

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50300196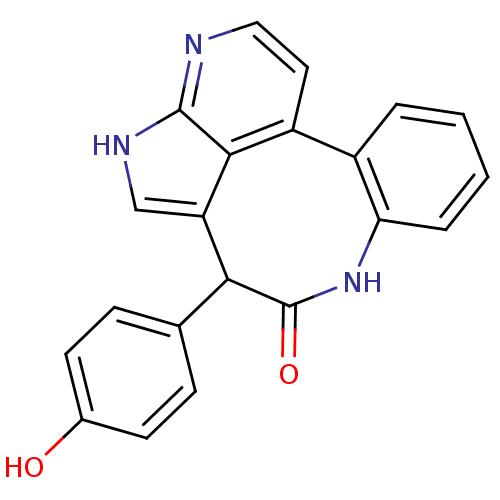 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK2 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK3 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK3 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50272298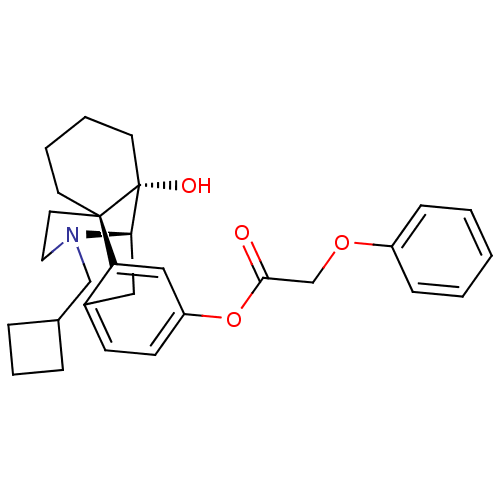 ((-)-N-cyclobutylmethylmorphinan-3-yl-14-ol phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135806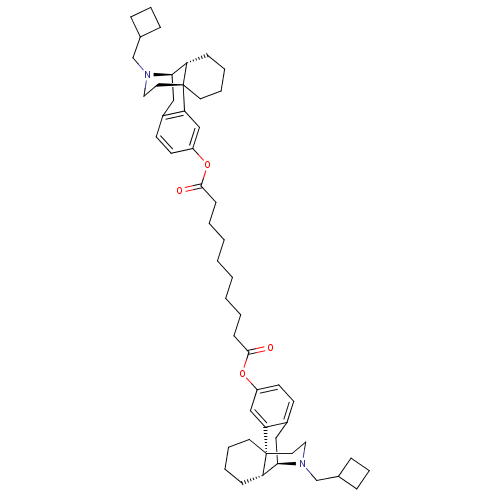 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272298 ((-)-N-cyclobutylmethylmorphinan-3-yl-14-ol phenoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135808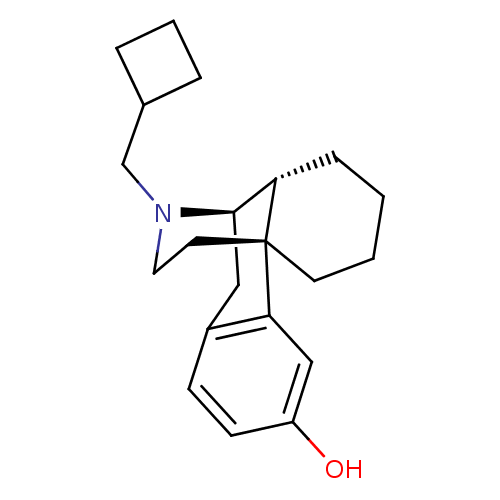 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240437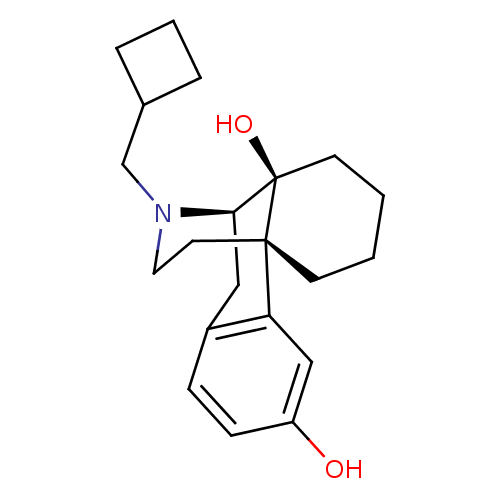 ((-)-17-(cyclobutylmethyl)morphinan-3,14-diol | (-)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50313228 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-[(5-{[(1R,9R,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50313237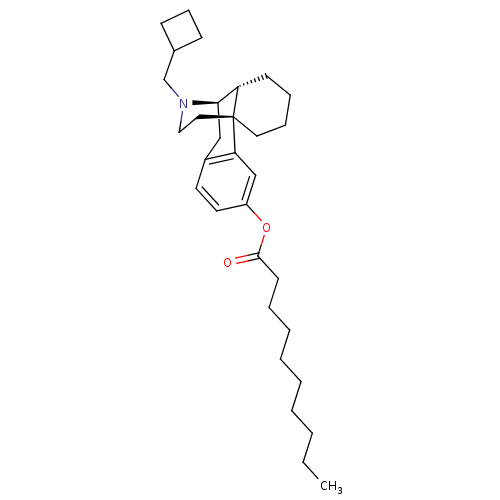 ((1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50272328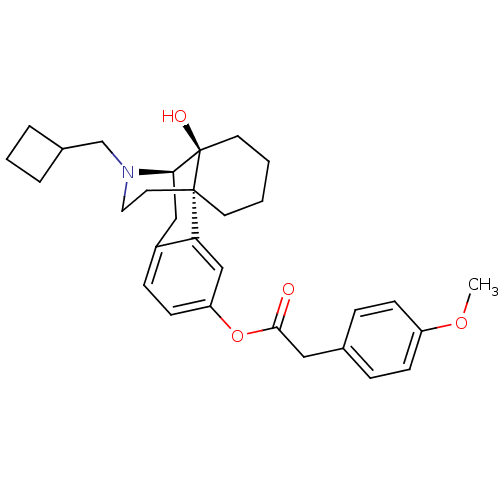 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50017233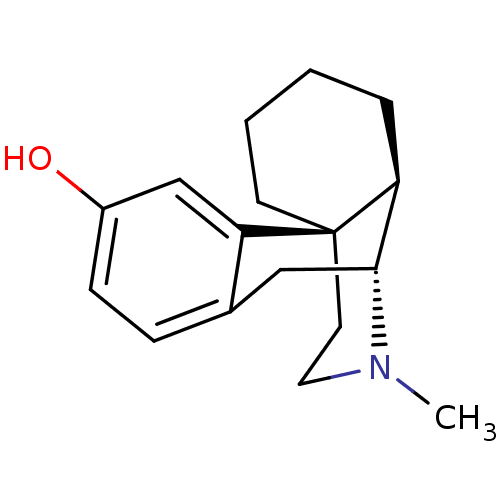 (CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313237 ((1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50240437 ((-)-17-(cyclobutylmethyl)morphinan-3,14-diol | (-)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272328 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313234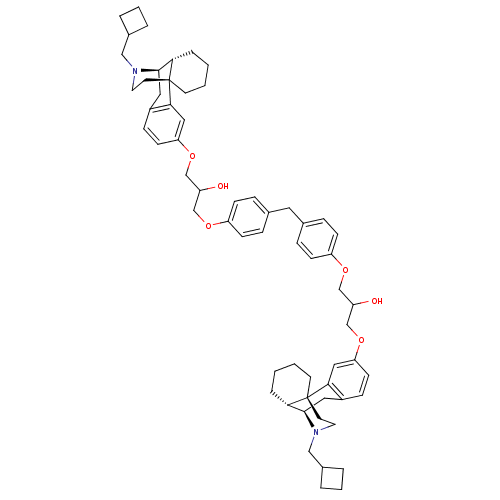 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-[3-(4-{[4-(3-{...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521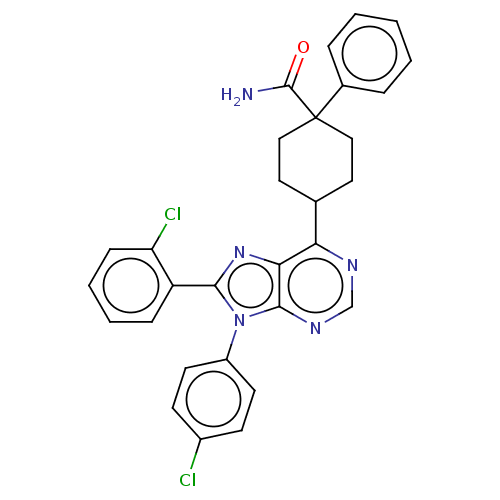 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300372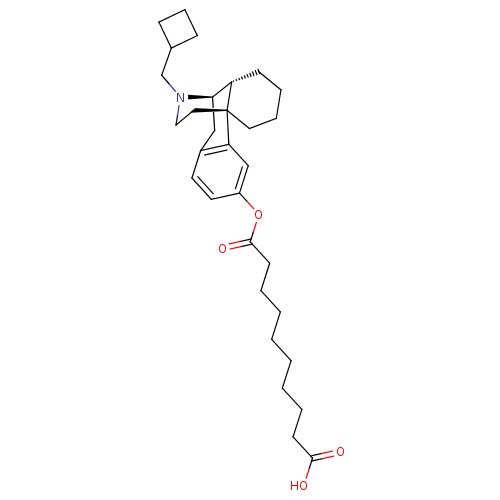 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272296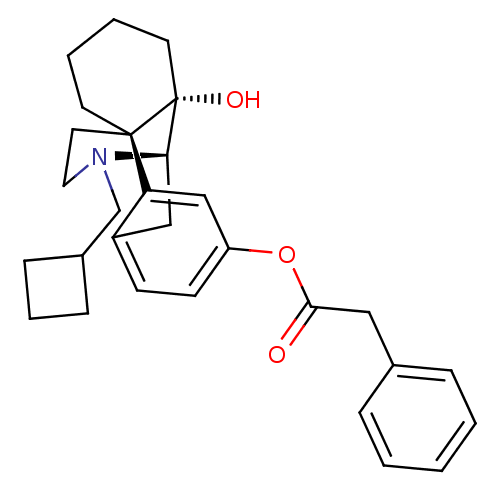 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50313234 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-[3-(4-{[4-(3-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300378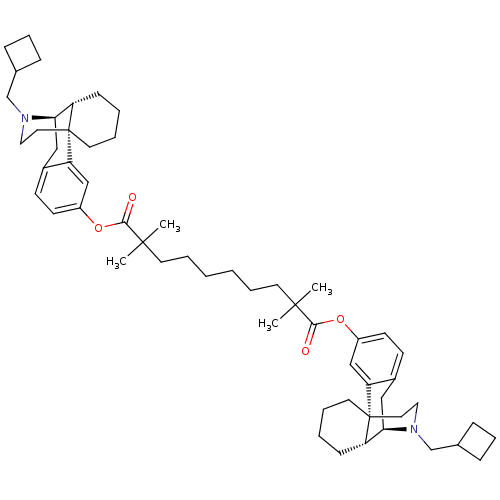 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300378 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50272296 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300377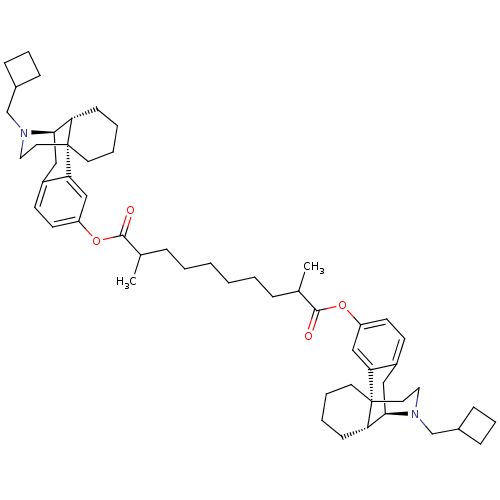 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300372 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50272257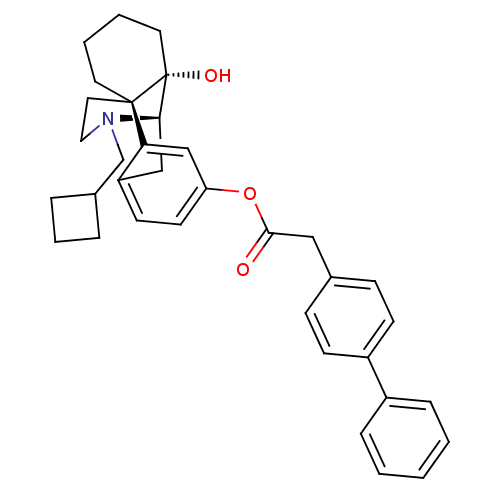 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300374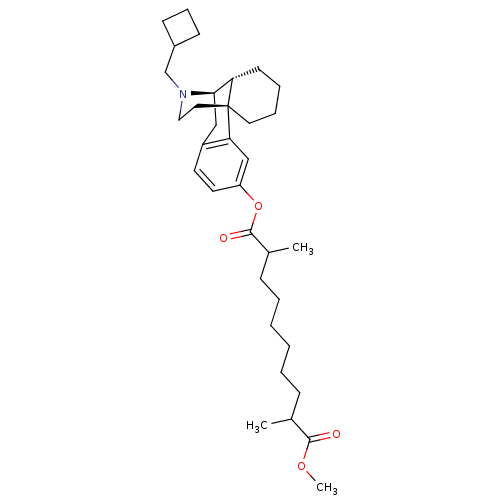 (CHEMBL566762 | Methyl 17-((-)-N-Cyclobutylmethyl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300377 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313227 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-(3-{[(1R,9R,10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50313227 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-(3-{[(1R,9R,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313228 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-[(5-{[(1R,9R,1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50272297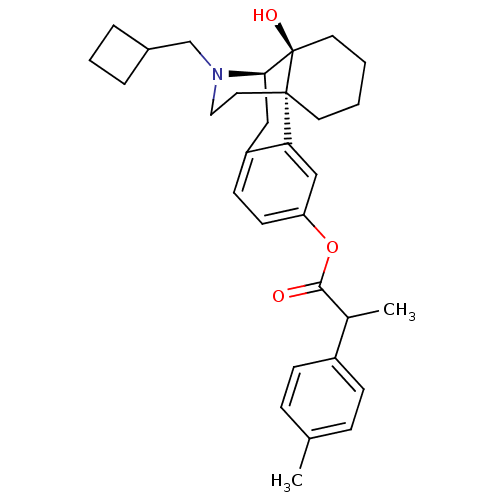 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50313233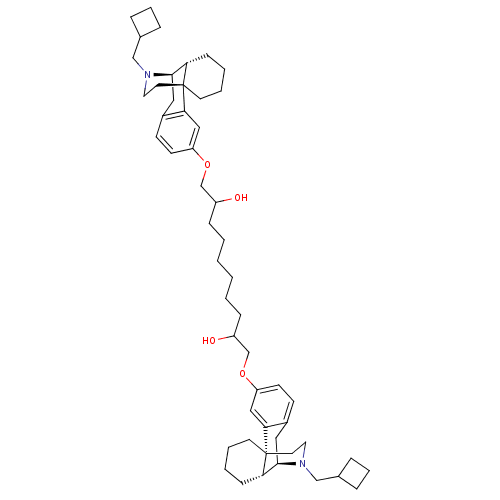 ((1R,9R,10R)-17-(cyclobutylmethyl)-4-[(10-{[(1R,9R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 1507-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.101 BindingDB Entry DOI: 10.7270/Q2251JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192536 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300374 (CHEMBL566762 | Methyl 17-((-)-N-Cyclobutylmethyl)m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192534 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192538 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300379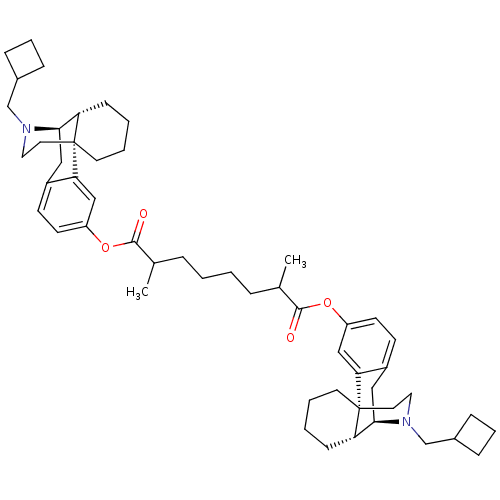 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,7-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272297 ((1S,9R,10S)-17-(cyclobutylmethyl)-10-hydroxy-17-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 4474-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.054 BindingDB Entry DOI: 10.7270/Q2K07423 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 900 total ) | Next | Last >> |