

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120757 (US8703761, 1-29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50042058 ((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472190 (CHEMBL167638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50143817 (CHEMBL3758618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant 6His-tagged DAGT1 expressed in fall armyworm Sf9 cells using diolein and oleoyl-CoA incubated for 30 mins by LC/MS/MS... | Bioorg Med Chem Lett 26: 1245-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.025 BindingDB Entry DOI: 10.7270/Q2N29ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120755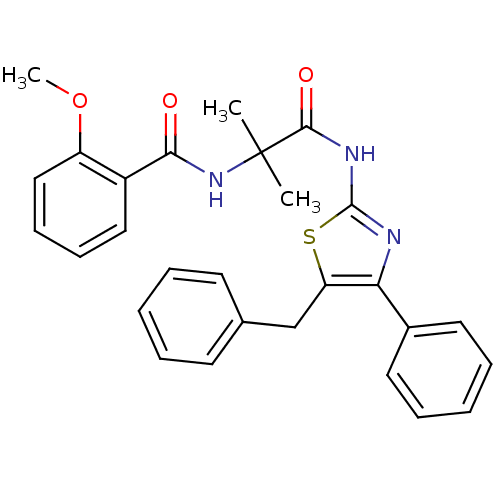 (US8703761, 1-8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239481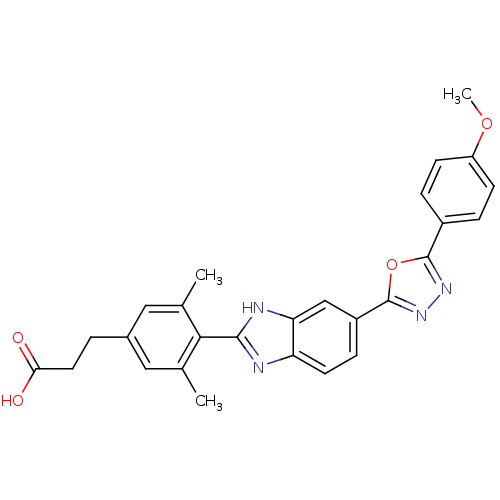 (CHEMBL4061308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50143818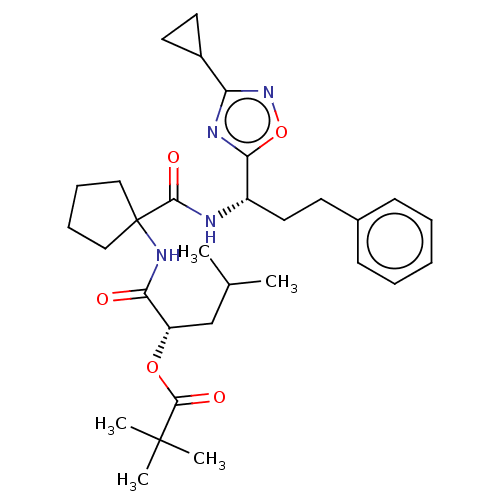 (CHEMBL3759363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant 6His-tagged DAGT1 expressed in fall armyworm Sf9 cells using diolein and oleoyl-CoA incubated for 30 mins by LC/MS/MS... | Bioorg Med Chem Lett 26: 1245-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.025 BindingDB Entry DOI: 10.7270/Q2N29ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120756 (US8703761, 1-28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239482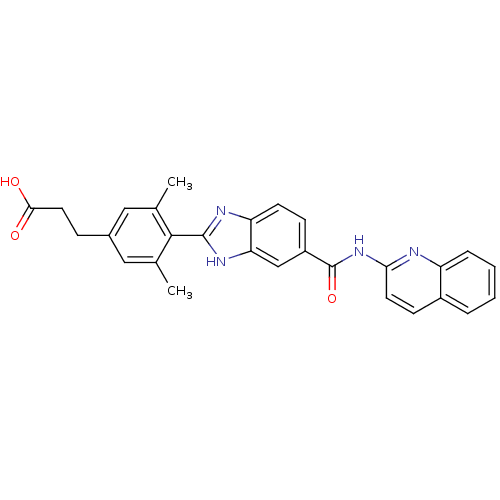 (CHEMBL1683001) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239480 (CHEMBL4074410) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239484 (CHEMBL4103025) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50143819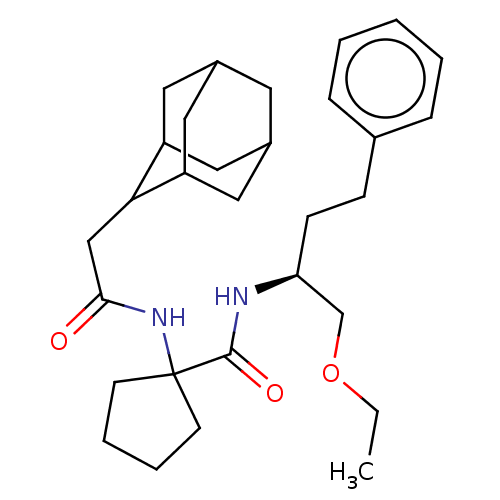 (CHEMBL3759790) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant 6His-tagged DAGT1 expressed in fall armyworm Sf9 cells using diolein and oleoyl-CoA incubated for 30 mins by LC/MS/MS... | Bioorg Med Chem Lett 26: 1245-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.025 BindingDB Entry DOI: 10.7270/Q2N29ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239483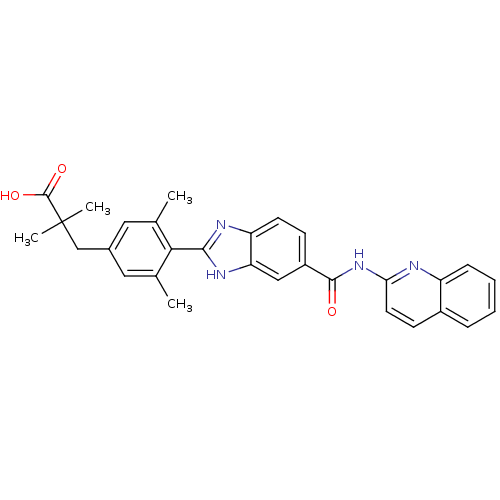 (CHEMBL4088335) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472191 (CHEMBL167166) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472193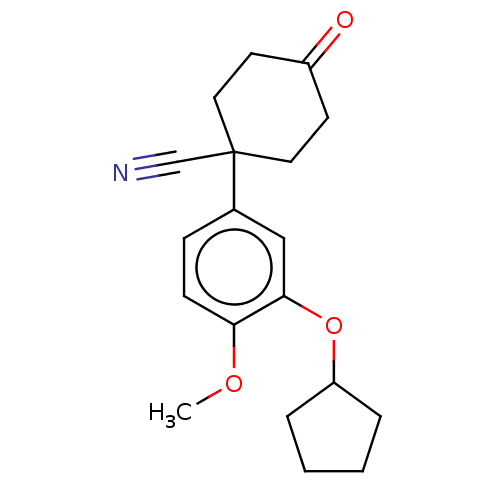 (CHEMBL352628) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50042056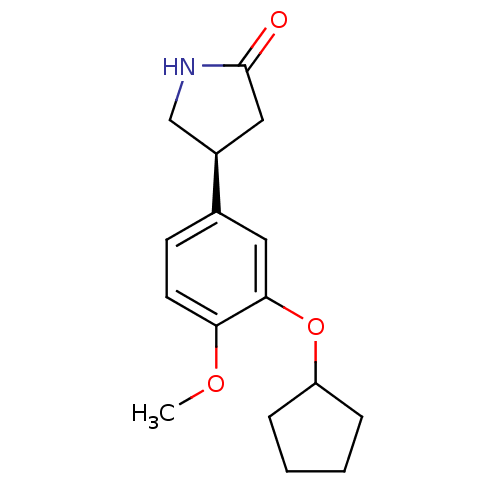 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472187 (CHEMBL169746) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239486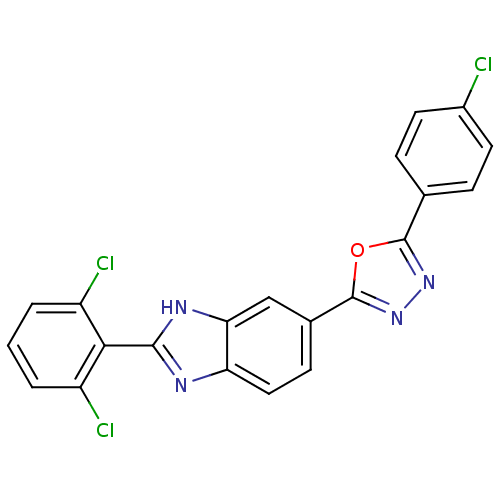 (CHEMBL4062866) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472198 (CHEMBL354447) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120759 (US8703761, 8-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120753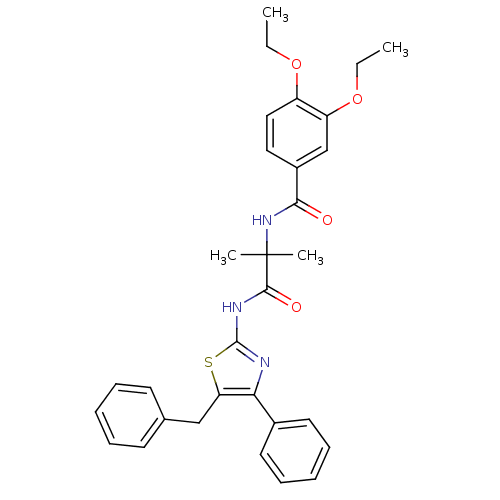 (US8703761, 1-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60.5 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50143883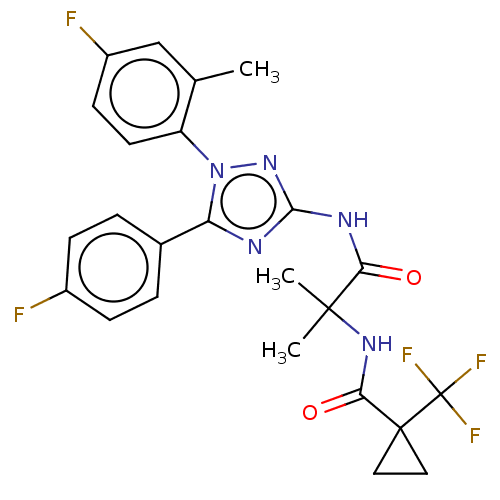 (CHEMBL3759471) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant 6His-tagged DAGT1 expressed in fall armyworm Sf9 cells using diolein and oleoyl-CoA incubated for 30 mins by LC/MS/MS... | Bioorg Med Chem Lett 26: 1245-8 (2016) Article DOI: 10.1016/j.bmcl.2016.01.025 BindingDB Entry DOI: 10.7270/Q2N29ZSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472189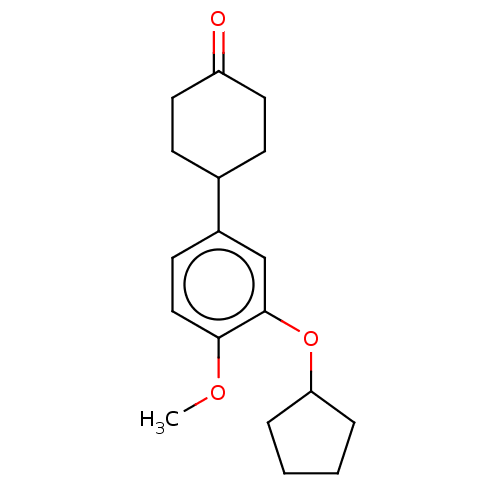 (CHEMBL167486) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472199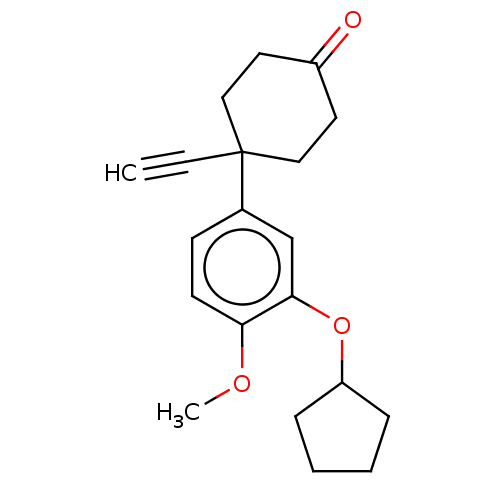 (CHEMBL169537) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472197 (CHEMBL352857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472192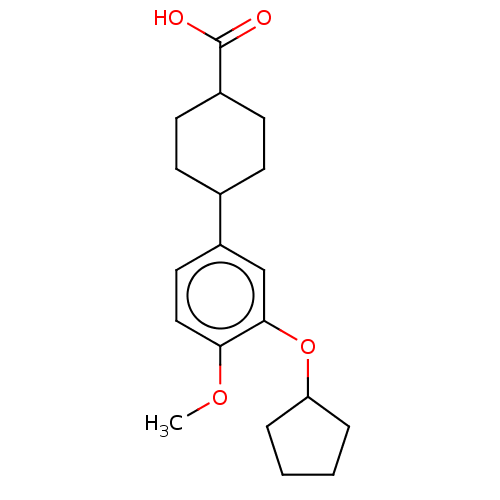 (CHEMBL167258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472193 (CHEMBL352628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472189 (CHEMBL167486) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239485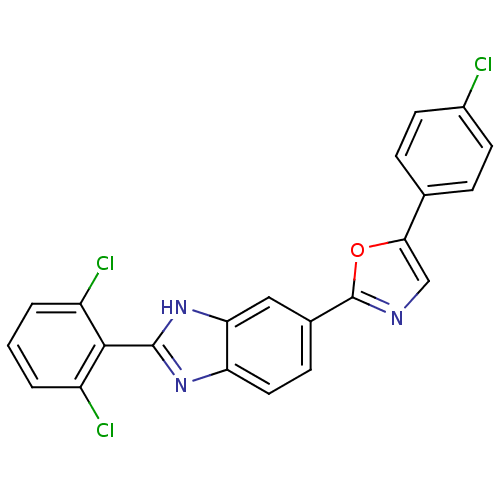 (CHEMBL4060245) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM50239480 (CHEMBL4074410) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against purine nucleoside phosphorylase (PNPase ) | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Mus musculus (mouse)) | BDBM50239481 (CHEMBL4061308) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DGTA1 in mouse C2C12 cells assessed as inhibition of triglyceride formation after 2 hrs by LC/MS/MS analysis | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472191 (CHEMBL167166) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120758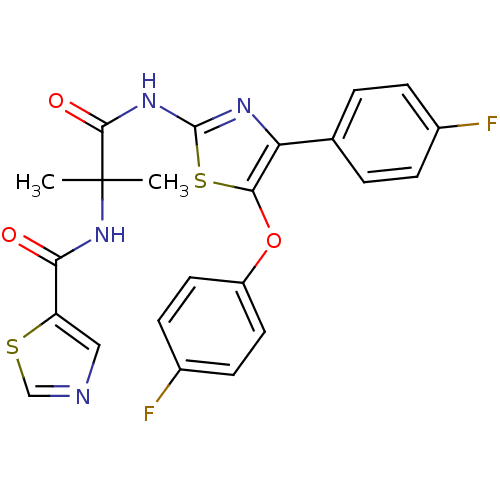 (US8703761, 6-14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 197 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120763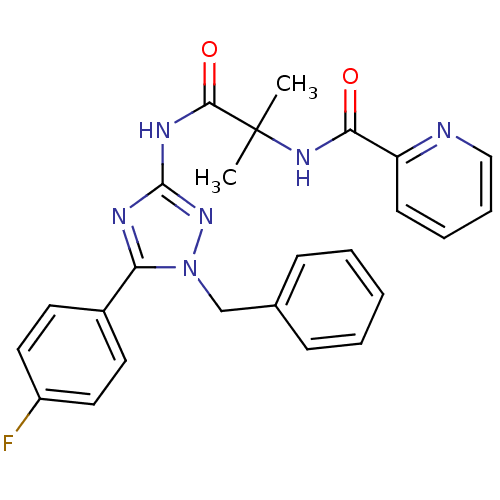 (US8703761, 14-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 225 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120760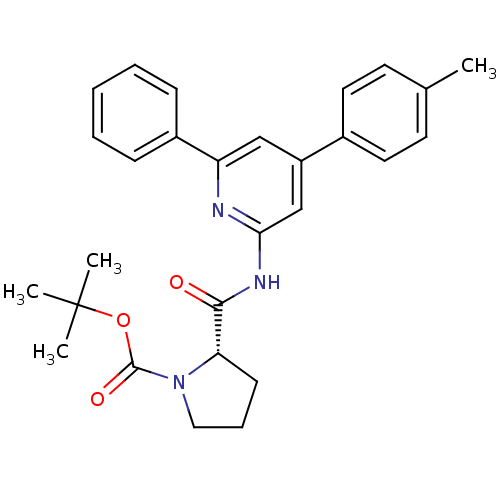 (US8703761, 8-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [89-488] (Homo sapiens (Human)) | BDBM120754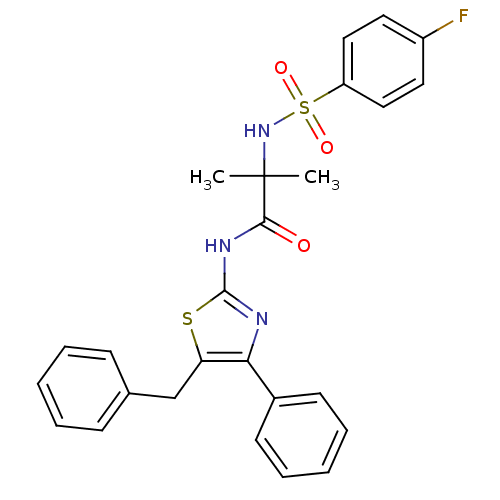 (US8703761, 1-6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 241 | n/a | n/a | n/a | n/a | 7.5 | 4 |
Novartis AG US Patent | Assay Description The enzyme preparation used in this assay is a membrane preparation from Sf9 cells overexpressing human (His)6DGAT1. During all steps samples were ch... | US Patent US8703761 (2014) BindingDB Entry DOI: 10.7270/Q2057DM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50239479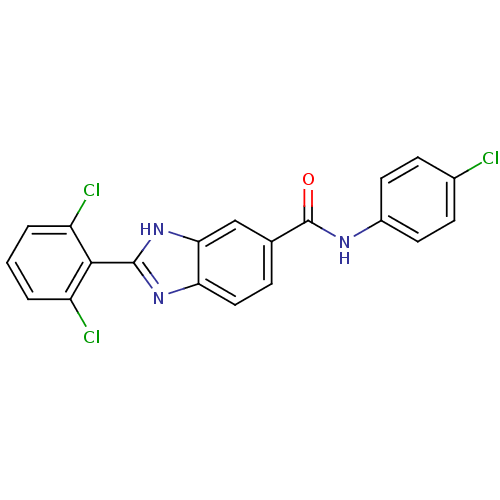 (CHEMBL4088473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged DGAT1 expressed in Sf9 insect cell membranes assessed as inhibition of triglyceride formation using diole... | J Med Chem 60: 4657-4664 (2017) Article DOI: 10.1021/acs.jmedchem.7b00173 BindingDB Entry DOI: 10.7270/Q2B27XF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472199 (CHEMBL169537) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50042058 ((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472195 (CHEMBL166529) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472198 (CHEMBL354447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472194 (CHEMBL169834) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472194 (CHEMBL169834) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50472187 (CHEMBL169746) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |