

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738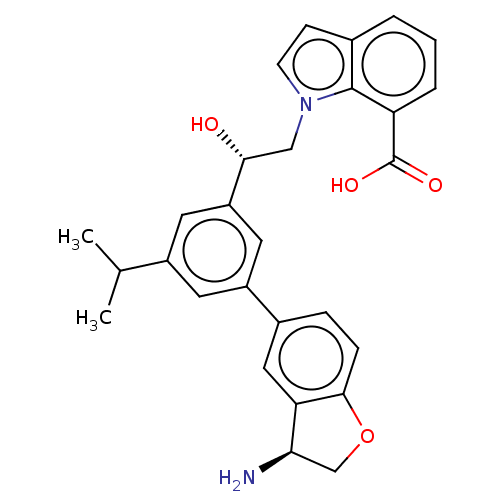 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542741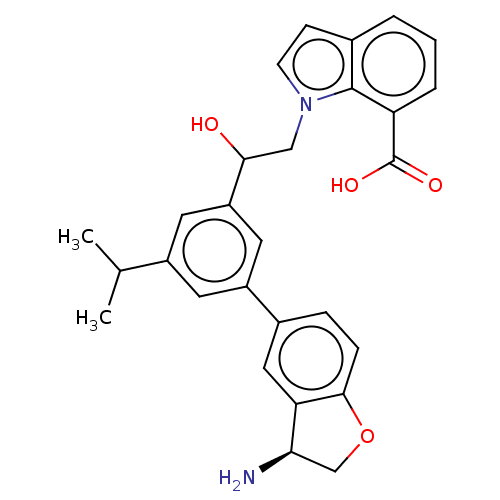 (CHEMBL4647950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731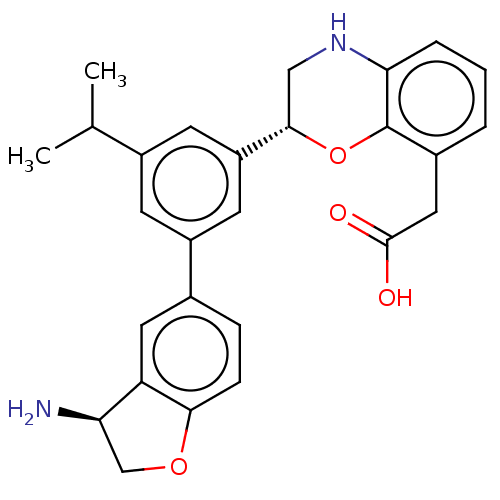 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24482 (7-methoxy-4-{[6-(4-methylthiophen-2-yl)-[1,2,4]tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50531540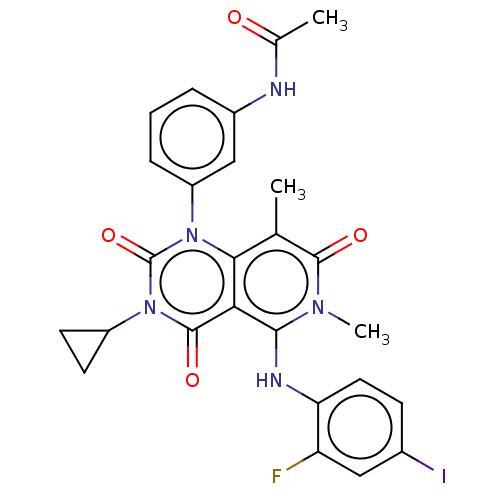 (CHEBI:75998 | GSK-1120212 | GSK1120212 | JTP 74057...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MEK in human KYSE-520 cells assessed as reduction in p-ERK levels | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24479 (2-chloro-4-(3-{[(7-methoxyquinolin-4-yl)oxy]methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24480 (7-methoxy-4-{[6-(thiophen-2-yl)-[1,2,4]triazolo[4,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-activated inward rectifier potassium channel 4 (Homo sapiens (Human)) | BDBM50613996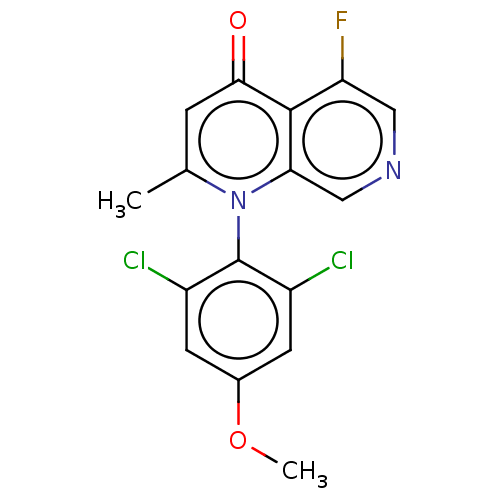 (CHEMBL5281961) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542740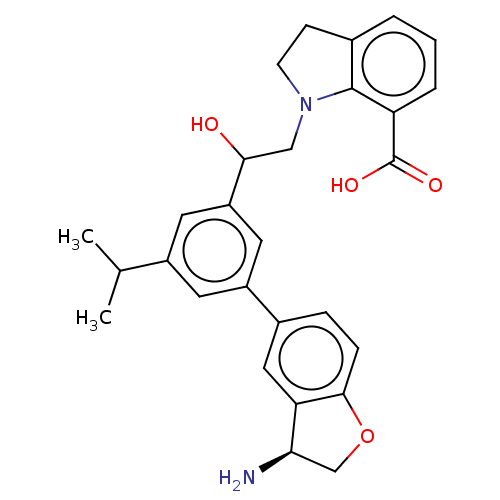 (CHEMBL4646398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542740 (CHEMBL4646398) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542724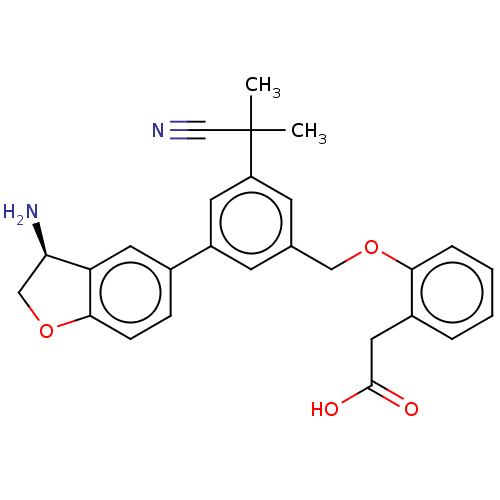 (CHEMBL4636415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542723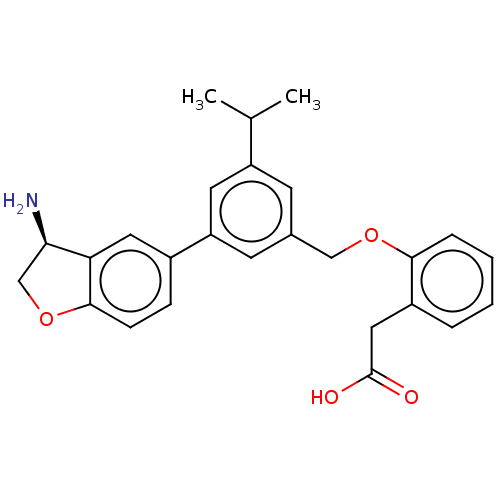 (CHEMBL4643449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542733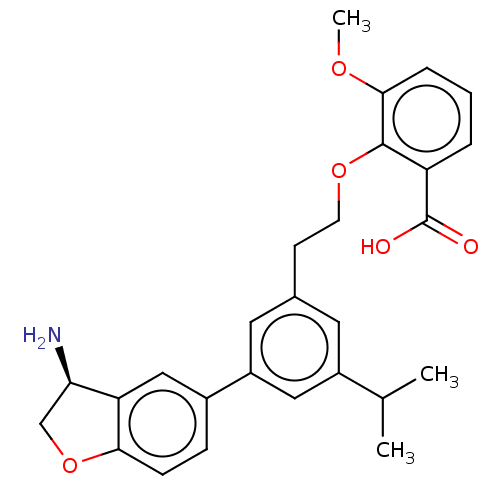 (CHEMBL4646141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542728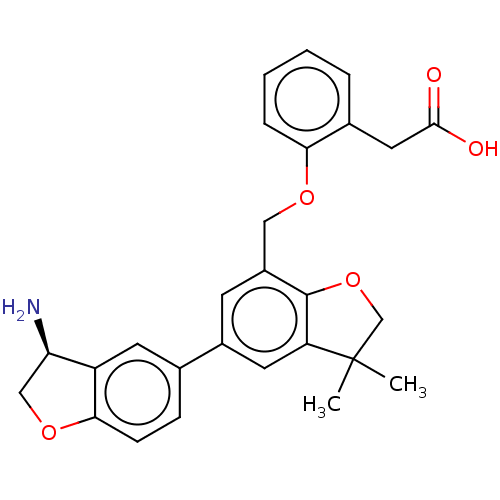 (CHEMBL4635912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24478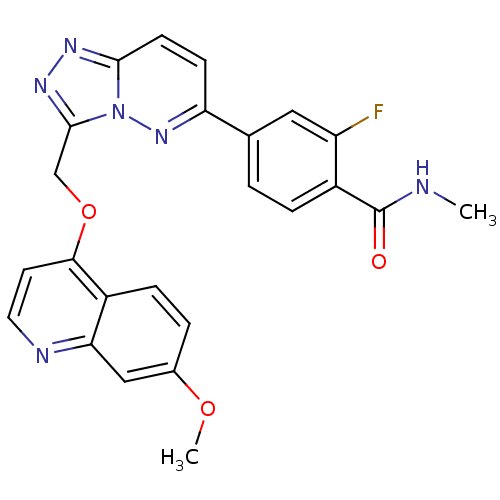 (2-fluoro-4-(3-{[(7-methoxyquinolin-4-yl)oxy]methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542723 (CHEMBL4643449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24483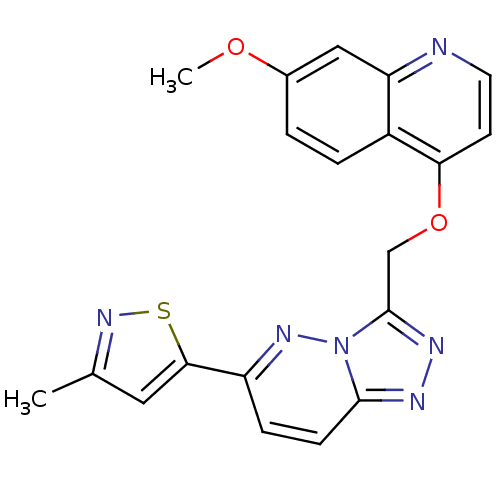 (5-(3-{[(7-methoxyquinolin-4-yl)oxy]methyl}-[1,2,4]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24476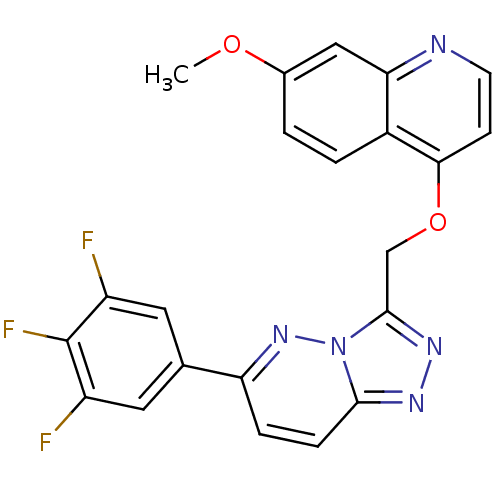 (7-methoxy-4-{[6-(3,4,5-trifluorophenyl)-[1,2,4]tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24481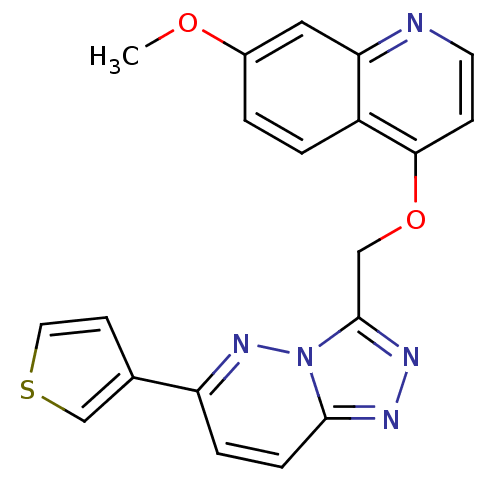 (7-methoxy-4-{[6-(thiophen-3-yl)-[1,2,4]triazolo[4,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542724 (CHEMBL4636415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542725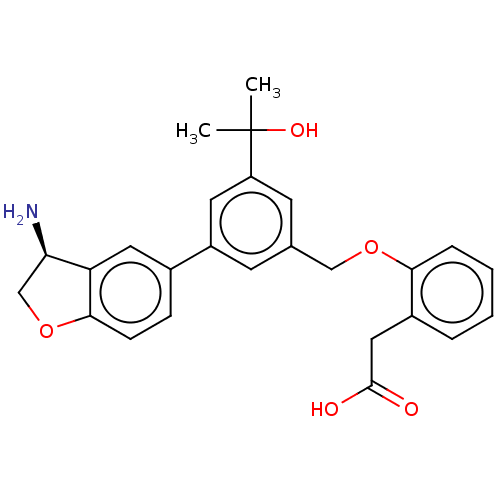 (CHEMBL4637683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542730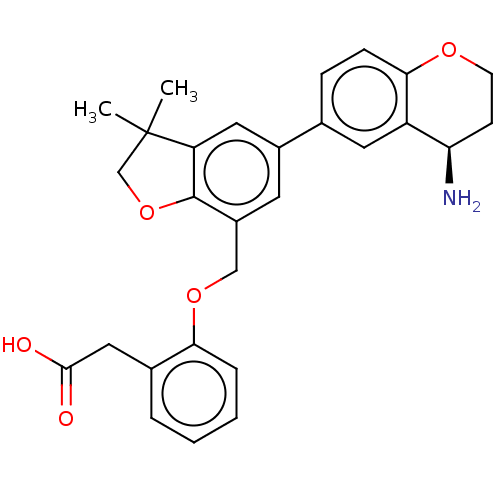 (CHEMBL4647909) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24477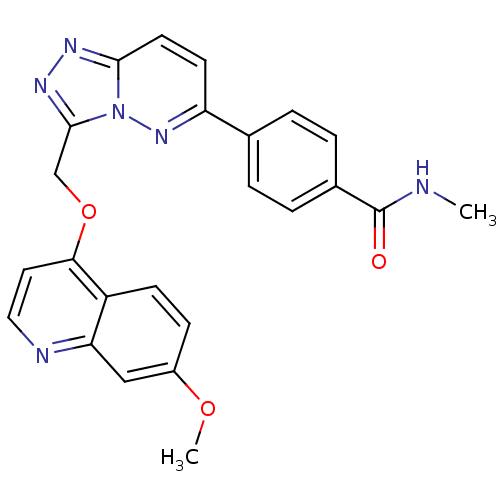 (4-(3-{[(7-methoxyquinolin-4-yl)oxy]methyl}-[1,2,4]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542735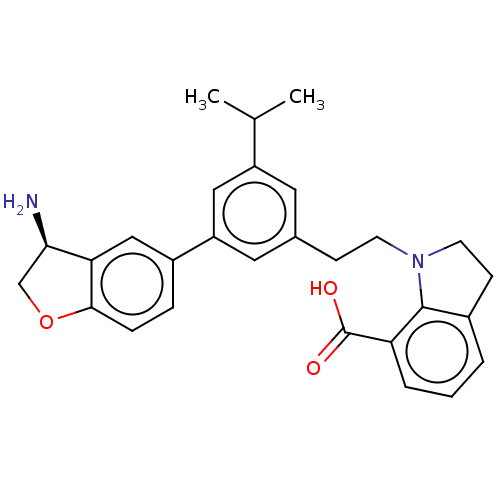 (CHEMBL4635286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542730 (CHEMBL4647909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542725 (CHEMBL4637683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408067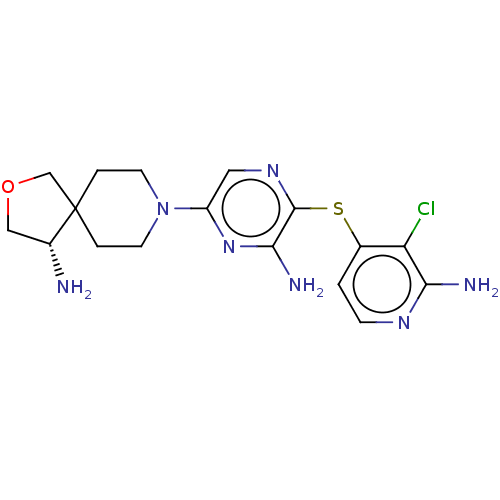 (US10336774, Example 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542728 (CHEMBL4635912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24475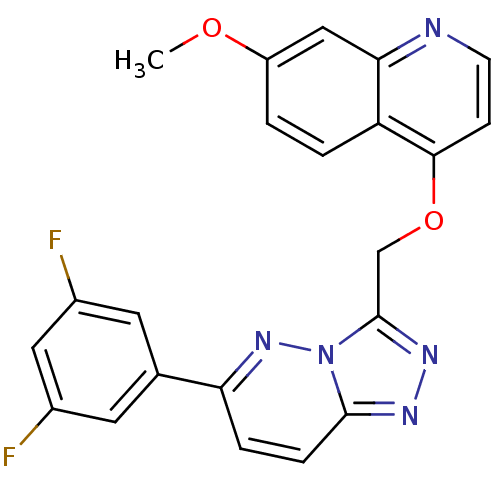 (4-{[6-(3,5-difluorophenyl)-[1,2,4]triazolo[4,3-a]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24472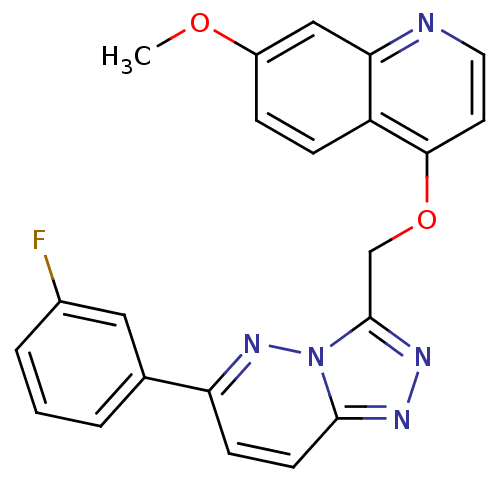 (4-{[6-(3-fluorophenyl)-[1,2,4]triazolo[4,3-a]pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50524338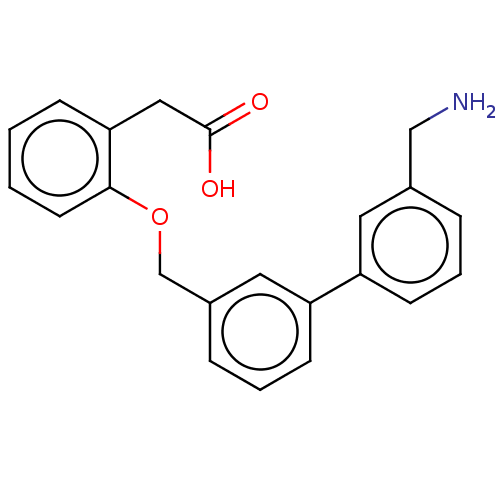 (CHEMBL4468000) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human complement FD by TR-FRET assay | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-activated inward rectifier potassium channel 4 (Homo sapiens (Human)) | BDBM585727 (8-chloro-1-(2,6-dichlorophenyl)-5- (2-hydroxyethox...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24470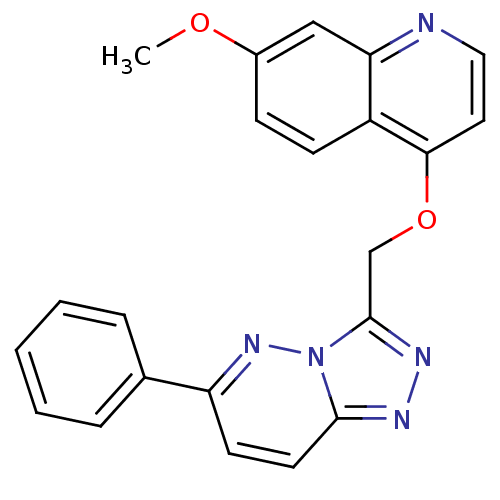 (7-methoxy-4-({6-phenyl-[1,2,4]triazolo[4,3-a]pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542733 (CHEMBL4646141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542726 (CHEMBL4642766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24474 (4-{[6-(3,4-difluorophenyl)-[1,2,4]triazolo[4,3-a]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553790 (CHEMBL4763213) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50539763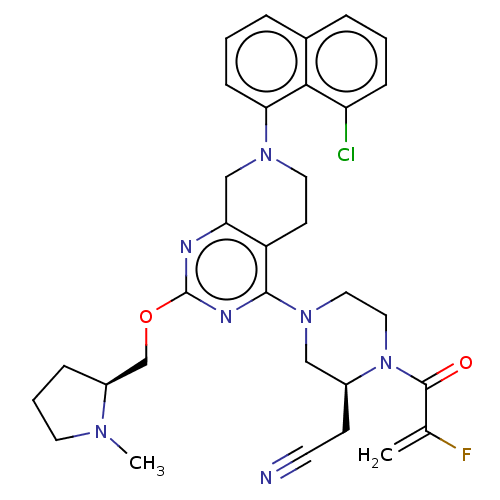 (Adagrasib | Mrtx-849 | Mrtx849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KRAS G12C mutant in human MIA PaCa-2 cells assessed as reduction in p-ERK levels | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553783 (Ptpn11 inhibitor tno155 | Shp2 inhibitor tno155 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542726 (CHEMBL4642766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-activated inward rectifier potassium channel 4 (Homo sapiens (Human)) | BDBM50613997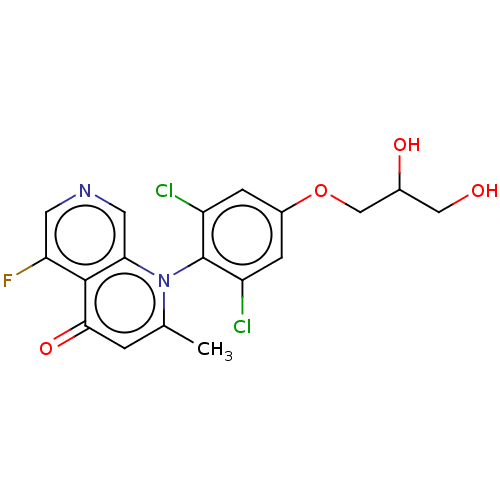 (CHEMBL5280047) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553783 (Ptpn11 inhibitor tno155 | Shp2 inhibitor tno155 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553787 (CHEMBL4755819) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542732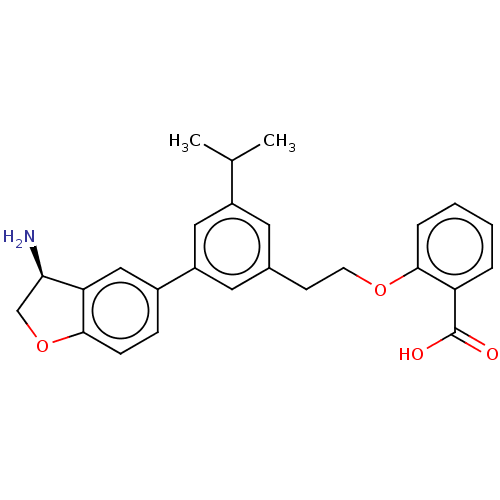 (CHEMBL4647925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24471 (4-{[6-(4-fluorophenyl)-[1,2,4]triazolo[4,3-a]pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. Mole... | J Med Chem 51: 2879-82 (2008) Article DOI: 10.1021/jm800043g BindingDB Entry DOI: 10.7270/Q29P2ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553790 (CHEMBL4763213) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 345 total ) | Next | Last >> |