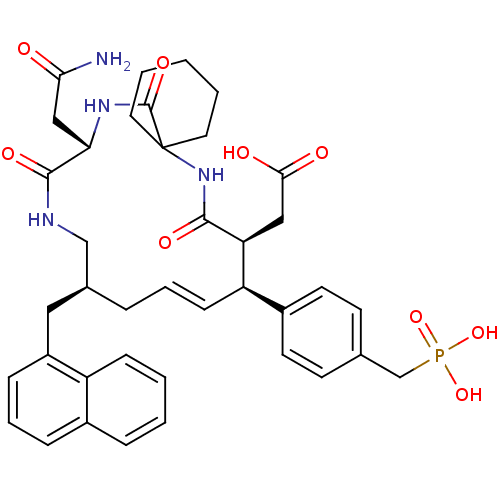
(CHEMBL306657 | [(E)-(9S,10S,14S,18S)-18-Carbamoylm...)Show SMILES NC(=O)C[C@@H]1NC(=O)C2(CCCCC2)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C[C@@H](Cc2cccc3ccccc23)CNC1=O)c1ccc(CP(O)(O)=O)cc1 |t:24| Show InChI InChI=1S/C39H47N4O9P/c40-34(44)22-33-37(48)41-23-26(20-29-11-7-10-27-9-2-3-12-30(27)29)8-6-13-31(28-16-14-25(15-17-28)24-53(50,51)52)32(21-35(45)46)36(47)43-39(38(49)42-33)18-4-1-5-19-39/h2-3,6-7,9-17,26,31-33H,1,4-5,8,18-24H2,(H2,40,44)(H,41,48)(H,42,49)(H,43,47)(H,45,46)(H2,50,51,52)/b13-6+/t26-,31+,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in in surface plasmon resonance (SPR) method |
J Med Chem 47: 2166-9 (2004)
Article DOI: 10.1021/jm030510e
BindingDB Entry DOI: 10.7270/Q2668CNN |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50139763

(CHEMBL306657 | [(E)-(9S,10S,14S,18S)-18-Carbamoylm...)Show SMILES NC(=O)C[C@@H]1NC(=O)C2(CCCCC2)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C[C@@H](Cc2cccc3ccccc23)CNC1=O)c1ccc(CP(O)(O)=O)cc1 |t:24| Show InChI InChI=1S/C39H47N4O9P/c40-34(44)22-33-37(48)41-23-26(20-29-11-7-10-27-9-2-3-12-30(27)29)8-6-13-31(28-16-14-25(15-17-28)24-53(50,51)52)32(21-35(45)46)36(47)43-39(38(49)42-33)18-4-1-5-19-39/h2-3,6-7,9-17,26,31-33H,1,4-5,8,18-24H2,(H2,40,44)(H,41,48)(H,42,49)(H,43,47)(H,45,46)(H2,50,51,52)/b13-6+/t26-,31+,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in in surface plasmon resonance (SPR) method |
J Med Chem 47: 2166-9 (2004)
Article DOI: 10.1021/jm030510e
BindingDB Entry DOI: 10.7270/Q2668CNN |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50139763

(CHEMBL306657 | [(E)-(9S,10S,14S,18S)-18-Carbamoylm...)Show SMILES NC(=O)C[C@@H]1NC(=O)C2(CCCCC2)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C[C@@H](Cc2cccc3ccccc23)CNC1=O)c1ccc(CP(O)(O)=O)cc1 |t:24| Show InChI InChI=1S/C39H47N4O9P/c40-34(44)22-33-37(48)41-23-26(20-29-11-7-10-27-9-2-3-12-30(27)29)8-6-13-31(28-16-14-25(15-17-28)24-53(50,51)52)32(21-35(45)46)36(47)43-39(38(49)42-33)18-4-1-5-19-39/h2-3,6-7,9-17,26,31-33H,1,4-5,8,18-24H2,(H2,40,44)(H,41,48)(H,42,49)(H,43,47)(H,45,46)(H2,50,51,52)/b13-6+/t26-,31+,32-,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity in extracellular ELISA-based assay for Grb2 SH2 domain |
J Med Chem 47: 788-91 (2004)
Article DOI: 10.1021/jm030440b
BindingDB Entry DOI: 10.7270/Q2XD114Q |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50168312
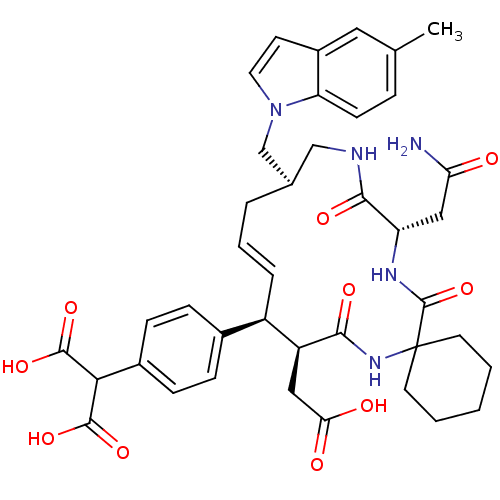
(2-{4-[(16R,20S)-18-((S)-Carbamoylmethyl)-9-carboxy...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(cc3)C(C(O)=O)C(O)=O)ccc2c1 |t:36| Show InChI InChI=1S/C40H47N5O10/c1-23-8-13-31-27(18-23)14-17-45(31)22-24-6-5-7-28(25-9-11-26(12-10-25)34(37(51)52)38(53)54)29(19-33(47)48)35(49)44-40(15-3-2-4-16-40)39(55)43-30(20-32(41)46)36(50)42-21-24/h5,7-14,17-18,24,28-30,34H,2-4,6,15-16,19-22H2,1H3,(H2,41,46)(H,42,50)(H,43,55)(H,44,49)(H,47,48)(H,51,52)(H,53,54)/b7-5+/t24-,28-,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against growth factor receptor bound protein 2 |
J Med Chem 48: 3945-8 (2005)
Article DOI: 10.1021/jm050059m
BindingDB Entry DOI: 10.7270/Q2V69J48 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50143944
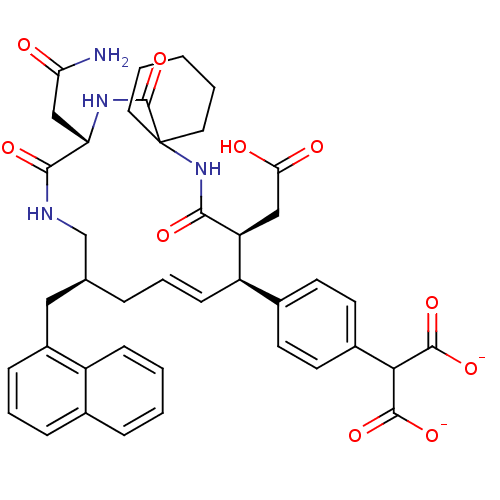
(2-[4-((E)-(9S,10S,14S,18S)-18-Carbamoylmethyl-9-ca...)Show SMILES NC(=O)C[C@@H]1NC(=O)C2(CCCCC2)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C[C@@H](Cc2cccc3ccccc23)CNC1=O)c1ccc(cc1)C(C([O-])=O)C([O-])=O |t:24| Show InChI InChI=1S/C41H46N4O10/c42-33(46)22-32-37(50)43-23-24(20-28-11-7-10-25-9-2-3-12-29(25)28)8-6-13-30(26-14-16-27(17-15-26)35(38(51)52)39(53)54)31(21-34(47)48)36(49)45-41(40(55)44-32)18-4-1-5-19-41/h2-3,6-7,9-17,24,30-32,35H,1,4-5,8,18-23H2,(H2,42,46)(H,43,50)(H,44,55)(H,45,49)(H,47,48)(H,51,52)(H,53,54)/p-2/b13-6+/t24-,30+,31-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in ELISA method |
J Med Chem 47: 2166-9 (2004)
Article DOI: 10.1021/jm030510e
BindingDB Entry DOI: 10.7270/Q2668CNN |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50168318
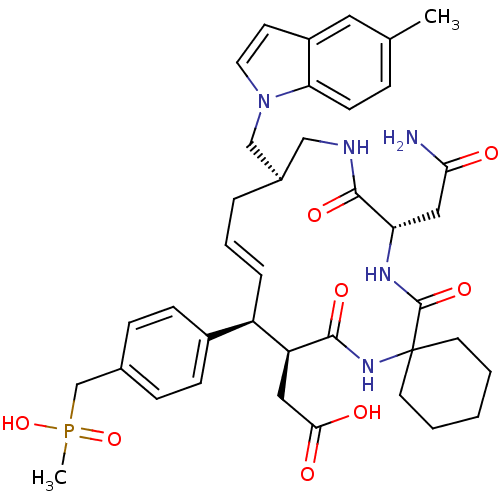
(CHEMBL424823 | [(15R,20S)-18-Carbamoylmethyl-10-[4...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(CP(C)(O)=O)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C39H50N5O8P/c1-25-9-14-33-29(19-25)15-18-44(33)23-27-7-6-8-30(28-12-10-26(11-13-28)24-53(2,51)52)31(20-35(46)47)36(48)43-39(16-4-3-5-17-39)38(50)42-32(21-34(40)45)37(49)41-22-27/h6,8-15,18-19,27,30-32H,3-5,7,16-17,20-24H2,1-2H3,(H2,40,45)(H,41,49)(H,42,50)(H,43,48)(H,46,47)(H,51,52)/b8-6+/t27-,30-,31+,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against growth factor receptor bound protein 2 |
J Med Chem 48: 3945-8 (2005)
Article DOI: 10.1021/jm050059m
BindingDB Entry DOI: 10.7270/Q2V69J48 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50168317
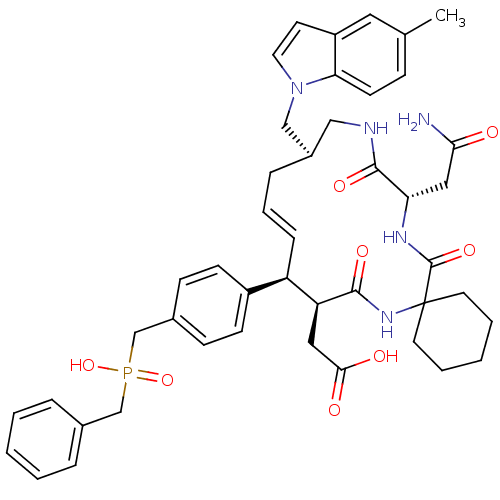
(CHEMBL193281 | [(15R,20S)-10-[4-(Benzyl-hydroxy-ph...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(CP(O)(=O)Cc4ccccc4)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C45H54N5O8P/c1-30-13-18-39-35(23-30)19-22-50(39)27-33-11-8-12-36(34-16-14-32(15-17-34)29-59(57,58)28-31-9-4-2-5-10-31)37(24-41(52)53)42(54)49-45(20-6-3-7-21-45)44(56)48-38(25-40(46)51)43(55)47-26-33/h2,4-5,8-10,12-19,22-23,33,36-38H,3,6-7,11,20-21,24-29H2,1H3,(H2,46,51)(H,47,55)(H,48,56)(H,49,54)(H,52,53)(H,57,58)/b12-8+/t33-,36-,37+,38+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against growth factor receptor bound protein 2 |
J Med Chem 48: 3945-8 (2005)
Article DOI: 10.1021/jm050059m
BindingDB Entry DOI: 10.7270/Q2V69J48 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50168316

(CHEMBL263328 | N-{4-[(7S,14R,18S)-18-Carbamoylmeth...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(NC(=O)C(O)=O)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C39H46N6O9/c1-23-8-13-31-26(18-23)14-17-45(31)22-24-6-5-7-28(25-9-11-27(12-10-25)42-36(51)37(52)53)29(19-33(47)48)34(49)44-39(15-3-2-4-16-39)38(54)43-30(20-32(40)46)35(50)41-21-24/h5,7-14,17-18,24,28-30H,2-4,6,15-16,19-22H2,1H3,(H2,40,46)(H,41,50)(H,42,51)(H,43,54)(H,44,49)(H,47,48)(H,52,53)/b7-5+/t24-,28-,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against growth factor receptor bound protein 2 |
J Med Chem 48: 3945-8 (2005)
Article DOI: 10.1021/jm050059m
BindingDB Entry DOI: 10.7270/Q2V69J48 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50072865
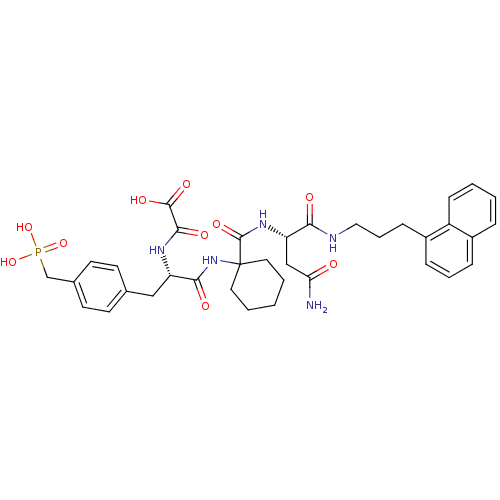
(CHEMBL55627 | N-[(S)-1-{1-[(S)-2-Carbamoyl-1-(3-na...)Show SMILES NC(=O)C[C@H](NC(=O)C1(CCCCC1)NC(=O)[C@H](Cc1ccc(CP(O)(O)=O)cc1)NC(=O)C(O)=O)C(=O)NCCCc1cccc2ccccc12 Show InChI InChI=1S/C36H44N5O10P/c37-30(42)21-29(31(43)38-19-7-11-26-10-6-9-25-8-2-3-12-27(25)26)40-35(48)36(17-4-1-5-18-36)41-32(44)28(39-33(45)34(46)47)20-23-13-15-24(16-14-23)22-52(49,50)51/h2-3,6,8-10,12-16,28-29H,1,4-5,7,11,17-22H2,(H2,37,42)(H,38,43)(H,39,45)(H,40,48)(H,41,44)(H,46,47)(H2,49,50,51)/t28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in ELISA method |
J Med Chem 47: 2166-9 (2004)
Article DOI: 10.1021/jm030510e
BindingDB Entry DOI: 10.7270/Q2668CNN |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50143944

(2-[4-((E)-(9S,10S,14S,18S)-18-Carbamoylmethyl-9-ca...)Show SMILES NC(=O)C[C@@H]1NC(=O)C2(CCCCC2)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C[C@@H](Cc2cccc3ccccc23)CNC1=O)c1ccc(cc1)C(C([O-])=O)C([O-])=O |t:24| Show InChI InChI=1S/C41H46N4O10/c42-33(46)22-32-37(50)43-23-24(20-28-11-7-10-25-9-2-3-12-29(25)28)8-6-13-30(26-14-16-27(17-15-26)35(38(51)52)39(53)54)31(21-34(47)48)36(49)45-41(40(55)44-32)18-4-1-5-19-41/h2-3,6-7,9-17,24,30-32,35H,1,4-5,8,18-23H2,(H2,42,46)(H,43,50)(H,44,55)(H,45,49)(H,47,48)(H,51,52)(H,53,54)/p-2/b13-6+/t24-,30+,31-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in in surface plasmon resonance (SPR) method |
J Med Chem 47: 2166-9 (2004)
Article DOI: 10.1021/jm030510e
BindingDB Entry DOI: 10.7270/Q2668CNN |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50139762
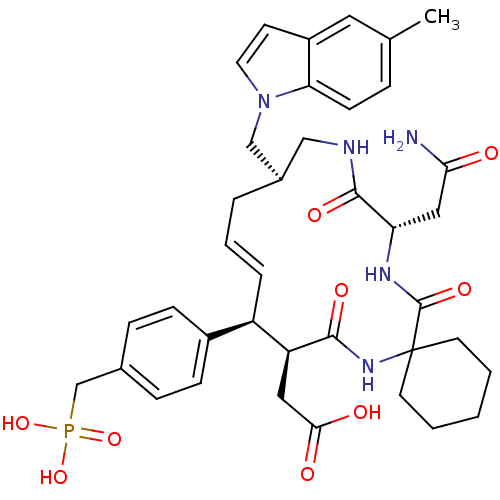
(CHEMBL349373 | [(16R,20S)-18-((S)-Carbamoylmethyl)...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(CP(O)(O)=O)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C38H48N5O9P/c1-24-8-13-32-28(18-24)14-17-43(32)22-26-6-5-7-29(27-11-9-25(10-12-27)23-53(50,51)52)30(19-34(45)46)35(47)42-38(15-3-2-4-16-38)37(49)41-31(20-33(39)44)36(48)40-21-26/h5,7-14,17-18,26,29-31H,2-4,6,15-16,19-23H2,1H3,(H2,39,44)(H,40,48)(H,41,49)(H,42,47)(H,45,46)(H2,50,51,52)/b7-5+/t26-,29-,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against growth factor receptor bound protein 2 |
J Med Chem 48: 3945-8 (2005)
Article DOI: 10.1021/jm050059m
BindingDB Entry DOI: 10.7270/Q2V69J48 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50102025
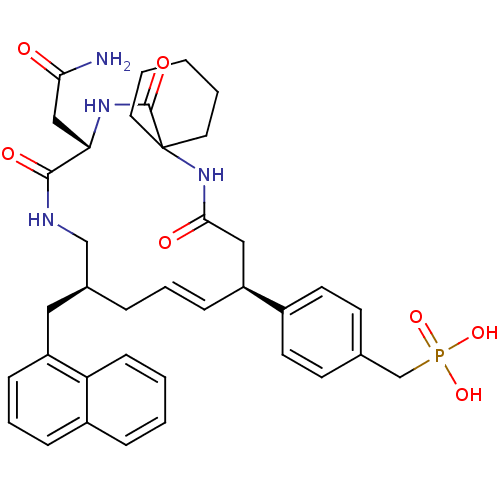
(4-[(10S,14S,18S)-18-(2-AMINO-2-OXOETHYL)-14-(1-NAP...)Show SMILES NC(=O)C[C@@H]1NC(=O)C2(CCCCC2)NC(=O)C[C@H](\C=C\C[C@@H](Cc2cccc3ccccc23)CNC1=O)c1ccc(CP(O)(O)=O)cc1 |t:20| Show InChI InChI=1S/C37H45N4O7P/c38-33(42)22-32-35(44)39-23-26(20-30-12-7-10-28-9-2-3-13-31(28)30)8-6-11-29(27-16-14-25(15-17-27)24-49(46,47)48)21-34(43)41-37(36(45)40-32)18-4-1-5-19-37/h2-3,6-7,9-17,26,29,32H,1,4-5,8,18-24H2,(H2,38,42)(H,39,44)(H,40,45)(H,41,43)(H2,46,47,48)/b11-6+/t26-,29-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity in extracellular ELISA-based assay for Grb2 SH2 domain |
J Med Chem 47: 788-91 (2004)
Article DOI: 10.1021/jm030440b
BindingDB Entry DOI: 10.7270/Q2XD114Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50085867
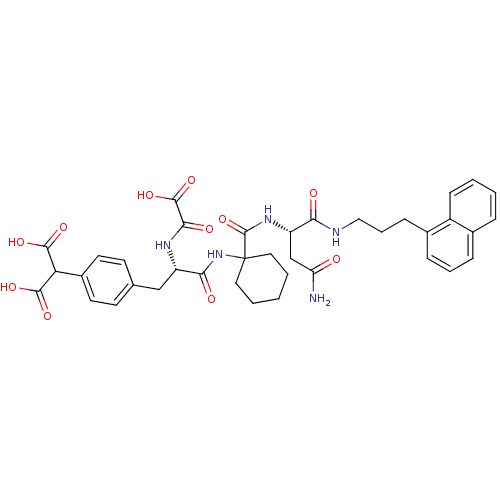
(2-(4-((S)-3-(1-((S)-4-amino-1-(3-(naphthalen-1-yl)...)Show SMILES NC(=O)C[C@H](NC(=O)C1(CCCCC1)NC(=O)[C@H](Cc1ccc(cc1)C(C(O)=O)C(O)=O)NC(=O)C(O)=O)C(=O)NCCCc1cccc2ccccc12 |r| Show InChI InChI=1S/C38H43N5O11/c39-29(44)21-28(31(45)40-19-7-11-24-10-6-9-23-8-2-3-12-26(23)24)42-37(54)38(17-4-1-5-18-38)43-32(46)27(41-33(47)36(52)53)20-22-13-15-25(16-14-22)30(34(48)49)35(50)51/h2-3,6,8-10,12-16,27-28,30H,1,4-5,7,11,17-21H2,(H2,39,44)(H,40,45)(H,41,47)(H,42,54)(H,43,46)(H,48,49)(H,50,51)(H,52,53)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in ELISA method |
J Med Chem 47: 2166-9 (2004)
Article DOI: 10.1021/jm030510e
BindingDB Entry DOI: 10.7270/Q2668CNN |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50168314

(CHEMBL193015 | [(7S,14R,18S)-18-Carbamoylmethyl-10...)Show SMILES Cc1ccc2n(C[C@H]3CNC(=O)[C@H](CC(N)=O)NC(=O)C4(CCCCC4)NC(=O)[C@@H](CC(O)=O)[C@H](\C=C\C3)c3ccc(CC(O)=O)cc3)ccc2c1 |t:36| Show InChI InChI=1S/C39H47N5O8/c1-24-8-13-32-28(18-24)14-17-44(32)23-26-6-5-7-29(27-11-9-25(10-12-27)19-34(46)47)30(20-35(48)49)36(50)43-39(15-3-2-4-16-39)38(52)42-31(21-33(40)45)37(51)41-22-26/h5,7-14,17-18,26,29-31H,2-4,6,15-16,19-23H2,1H3,(H2,40,45)(H,41,51)(H,42,52)(H,43,50)(H,46,47)(H,48,49)/b7-5+/t26-,29-,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against growth factor receptor bound protein 2 |
J Med Chem 48: 3945-8 (2005)
Article DOI: 10.1021/jm050059m
BindingDB Entry DOI: 10.7270/Q2V69J48 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50072862
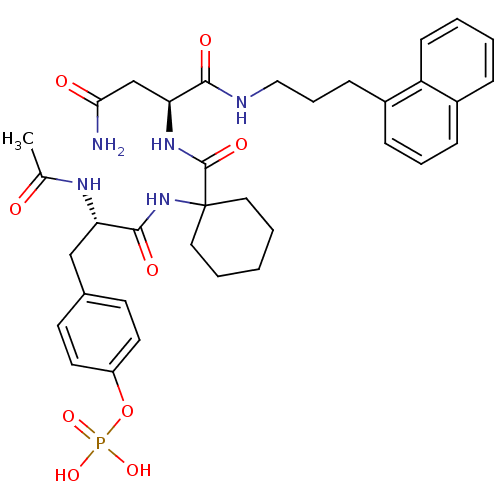
(CHEMBL117807 | Phosphoric acid mono-[4-((S)-2-acet...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)NC1(CCCCC1)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCc1cccc2ccccc12 Show InChI InChI=1S/C35H44N5O9P/c1-23(41)38-29(21-24-14-16-27(17-15-24)49-50(46,47)48)33(44)40-35(18-5-2-6-19-35)34(45)39-30(22-31(36)42)32(43)37-20-8-12-26-11-7-10-25-9-3-4-13-28(25)26/h3-4,7,9-11,13-17,29-30H,2,5-6,8,12,18-22H2,1H3,(H2,36,42)(H,37,43)(H,38,41)(H,39,45)(H,40,44)(H2,46,47,48)/t29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of Grb2 SH2 domain binding in ELISA |
J Med Chem 48: 5369-72 (2005)
Article DOI: 10.1021/jm050154v
BindingDB Entry DOI: 10.7270/Q2513XRP |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50085867

(2-(4-((S)-3-(1-((S)-4-amino-1-(3-(naphthalen-1-yl)...)Show SMILES NC(=O)C[C@H](NC(=O)C1(CCCCC1)NC(=O)[C@H](Cc1ccc(cc1)C(C(O)=O)C(O)=O)NC(=O)C(O)=O)C(=O)NCCCc1cccc2ccccc12 |r| Show InChI InChI=1S/C38H43N5O11/c39-29(44)21-28(31(45)40-19-7-11-24-10-6-9-23-8-2-3-12-26(23)24)42-37(54)38(17-4-1-5-18-38)43-32(46)27(41-33(47)36(52)53)20-22-13-15-25(16-14-22)30(34(48)49)35(50)51/h2-3,6,8-10,12-16,27-28,30H,1,4-5,7,11,17-21H2,(H2,39,44)(H,40,45)(H,41,47)(H,42,54)(H,43,46)(H,48,49)(H,50,51)(H,52,53)/t27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of Grb2 SH2 domain binding in ELISA |
J Med Chem 48: 5369-72 (2005)
Article DOI: 10.1021/jm050154v
BindingDB Entry DOI: 10.7270/Q2513XRP |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50161466
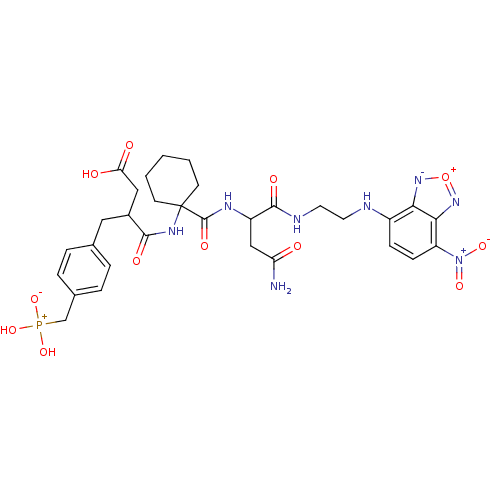
((R)-N-(1-{(S)-2-Carbamoyl-1-[2-(7-nitro-benzo[1,2,...)Show SMILES NC(=O)CC(NC(=O)C1(CCCCC1)NC(=O)C(CC(O)=O)Cc1ccc(C[P+](O)(O)[O-])cc1)C(=O)NCCNc1ccc([N+]([O-])=O)c2n[o+][n-]c12 Show InChI InChI=1S/C31H39N8O12P/c32-24(40)16-22(29(44)34-13-12-33-21-8-9-23(39(46)47)27-26(21)37-51-38-27)35-30(45)31(10-2-1-3-11-31)36-28(43)20(15-25(41)42)14-18-4-6-19(7-5-18)17-52(48,49)50/h4-9,20,22,33H,1-3,10-17H2,(H2,32,40)(H,34,44)(H,35,45)(H,36,43)(H,41,42)(H2,48,49,50) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Apparent dissociation constant for human growth factor receptor bound protein 2 |
Bioorg Med Chem Lett 15: 1385-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.017
BindingDB Entry DOI: 10.7270/Q22R3R59 |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50085877
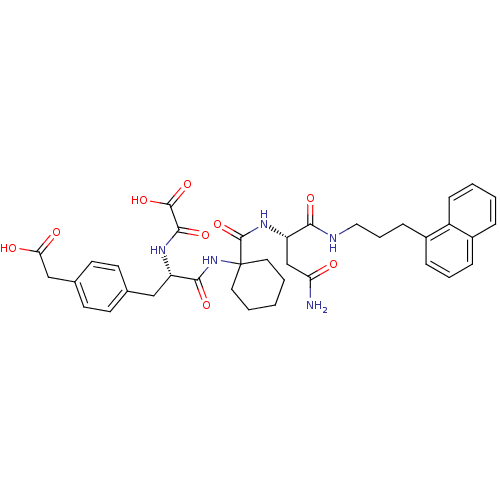
(CHEMBL355066 | N-[(S)-1-{1-[(S)-2-Carbamoyl-1-(3-n...)Show SMILES NC(=O)C[C@H](NC(=O)C1(CCCCC1)NC(=O)[C@H](Cc1ccc(CC(O)=O)cc1)NC(=O)C(O)=O)C(=O)NCCCc1cccc2ccccc12 Show InChI InChI=1S/C37H43N5O9/c38-30(43)22-29(32(46)39-19-7-11-26-10-6-9-25-8-2-3-12-27(25)26)41-36(51)37(17-4-1-5-18-37)42-33(47)28(40-34(48)35(49)50)20-23-13-15-24(16-14-23)21-31(44)45/h2-3,6,8-10,12-16,28-29H,1,4-5,7,11,17-22H2,(H2,38,43)(H,39,46)(H,40,48)(H,41,51)(H,42,47)(H,44,45)(H,49,50)/t28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of Grb2 SH2 domain binding |
J Med Chem 48: 5369-72 (2005)
Article DOI: 10.1021/jm050154v
BindingDB Entry DOI: 10.7270/Q2513XRP |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50085877

(CHEMBL355066 | N-[(S)-1-{1-[(S)-2-Carbamoyl-1-(3-n...)Show SMILES NC(=O)C[C@H](NC(=O)C1(CCCCC1)NC(=O)[C@H](Cc1ccc(CC(O)=O)cc1)NC(=O)C(O)=O)C(=O)NCCCc1cccc2ccccc12 Show InChI InChI=1S/C37H43N5O9/c38-30(43)22-29(32(46)39-19-7-11-26-10-6-9-25-8-2-3-12-27(25)26)41-36(51)37(17-4-1-5-18-37)42-33(47)28(40-34(48)35(49)50)20-23-13-15-24(16-14-23)21-31(44)45/h2-3,6,8-10,12-16,28-29H,1,4-5,7,11,17-22H2,(H2,38,43)(H,39,46)(H,40,48)(H,41,51)(H,42,47)(H,44,45)(H,49,50)/t28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of Grb2 SH2 domain binding in ELISA |
J Med Chem 48: 5369-72 (2005)
Article DOI: 10.1021/jm050154v
BindingDB Entry DOI: 10.7270/Q2513XRP |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388544
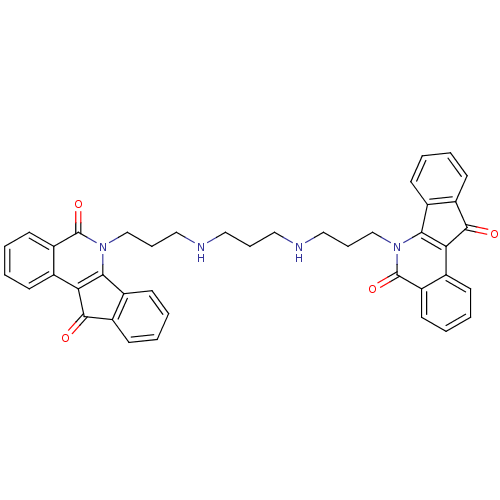
(CHEMBL2057323)Show SMILES O=C1c2ccccc2-c2c1c1ccccc1c(=O)n2CCCNCCCNCCCn1c2-c3ccccc3C(=O)c2c2ccccc2c1=O Show InChI InChI=1S/C41H36N4O4/c46-38-30-16-5-3-14-28(30)36-34(38)26-12-1-7-18-32(26)40(48)44(36)24-10-22-42-20-9-21-43-23-11-25-45-37-29-15-4-6-17-31(29)39(47)35(37)27-13-2-8-19-33(27)41(45)49/h1-8,12-19,42-43H,9-11,20-25H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388544

(CHEMBL2057323)Show SMILES O=C1c2ccccc2-c2c1c1ccccc1c(=O)n2CCCNCCCNCCCn1c2-c3ccccc3C(=O)c2c2ccccc2c1=O Show InChI InChI=1S/C41H36N4O4/c46-38-30-16-5-3-14-28(30)36-34(38)26-12-1-7-18-32(26)40(48)44(36)24-10-22-42-20-9-21-43-23-11-25-45-37-29-15-4-6-17-31(29)39(47)35(37)27-13-2-8-19-33(27)41(45)49/h1-8,12-19,42-43H,9-11,20-25H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human Tdp1 in Tdp1-deficient chicken DT40 whole cell extract using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-p... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
SHC-transforming protein 1
(Homo sapiens (Human)) | BDBM50276388
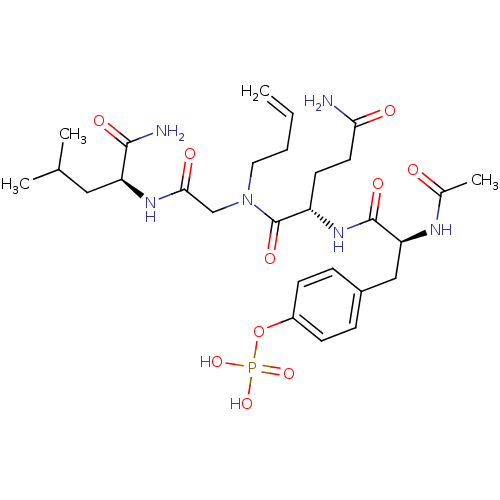
(4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...)Show SMILES CC(C)C[C@H](NC(=O)CN(CCC=C)C(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C28H43N6O10P/c1-5-6-13-34(16-25(37)32-22(26(30)38)14-17(2)3)28(40)21(11-12-24(29)36)33-27(39)23(31-18(4)35)15-19-7-9-20(10-8-19)44-45(41,42)43/h5,7-10,17,21-23H,1,6,11-16H2,2-4H3,(H2,29,36)(H2,30,38)(H,31,35)(H,32,37)(H,33,39)(H2,41,42,43)/t21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... |
J Med Chem 52: 1612-8 (2009)
Article DOI: 10.1021/jm800789h
BindingDB Entry DOI: 10.7270/Q2XP74TZ |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50158383
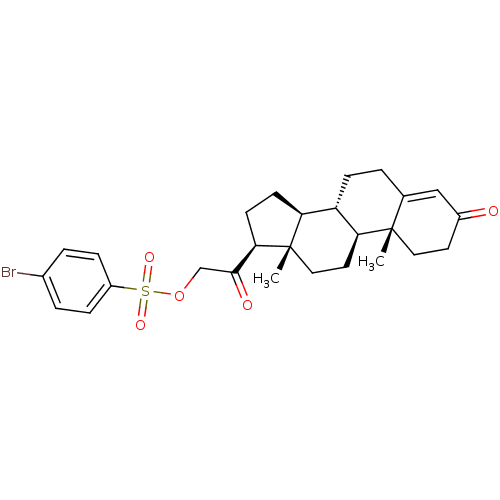
(2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)COS(=O)(=O)c1ccc(Br)cc1 |r,t:8| Show InChI InChI=1S/C27H33BrO5S/c1-26-13-11-19(29)15-17(26)3-8-21-22-9-10-24(27(22,2)14-12-23(21)26)25(30)16-33-34(31,32)20-6-4-18(28)5-7-20/h4-7,15,21-24H,3,8-14,16H2,1-2H3/t21-,22-,23-,24+,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Tdp1 expressed in Escherichia coli assessed as blockade of enzyme-mediated hydrolysis of phosphodiester linkage between tyrosine ... |
J Med Chem 52: 7122-31 (2009)
Article DOI: 10.1021/jm901061s
BindingDB Entry DOI: 10.7270/Q2H1325T |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50158383

(2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)COS(=O)(=O)c1ccc(Br)cc1 |r,t:8| Show InChI InChI=1S/C27H33BrO5S/c1-26-13-11-19(29)15-17(26)3-8-21-22-9-10-24(27(22,2)14-12-23(21)26)25(30)16-33-34(31,32)20-6-4-18(28)5-7-20/h4-7,15,21-24H,3,8-14,16H2,1-2H3/t21-,22-,23-,24+,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
SHC-transforming protein 1
(Homo sapiens (Human)) | BDBM50276339
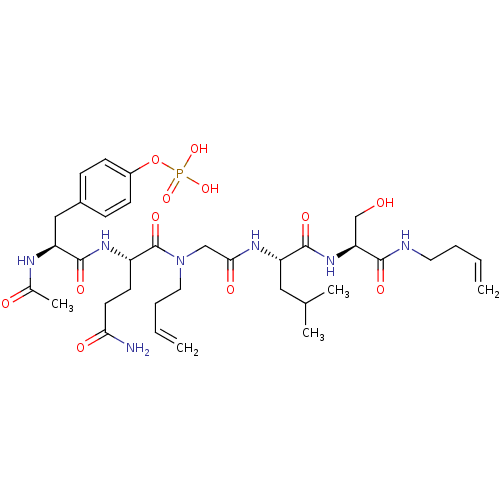
(4-((2S,5S,11S,14S)-2-acetamido-5-(3-amino-3-oxopro...)Show SMILES CC(C)C[C@H](NC(=O)CN(CCC=C)C(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(=O)N[C@@H](CO)C(=O)NCCC=C |r| Show InChI InChI=1S/C35H54N7O12P/c1-6-8-16-37-32(47)29(21-43)41-33(48)27(18-22(3)4)39-31(46)20-42(17-9-7-2)35(50)26(14-15-30(36)45)40-34(49)28(38-23(5)44)19-24-10-12-25(13-11-24)54-55(51,52)53/h6-7,10-13,22,26-29,43H,1-2,8-9,14-21H2,3-5H3,(H2,36,45)(H,37,47)(H,38,44)(H,39,46)(H,40,49)(H,41,48)(H2,51,52,53)/t26-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... |
J Med Chem 52: 1612-8 (2009)
Article DOI: 10.1021/jm800789h
BindingDB Entry DOI: 10.7270/Q2XP74TZ |
More data for this
Ligand-Target Pair | |
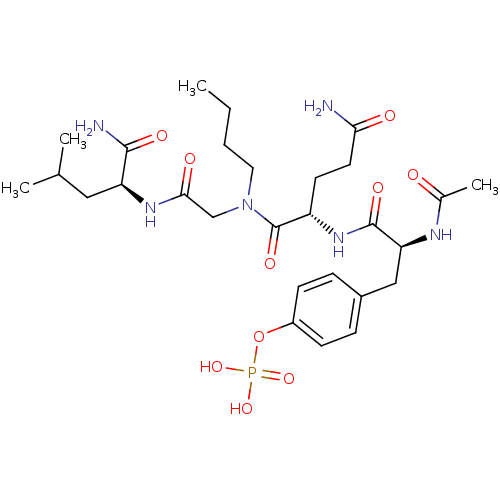
SHC-transforming protein 1
(Homo sapiens (Human)) | BDBM50276387
(4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(C)C)C(N)=O)C(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O |r| Show InChI InChI=1S/C28H45N6O10P/c1-5-6-13-34(16-25(37)32-22(26(30)38)14-17(2)3)28(40)21(11-12-24(29)36)33-27(39)23(31-18(4)35)15-19-7-9-20(10-8-19)44-45(41,42)43/h7-10,17,21-23H,5-6,11-16H2,1-4H3,(H2,29,36)(H2,30,38)(H,31,35)(H,32,37)(H,33,39)(H2,41,42,43)/t21-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... |
J Med Chem 52: 1612-8 (2009)
Article DOI: 10.1021/jm800789h
BindingDB Entry DOI: 10.7270/Q2XP74TZ |
More data for this
Ligand-Target Pair | |
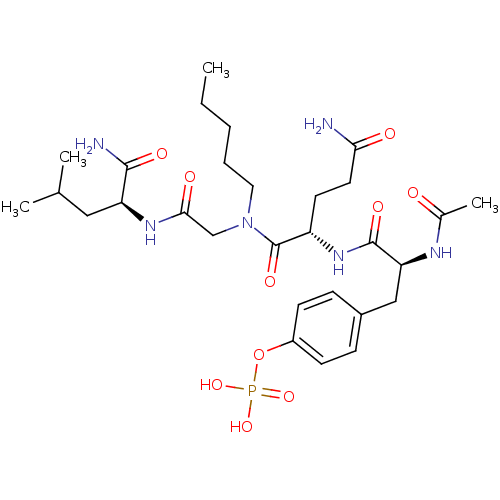
SHC-transforming protein 1
(Homo sapiens (Human)) | BDBM50276396
(4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...)Show SMILES CCCCCN(CC(=O)N[C@@H](CC(C)C)C(N)=O)C(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O |r| Show InChI InChI=1S/C29H47N6O10P/c1-5-6-7-14-35(17-26(38)33-23(27(31)39)15-18(2)3)29(41)22(12-13-25(30)37)34-28(40)24(32-19(4)36)16-20-8-10-21(11-9-20)45-46(42,43)44/h8-11,18,22-24H,5-7,12-17H2,1-4H3,(H2,30,37)(H2,31,39)(H,32,36)(H,33,38)(H,34,40)(H2,42,43,44)/t22-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... |
J Med Chem 52: 1612-8 (2009)
Article DOI: 10.1021/jm800789h
BindingDB Entry DOI: 10.7270/Q2XP74TZ |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230460
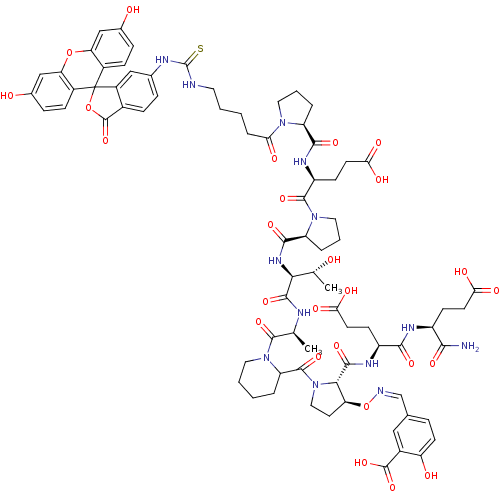
(5-[(1Z)-({[(2S,3S)-2-{[(1S)-1-{[(1S)-1-carbamoyl-3...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1CC[C@H](O\N=C/c2ccc(O)c(c2)C(O)=O)[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O Show InChI InChI=1S/C77H91N13O26S/c1-38(71(108)89-29-6-4-9-54(89)73(110)90-32-27-56(116-80-37-40-13-23-55(94)45(33-40)74(111)112)64(90)70(107)84-50(21-25-61(98)99)66(103)83-49(65(78)102)20-24-60(96)97)81-69(106)63(39(2)91)86-68(105)53-11-8-31-88(53)72(109)51(22-26-62(100)101)85-67(104)52-10-7-30-87(52)59(95)12-3-5-28-79-76(117)82-41-14-17-44-48(34-41)77(115-75(44)113)46-18-15-42(92)35-57(46)114-58-36-43(93)16-19-47(58)77/h13-19,23,33-39,49-54,56,63-64,91-94H,3-12,20-22,24-32H2,1-2H3,(H2,78,102)(H,81,106)(H,83,103)(H,84,107)(H,85,104)(H,86,105)(H,96,97)(H,98,99)(H,100,101)(H,111,112)(H2,79,82,117)/b80-37-/t38-,39+,49-,50-,51-,52-,53-,54?,56-,63-,64-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230449
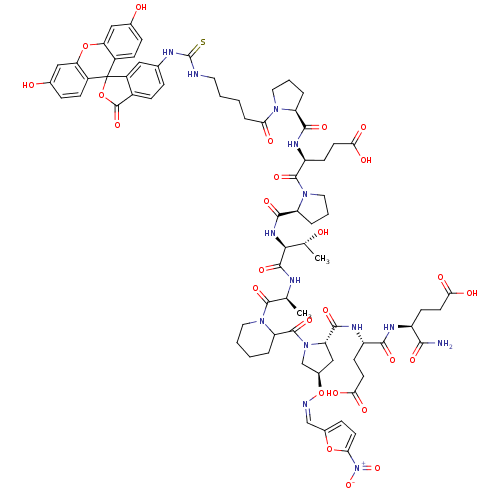
((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O)O\N=C/c1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C74H88N14O26S/c1-37(69(105)86-28-6-4-9-53(86)71(107)87-36-43(114-77-35-42-16-23-58(111-42)88(109)110)34-54(87)67(103)81-49(21-25-60(95)96)64(100)80-48(63(75)99)20-24-59(93)94)78-68(104)62(38(2)89)83-66(102)52-11-8-30-85(52)70(106)50(22-26-61(97)98)82-65(101)51-10-7-29-84(51)57(92)12-3-5-27-76-73(115)79-39-13-17-44-47(31-39)74(113-72(44)108)45-18-14-40(90)32-55(45)112-56-33-41(91)15-19-46(56)74/h13-19,23,31-33,35,37-38,43,48-54,62,89-91H,3-12,20-22,24-30,34,36H2,1-2H3,(H2,75,99)(H,78,104)(H,80,100)(H,81,103)(H,82,101)(H,83,102)(H,93,94)(H,95,96)(H,97,98)(H2,76,79,115)/b77-35-/t37-,38+,43+,48-,49-,50-,51-,52-,53?,54-,62-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50362891

(CHEMBL1946260)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(C)=O)O\N=C\c1ccc(OC(=O)c2ccc3OCOc3c2)cc1)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r| Show InChI InChI=1S/C59H75N11O22/c1-30(56(85)69-24-6-9-42(69)58(87)68-23-5-8-41(68)53(82)64-38(16-20-47(75)76)51(80)63-37(50(60)79)15-19-46(73)74)62-55(84)49(31(2)71)66-54(83)43-26-36(28-70(43)57(86)39(17-21-48(77)78)65-52(81)40-7-4-22-67(40)32(3)72)92-61-27-33-10-13-35(14-11-33)91-59(88)34-12-18-44-45(25-34)90-29-89-44/h10-14,18,25,27,30-31,36-43,49,71H,4-9,15-17,19-24,26,28-29H2,1-3H3,(H2,60,79)(H,62,84)(H,63,80)(H,64,82)(H,65,81)(H,66,83)(H,73,74)(H,75,76)(H,77,78)/b61-27+/t30-,31+,36+,37-,38-,39-,40-,41-,42-,43-,49-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of human Tsg101 binding to GST-tagged P6 protein by surface plasmon resonance method |
ACS Med Chem Lett 2: 337-341 (2011)
Article DOI: 10.1021/ml1002579
BindingDB Entry DOI: 10.7270/Q22B8ZF4 |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230450

(4-carbamoyl-4-(4-carboxy-2-{[(2S,3S)-1-({1-[(2S)-2...)Show SMILES CC(O)C(NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1CC[C@H](O\N=C/c2ccc(cc2)N(C)C)[C@H]1C(=O)NC(CCC(O)=O)C(=O)NC(CCC(O)=O)C(N)=O Show InChI InChI=1S/C78H96N14O23S/c1-41(73(109)91-33-8-6-11-57(91)75(111)92-36-31-58(115-81-40-43-15-18-45(19-16-43)88(3)4)66(92)72(108)85-53(26-29-63(99)100)68(104)84-52(67(79)103)25-28-62(97)98)82-71(107)65(42(2)93)87-70(106)56-13-10-35-90(56)74(110)54(27-30-64(101)102)86-69(105)55-12-9-34-89(55)61(96)14-5-7-32-80-77(116)83-44-17-22-48-51(37-44)78(114-76(48)112)49-23-20-46(94)38-59(49)113-60-39-47(95)21-24-50(60)78/h15-24,37-42,52-58,65-66,93-95H,5-14,25-36H2,1-4H3,(H2,79,103)(H,82,107)(H,84,104)(H,85,108)(H,86,105)(H,87,106)(H,97,98)(H,99,100)(H,101,102)(H2,80,83,116)/b81-40-/t41-,42?,52?,53?,54-,55-,56-,57?,58-,65?,66-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230470

((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1CC[C@H](O\N=C/C(C)=Cc2ccccc2)[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O |w:87.95| Show InChI InChI=1S/C79H95N13O23S/c1-42(37-45-13-5-4-6-14-45)41-82-115-59-31-36-92(67(59)73(108)86-54(26-29-64(99)100)69(104)85-53(68(80)103)25-28-63(97)98)76(111)58-15-8-10-33-91(58)74(109)43(2)83-72(107)66(44(3)93)88-71(106)57-17-12-35-90(57)75(110)55(27-30-65(101)102)87-70(105)56-16-11-34-89(56)62(96)18-7-9-32-81-78(116)84-46-19-22-49-52(38-46)79(114-77(49)112)50-23-20-47(94)39-60(50)113-61-40-48(95)21-24-51(61)79/h4-6,13-14,19-24,37-41,43-44,53-59,66-67,93-95H,7-12,15-18,25-36H2,1-3H3,(H2,80,103)(H,83,107)(H,85,104)(H,86,108)(H,87,105)(H,88,106)(H,97,98)(H,99,100)(H,101,102)(H2,81,84,116)/b42-37?,82-41-/t43-,44+,53-,54-,55-,56-,57-,58?,59-,66-,67-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388554

(CHEMBL223545)Show SMILES NCCCCCCCCCCCn1c2-c3ccccc3C(=O)c2c2ccccc2c1=O Show InChI InChI=1S/C27H32N2O2/c28-18-12-6-4-2-1-3-5-7-13-19-29-25-21-15-9-10-16-22(21)26(30)24(25)20-14-8-11-17-23(20)27(29)31/h8-11,14-17H,1-7,12-13,18-19,28H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230469
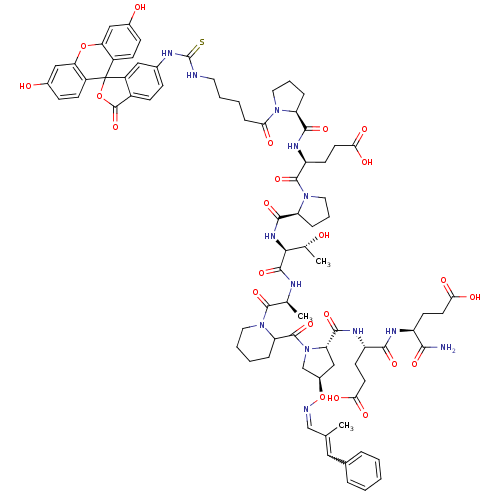
((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O)O\N=C/C(C)=Cc1ccccc1 |w:109.118| Show InChI InChI=1S/C79H95N13O23S/c1-42(35-45-13-5-4-6-14-45)40-82-115-49-39-60(72(107)86-55(26-29-65(99)100)69(104)85-54(68(80)103)25-28-64(97)98)92(41-49)76(111)59-15-8-10-32-91(59)74(109)43(2)83-73(108)67(44(3)93)88-71(106)58-17-12-34-90(58)75(110)56(27-30-66(101)102)87-70(105)57-16-11-33-89(57)63(96)18-7-9-31-81-78(116)84-46-19-22-50-53(36-46)79(114-77(50)112)51-23-20-47(94)37-61(51)113-62-38-48(95)21-24-52(62)79/h4-6,13-14,19-24,35-38,40,43-44,49,54-60,67,93-95H,7-12,15-18,25-34,39,41H2,1-3H3,(H2,80,103)(H,83,108)(H,85,104)(H,86,107)(H,87,105)(H,88,106)(H,97,98)(H,99,100)(H,101,102)(H2,81,84,116)/b42-35?,82-40-/t43-,44+,49+,54-,55-,56-,57-,58-,59?,60-,67-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50371256
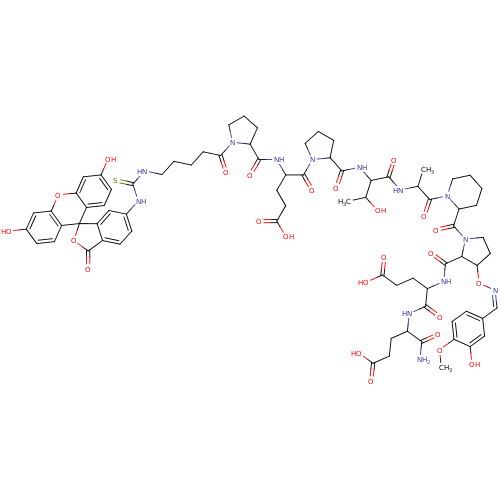
(CHEMBL1162967)Show SMILES COc1ccc(\C=N/OC2CCN(C2C(=O)NC(CCC(O)=O)C(=O)NC(CCC(O)=O)C(N)=O)C(=O)C2CCCCN2C(=O)C(C)NC(=O)C(NC(=O)C2CCCN2C(=O)C(CCC(O)=O)NC(=O)C2CCCN2C(=O)CCCCNC(=S)Nc2ccc3C(=O)OC4(c3c2)c2ccc(O)cc2Oc2cc(O)ccc42)C(C)O)cc1O Show InChI InChI=1S/C77H93N13O25S/c1-39(72(108)89-30-7-5-10-54(89)74(110)90-33-28-57(115-80-38-41-14-24-56(112-3)55(94)34-41)65(90)71(107)84-50(22-26-62(98)99)67(103)83-49(66(78)102)21-25-61(96)97)81-70(106)64(40(2)91)86-69(105)53-12-9-32-88(53)73(109)51(23-27-63(100)101)85-68(104)52-11-8-31-87(52)60(95)13-4-6-29-79-76(116)82-42-15-18-45-48(35-42)77(114-75(45)111)46-19-16-43(92)36-58(46)113-59-37-44(93)17-20-47(59)77/h14-20,24,34-40,49-54,57,64-65,91-94H,4-13,21-23,25-33H2,1-3H3,(H2,78,102)(H,81,106)(H,83,103)(H,84,107)(H,85,104)(H,86,105)(H,96,97)(H,98,99)(H,100,101)(H2,79,82,116)/b80-38- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
SHC-transforming protein 1
(Homo sapiens (Human)) | BDBM50276402
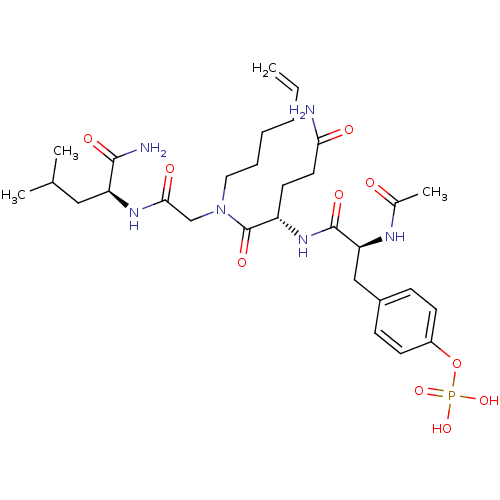
(4-((S)-2-acetamido-3-((S)-5-amino-1-((2-((S)-1-ami...)Show SMILES CC(C)C[C@H](NC(=O)CN(CCCCC=C)C(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C30H47N6O10P/c1-5-6-7-8-15-36(18-27(39)34-24(28(32)40)16-19(2)3)30(42)23(13-14-26(31)38)35-29(41)25(33-20(4)37)17-21-9-11-22(12-10-21)46-47(43,44)45/h5,9-12,19,23-25H,1,6-8,13-18H2,2-4H3,(H2,31,38)(H2,32,40)(H,33,37)(H,34,39)(H,35,41)(H2,43,44,45)/t23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... |
J Med Chem 52: 1612-8 (2009)
Article DOI: 10.1021/jm800789h
BindingDB Entry DOI: 10.7270/Q2XP74TZ |
More data for this
Ligand-Target Pair | |
SHC-transforming protein 1
(Homo sapiens (Human)) | BDBM50276346
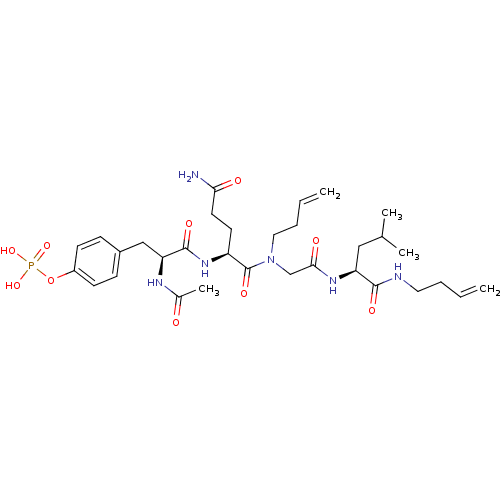
(4-((2S,5S,11S)-2-acetamido-5-(3-amino-3-oxopropyl)...)Show SMILES CC(C)C[C@H](NC(=O)CN(CCC=C)C(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(C)=O)C(=O)NCCC=C |r| Show InChI InChI=1S/C32H49N6O10P/c1-6-8-16-34-30(42)26(18-21(3)4)36-29(41)20-38(17-9-7-2)32(44)25(14-15-28(33)40)37-31(43)27(35-22(5)39)19-23-10-12-24(13-11-23)48-49(45,46)47/h6-7,10-13,21,25-27H,1-2,8-9,14-20H2,3-5H3,(H2,33,40)(H,34,42)(H,35,39)(H,36,41)(H,37,43)(H2,45,46,47)/t25-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Displacement of FITC-tagged NH-(CH2)4-CO-phospho-tyrosine-glutamine-glycine-leucine-serine-amide from Shc Src homology 2 domain (unknown origin) by f... |
J Med Chem 52: 1612-8 (2009)
Article DOI: 10.1021/jm800789h
BindingDB Entry DOI: 10.7270/Q2XP74TZ |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388553
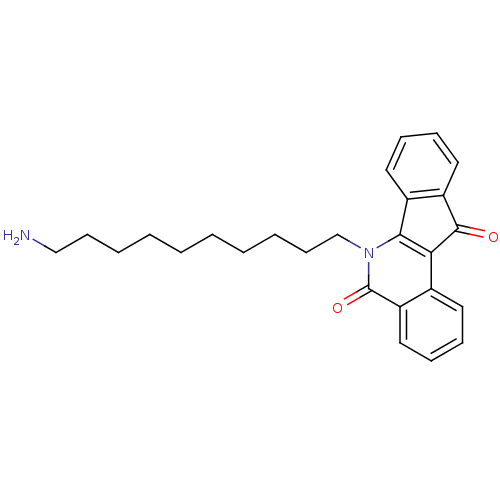
(CHEMBL223773)Show SMILES NCCCCCCCCCCn1c2-c3ccccc3C(=O)c2c2ccccc2c1=O Show InChI InChI=1S/C26H30N2O2/c27-17-11-5-3-1-2-4-6-12-18-28-24-20-14-8-9-15-21(20)25(29)23(24)19-13-7-10-16-22(19)26(28)30/h7-10,13-16H,1-6,11-12,17-18,27H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388554

(CHEMBL223545)Show SMILES NCCCCCCCCCCCn1c2-c3ccccc3C(=O)c2c2ccccc2c1=O Show InChI InChI=1S/C27H32N2O2/c28-18-12-6-4-2-1-3-5-7-13-19-29-25-21-15-9-10-16-22(21)26(30)24(25)20-14-8-11-17-23(20)27(29)31/h8-11,14-17H,1-7,12-13,18-19,28H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human Tdp1 in Tdp1-deficient chicken DT40 whole cell extract using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-p... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230462

((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1CC[C@H](O\N=C/c2ccccc2)[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O Show InChI InChI=1S/C76H91N13O23S/c1-40(71(106)88-32-9-7-14-55(88)73(108)89-35-30-56(112-79-39-42-12-4-3-5-13-42)64(89)70(105)83-51(25-28-61(96)97)66(101)82-50(65(77)100)24-27-60(94)95)80-69(104)63(41(2)90)85-68(103)54-16-11-34-87(54)72(107)52(26-29-62(98)99)84-67(102)53-15-10-33-86(53)59(93)17-6-8-31-78-75(113)81-43-18-21-46-49(36-43)76(111-74(46)109)47-22-19-44(91)37-57(47)110-58-38-45(92)20-23-48(58)76/h3-5,12-13,18-23,36-41,50-56,63-64,90-92H,6-11,14-17,24-35H2,1-2H3,(H2,77,100)(H,80,104)(H,82,101)(H,83,105)(H,84,102)(H,85,103)(H,94,95)(H,96,97)(H,98,99)(H2,78,81,113)/b79-39-/t40-,41+,50-,51-,52-,53-,54-,55?,56-,63-,64-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50124303

(3-[16-carbamoyl-31-carbamoylmethyl-10-(2-carboxyet...)Show SMILES CSCC[C@@H]1NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)C(N)=O)C(C)C Show InChI InChI=1S/C55H78N12O18S2/c1-27(2)20-36-51(81)64-37(21-29-6-10-31(68)11-7-29)52(82)61-34(15-17-45(75)76)49(79)65-39(23-41(56)70)54(84)67-46(28(3)4)55(85)58-24-42(71)59-35(18-19-86-5)50(80)63-38(22-30-8-12-32(69)13-9-30)53(83)66-40(47(57)77)25-87-26-43(72)60-33(48(78)62-36)14-16-44(73)74/h6-13,27-28,33-40,46,68-69H,14-26H2,1-5H3,(H2,56,70)(H2,57,77)(H,58,85)(H,59,71)(H,60,72)(H,61,82)(H,62,78)(H,63,80)(H,64,81)(H,65,79)(H,66,83)(H,67,84)(H,73,74)(H,75,76)/t33-,34-,35-,36-,37-,38-,39-,40-,46-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged recombinant Grb2 SH2 domain by Biacore assay |
Bioorg Med Chem Lett 19: 2693-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.134
BindingDB Entry DOI: 10.7270/Q2862HC2 |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388555

(CHEMBL388801)Show SMILES NCCCCCCCCCCCCn1c2-c3ccccc3C(=O)c2c2ccccc2c1=O Show InChI InChI=1S/C28H34N2O2/c29-19-13-7-5-3-1-2-4-6-8-14-20-30-26-22-16-10-11-17-23(22)27(31)25(26)21-15-9-12-18-24(21)28(30)32/h9-12,15-18H,1-8,13-14,19-20,29H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Tdp1 using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate assessed a... |
J Med Chem 55: 4457-78 (2012)
Article DOI: 10.1021/jm300335n
BindingDB Entry DOI: 10.7270/Q2SB46TZ |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
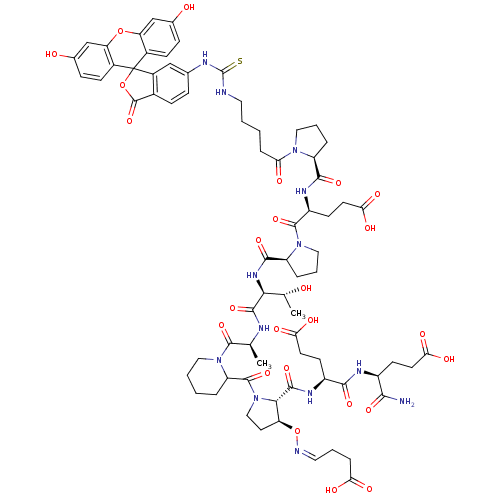
(Homo sapiens (Human)) | BDBM50230455
((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1CC[C@H](O\N=C/CCC(O)=O)[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O Show InChI InChI=1S/C73H91N13O25S/c1-37(68(105)85-30-6-4-10-51(85)70(107)86-33-27-52(111-76-29-7-14-56(91)92)61(86)67(104)80-47(22-25-58(95)96)63(100)79-46(62(74)99)21-24-57(93)94)77-66(103)60(38(2)87)82-65(102)50-12-9-32-84(50)69(106)48(23-26-59(97)98)81-64(101)49-11-8-31-83(49)55(90)13-3-5-28-75-72(112)78-39-15-18-42-45(34-39)73(110-71(42)108)43-19-16-40(88)35-53(43)109-54-36-41(89)17-20-44(54)73/h15-20,29,34-38,46-52,60-61,87-89H,3-14,21-28,30-33H2,1-2H3,(H2,74,99)(H,77,103)(H,79,100)(H,80,104)(H,81,101)(H,82,102)(H,91,92)(H,93,94)(H,95,96)(H,97,98)(H2,75,78,112)/b76-29-/t37-,38+,46-,47-,48-,49-,50-,51?,52-,60-,61-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
(Homo sapiens (Human)) | BDBM50230463

((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES COc1ccc(\C=N/O[C@@H]2C[C@H](N(C2)C(=O)C2CCCCN2C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)CCCCNC(=S)Nc2ccc3C(=O)OC4(c3c2)c2ccc(O)cc2Oc2cc(O)ccc42)[C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O)cc1OC Show InChI InChI=1S/C78H95N13O25S/c1-40(73(108)90-30-8-6-11-56(90)75(110)91-39-46(116-81-38-42-15-25-58(112-3)61(33-42)113-4)37-57(91)71(106)85-52(23-27-64(98)99)68(103)84-51(67(79)102)22-26-63(96)97)82-72(107)66(41(2)92)87-70(105)55-13-10-32-89(55)74(109)53(24-28-65(100)101)86-69(104)54-12-9-31-88(54)62(95)14-5-7-29-80-77(117)83-43-16-19-47-50(34-43)78(115-76(47)111)48-20-17-44(93)35-59(48)114-60-36-45(94)18-21-49(60)78/h15-21,25,33-36,38,40-41,46,51-57,66,92-94H,5-14,22-24,26-32,37,39H2,1-4H3,(H2,79,102)(H,82,107)(H,84,103)(H,85,106)(H,86,104)(H,87,105)(H,96,97)(H,98,99)(H,100,101)(H2,80,83,117)/b81-38-/t40-,41+,46+,51-,52-,53-,54-,55-,56?,57-,66-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |
Tumor susceptibility gene 101 protein
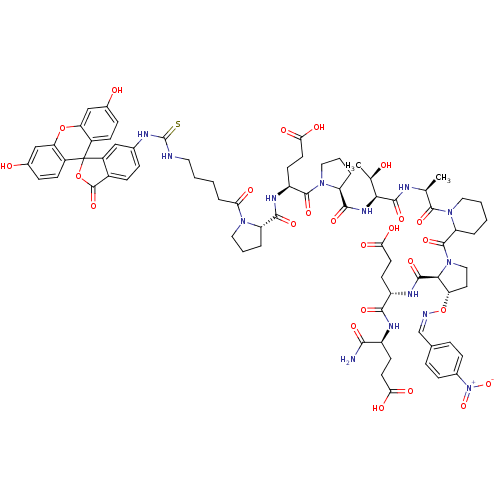
(Homo sapiens (Human)) | BDBM50230467
((4S)-4-{[(1S)-1-carbamoyl-3-carboxypropyl]carbamoy...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CCCCNC(=S)Nc1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)N[C@@H](C)C(=O)N1CCCCC1C(=O)N1CC[C@H](O\N=C/c2ccc(cc2)[N+]([O-])=O)[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O Show InChI InChI=1S/C76H90N14O25S/c1-39(71(107)88-31-6-4-9-55(88)73(109)89-34-29-56(115-79-38-41-13-16-43(17-14-41)90(111)112)64(89)70(106)83-51(24-27-61(97)98)66(102)82-50(65(77)101)23-26-60(95)96)80-69(105)63(40(2)91)85-68(104)54-11-8-33-87(54)72(108)52(25-28-62(99)100)84-67(103)53-10-7-32-86(53)59(94)12-3-5-30-78-75(116)81-42-15-20-46-49(35-42)76(114-74(46)110)47-21-18-44(92)36-57(47)113-58-37-45(93)19-22-48(58)76/h13-22,35-40,50-56,63-64,91-93H,3-12,23-34H2,1-2H3,(H2,77,101)(H,80,105)(H,82,102)(H,83,106)(H,84,103)(H,85,104)(H,95,96)(H,97,98)(H,99,100)(H2,78,81,116)/b79-38-/t39-,40+,50-,51-,52-,53-,54-,55?,56-,63-,64-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH
Curated by ChEMBL
| Assay Description
Binding affinity to Tsg101 |
Bioorg Med Chem Lett 18: 1096-101 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.003
BindingDB Entry DOI: 10.7270/Q2930V0S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data