

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200169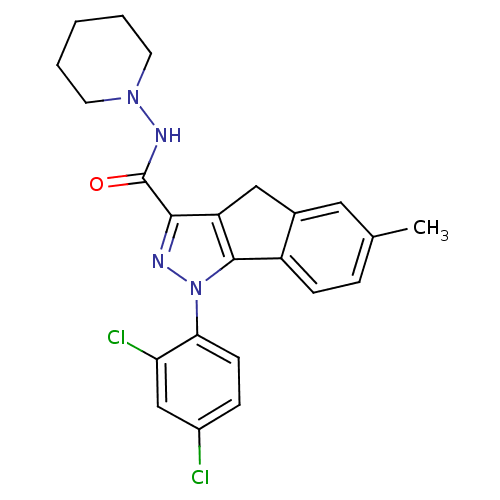 (6-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180022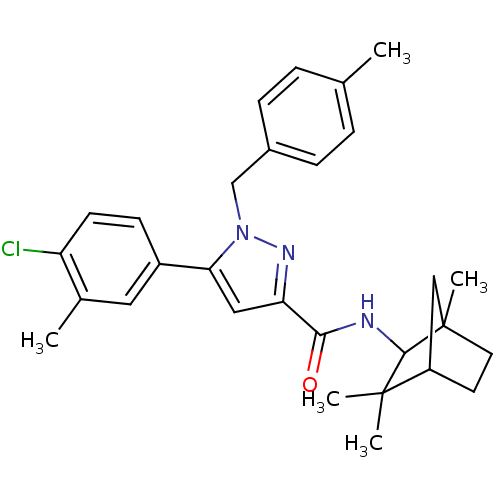 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor (unknown origin) | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020597 (CHEMBL3290447) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020600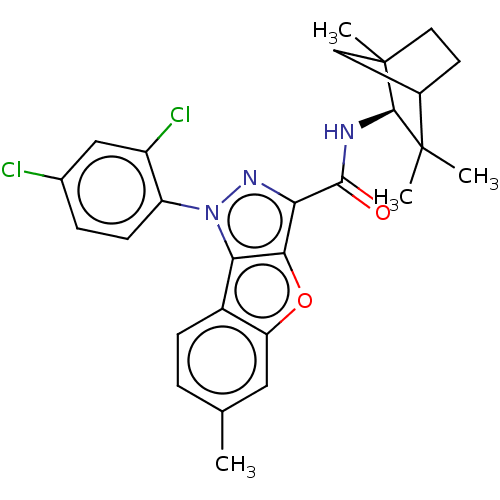 (CHEMBL3290450) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020599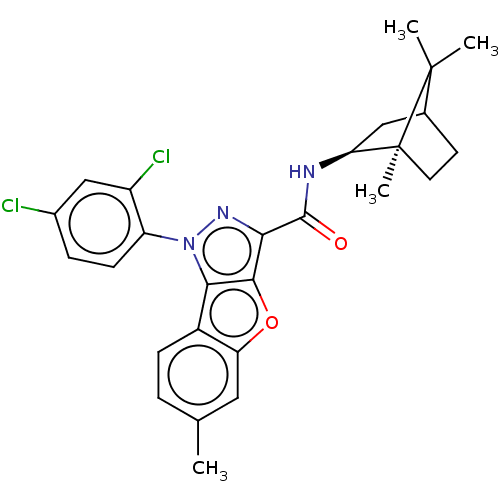 (CHEMBL3290449) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020598 (CHEMBL3290448) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020591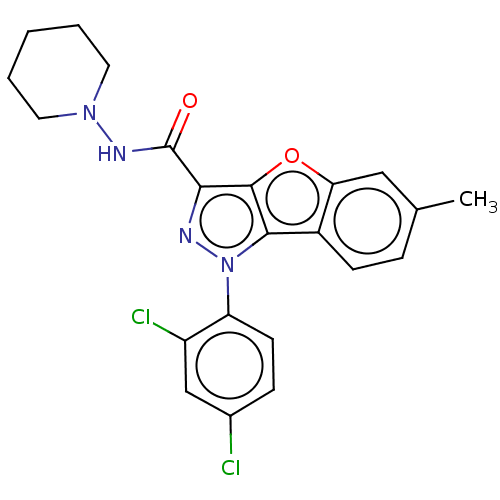 (CHEMBL3290442) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020594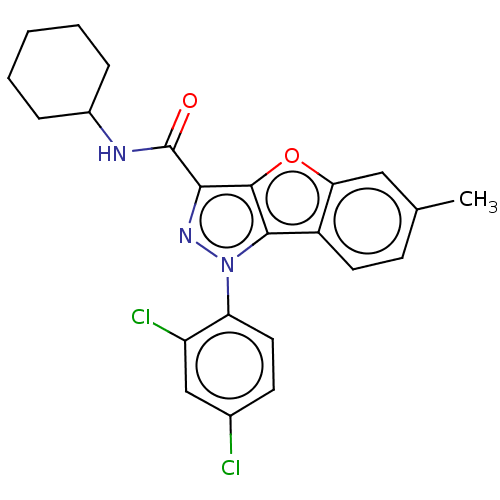 (CHEMBL3290444) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020595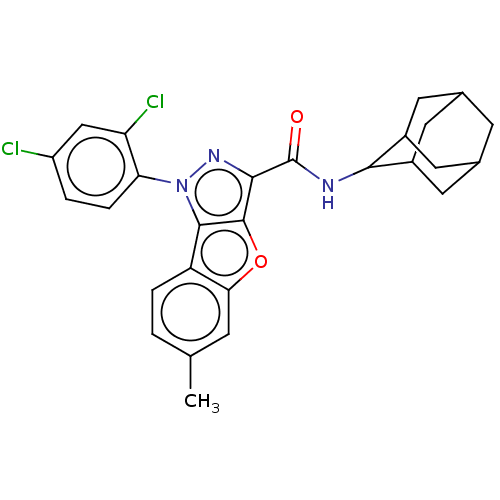 (CHEMBL3290445) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020596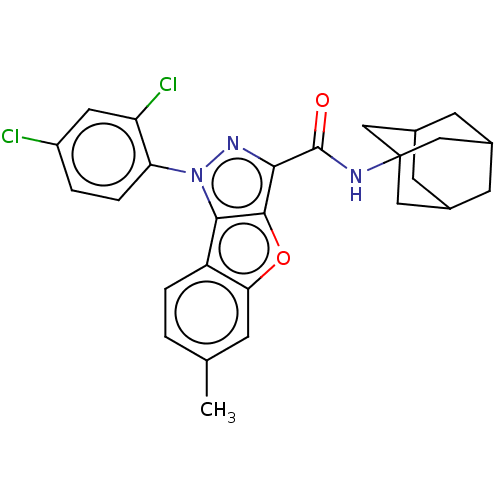 (CHEMBL3290446) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020592 (CHEMBL3286437) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50020593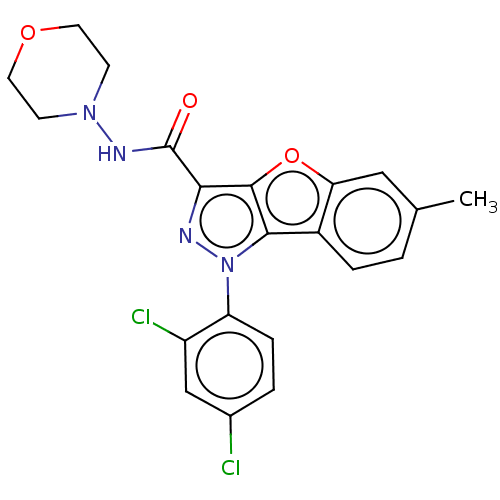 (CHEMBL3290443) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB2 receptor in CD1 mouse spleen homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020597 (CHEMBL3290447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50180022 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor (unknown origin) | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020600 (CHEMBL3290450) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200169 (6-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020596 (CHEMBL3290446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020599 (CHEMBL3290449) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50326197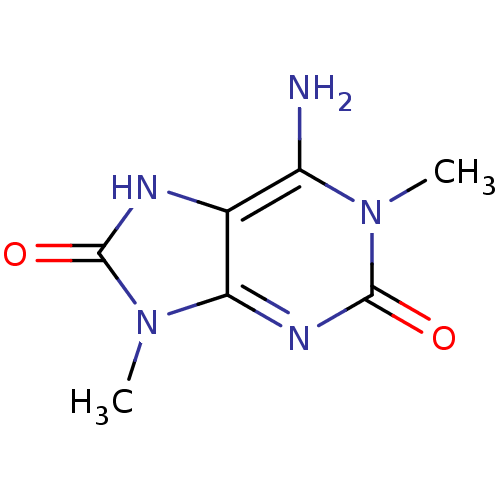 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha1D receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020595 (CHEMBL3290445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50326197 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 758 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha1B receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50326197 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 885 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha1A receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020594 (CHEMBL3290444) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020591 (CHEMBL3290442) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50326197 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020598 (CHEMBL3290448) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020592 (CHEMBL3286437) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50326197 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to 5HT1E receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50020593 (CHEMBL3290443) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP-55,940 from CB1 receptor in CD1 mouse cerebellum-less brain homogenate after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 82: 281-92 (2014) Article DOI: 10.1016/j.ejmech.2014.05.055 BindingDB Entry DOI: 10.7270/Q2D2206G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Homo sapiens (Human)) | BDBM50326197 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to nicotinic alpha3beta2 receptor receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50326197 (1,9-dimethyl-2,8-dioxo-2,3,8,9-tetrahydro-1H-purin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor | J Med Chem 53: 6089-99 (2010) Article DOI: 10.1021/jm100490m BindingDB Entry DOI: 10.7270/Q2GX4BS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426601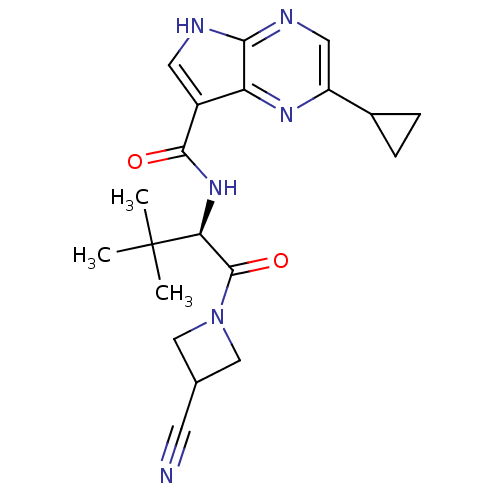 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426599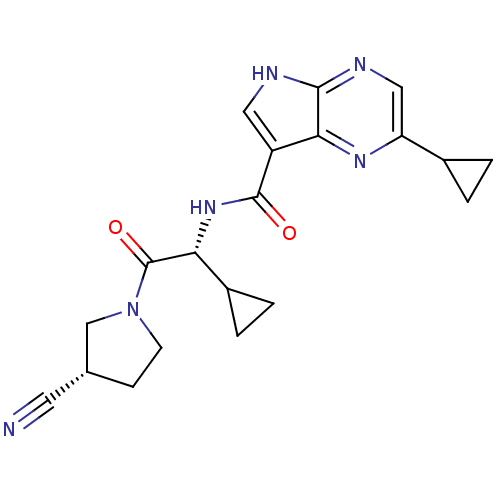 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426607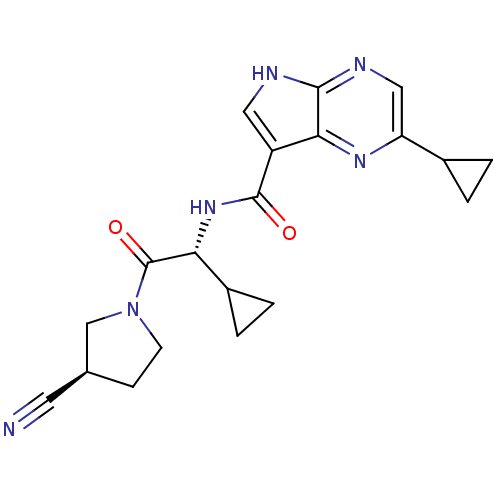 (CHEMBL2325897) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426599 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426599 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426601 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341370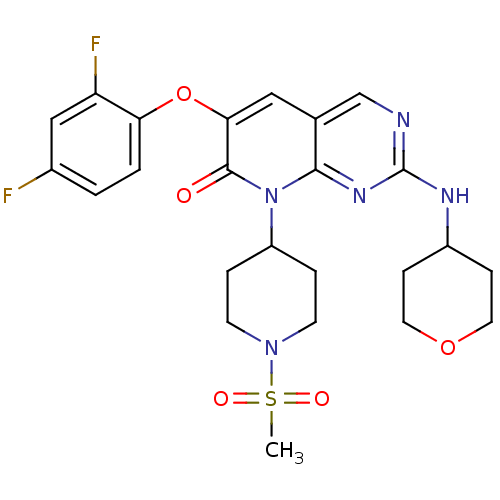 (6-(2,4-Difluorophenoxy)-8-(1-methanesulfonyl-piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341361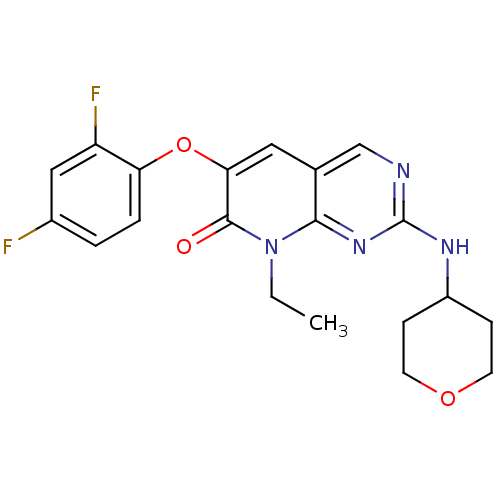 (6-(2,4-Difluorophenoxy)-8-ethyl-2-(tetrahydro-2H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341366 (6-(2,4-Difluorophenoxy)-8-(1,1-dioxo-hexahydro-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341350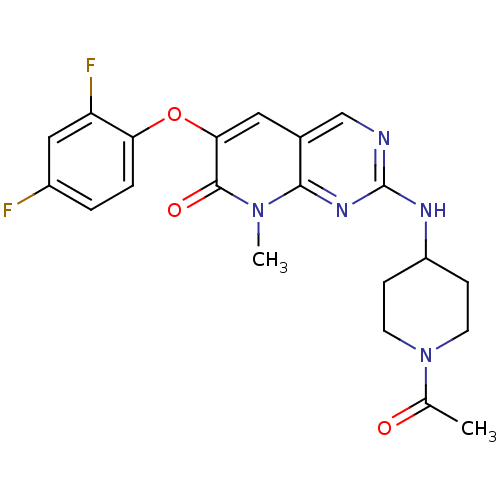 (2-(1-Acetyl-piperidin-4-ylamino)-6-(2,4-difluoroph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426606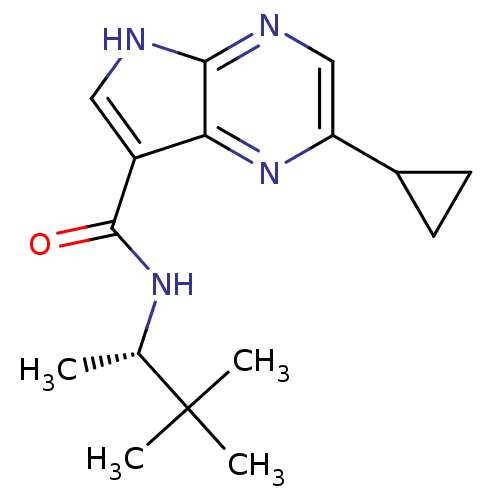 (CHEMBL2325903) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426604 (CHEMBL2325906) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341369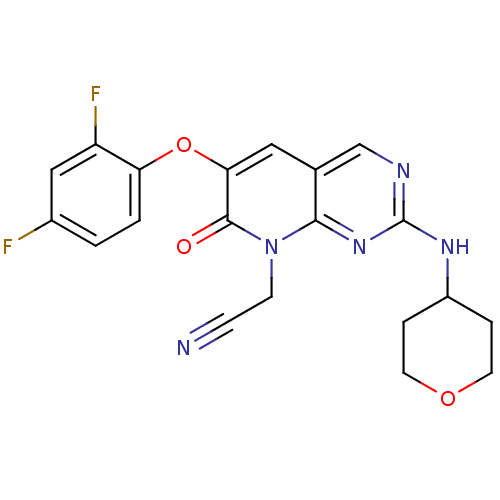 (CHEMBL1766506 | [6-(2,4-Difluorophenoxy)-7-oxo-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341363 (6-(2,4-Difluorophenoxy)-8-(3-hydroxy-propyl)-2-(te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50341356 (6-(2,4-Difluorophenoxy)-8-methyl-2-((S)-1-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha assessed as incorporation of 33P from gamma-[33P]ATP into myelin basic protein after 30 mins by scintillatio... | J Med Chem 54: 2255-65 (2011) Article DOI: 10.1021/jm101423y BindingDB Entry DOI: 10.7270/Q20P109X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426601 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 206 total ) | Next | Last >> |