 Found 455 hits with Last Name = 'gavaldà' and Initial = 'a'
Found 455 hits with Last Name = 'gavaldà' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50094037
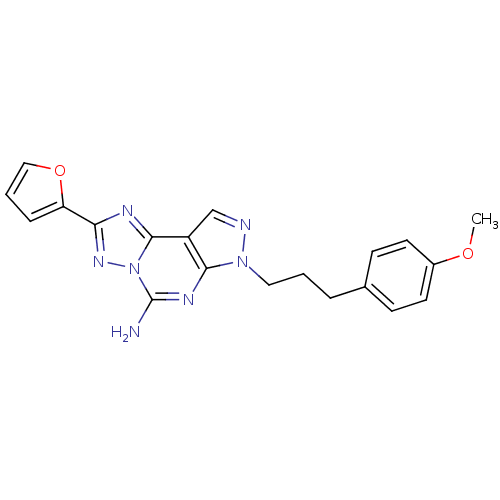
(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296336
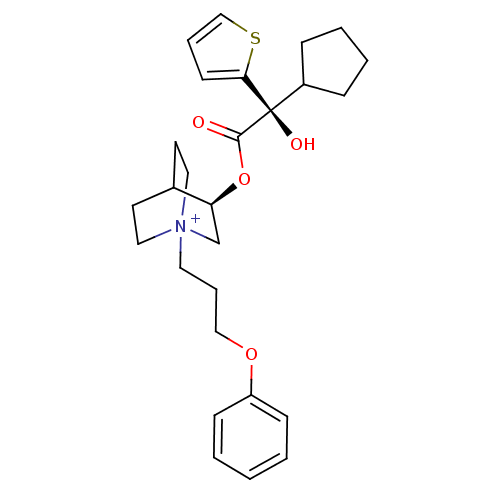
((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296336

((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296331
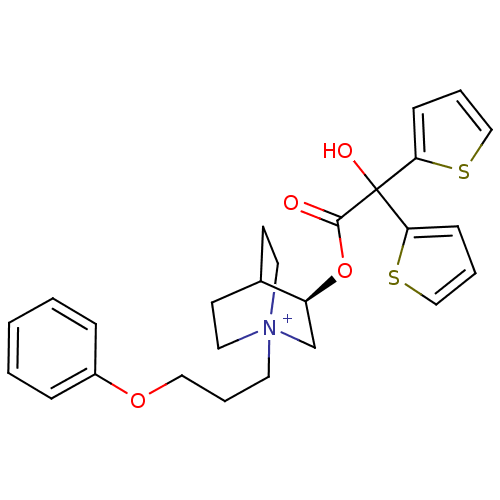
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296329
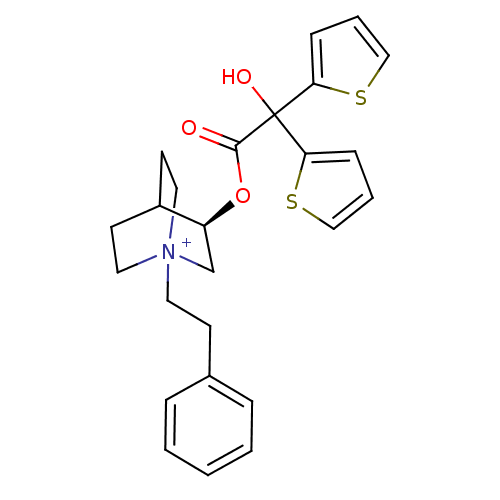
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296329

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296331

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296331

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296329

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296336

((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50378083
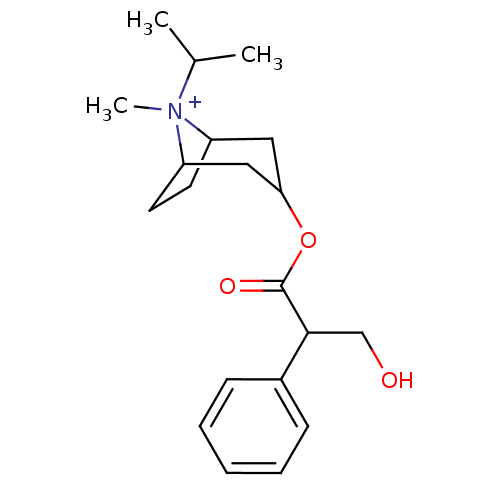
(Atrovent HFA | IPRATROPIUM BROMIDE)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:4:3:10.11.9:6.7,THB:12:10:3:6.7,1:3:10.11.9:6.7,(8.93,-5.03,;7.4,-5.04,;6.62,-3.72,;6.64,-6.39,;5.29,-5.61,;6.91,-7.92,;5.92,-9.27,;7.1,-8.56,;7.69,-7.22,;9.45,-7.19,;9.72,-8.74,;8.74,-7.92,;9.71,-10.27,;11.04,-11.06,;11.03,-12.6,;12.38,-10.29,;13.71,-11.07,;15.04,-10.31,;12.39,-8.75,;13.74,-8,;13.74,-6.46,;12.41,-5.67,;11.07,-6.44,;11.06,-7.98,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50378083

(Atrovent HFA | IPRATROPIUM BROMIDE)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:4:3:10.11.9:6.7,THB:12:10:3:6.7,1:3:10.11.9:6.7,(8.93,-5.03,;7.4,-5.04,;6.62,-3.72,;6.64,-6.39,;5.29,-5.61,;6.91,-7.92,;5.92,-9.27,;7.1,-8.56,;7.69,-7.22,;9.45,-7.19,;9.72,-8.74,;8.74,-7.92,;9.71,-10.27,;11.04,-11.06,;11.03,-12.6,;12.38,-10.29,;13.71,-11.07,;15.04,-10.31,;12.39,-8.75,;13.74,-8,;13.74,-6.46,;12.41,-5.67,;11.07,-6.44,;11.06,-7.98,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50378083

(Atrovent HFA | IPRATROPIUM BROMIDE)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:4:3:10.11.9:6.7,THB:12:10:3:6.7,1:3:10.11.9:6.7,(8.93,-5.03,;7.4,-5.04,;6.62,-3.72,;6.64,-6.39,;5.29,-5.61,;6.91,-7.92,;5.92,-9.27,;7.1,-8.56,;7.69,-7.22,;9.45,-7.19,;9.72,-8.74,;8.74,-7.92,;9.71,-10.27,;11.04,-11.06,;11.03,-12.6,;12.38,-10.29,;13.71,-11.07,;15.04,-10.31,;12.39,-8.75,;13.74,-8,;13.74,-6.46,;12.41,-5.67,;11.07,-6.44,;11.06,-7.98,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50039951
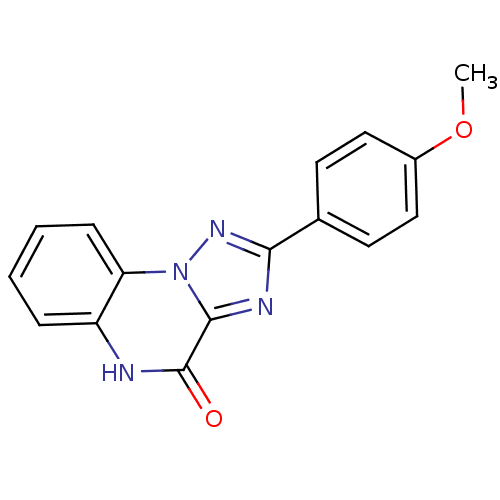
(2-(4-Methoxy-phenyl)-5H-[1,2,4]triazolo[1,5-a]quin...)Show InChI InChI=1S/C16H12N4O2/c1-22-11-8-6-10(7-9-11)14-18-15-16(21)17-12-4-2-3-5-13(12)20(15)19-14/h2-9H,1H3,(H,17,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330632
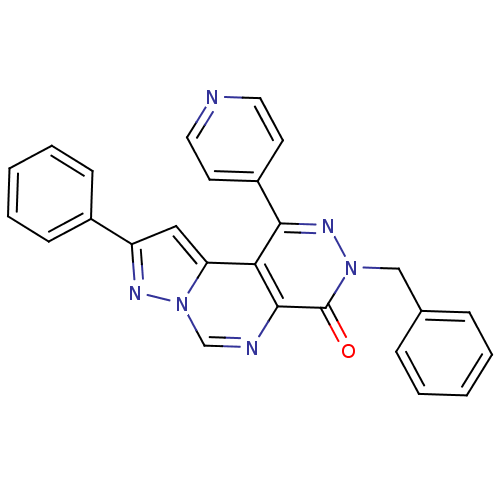
(3-Benzyl-9-phenyl-1-pyridin-4-yl-pyrazolo[1',5':1,...)Show SMILES O=c1n(Cc2ccccc2)nc(-c2ccncc2)c2c3cc(nn3cnc12)-c1ccccc1 Show InChI InChI=1S/C26H18N6O/c33-26-25-23(22-15-21(29-32(22)17-28-25)19-9-5-2-6-10-19)24(20-11-13-27-14-12-20)30-31(26)16-18-7-3-1-4-8-18/h1-15,17H,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330631

(3-Benzyl-9-phenyl-1-pyridin-3-yl-pyrazolo[1',5':1,...)Show SMILES O=c1n(Cc2ccccc2)nc(-c2cccnc2)c2c3cc(nn3cnc12)-c1ccccc1 Show InChI InChI=1S/C26H18N6O/c33-26-25-23(22-14-21(29-32(22)17-28-25)19-10-5-2-6-11-19)24(20-12-7-13-27-15-20)30-31(26)16-18-8-3-1-4-9-18/h1-15,17H,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50102282
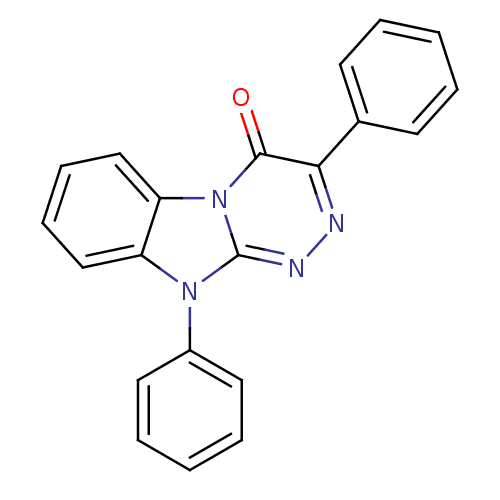
(3,10-Diphenyl-10H-benzo[4,5]imidazo[2,1-c][1,2,4]t...)Show SMILES O=c1c(nnc2n(-c3ccccc3)c3ccccc3n12)-c1ccccc1 Show InChI InChI=1S/C21H14N4O/c26-20-19(15-9-3-1-4-10-15)22-23-21-24(16-11-5-2-6-12-16)17-13-7-8-14-18(17)25(20)21/h1-14H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to bovine adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50133368
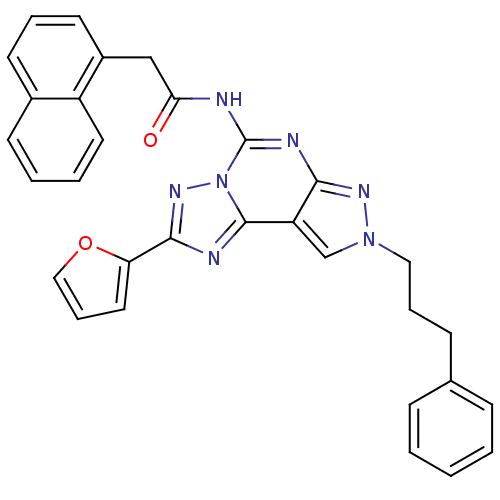
(CHEMBL335950 | N-(2-(furan-2-yl)-8-(3-phenylpropyl...)Show SMILES O=C(Cc1cccc2ccccc12)Nc1nc2nn(CCCc3ccccc3)cc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C31H25N7O2/c39-27(19-23-14-6-13-22-12-4-5-15-24(22)23)32-31-34-28-25(30-33-29(36-38(30)31)26-16-8-18-40-26)20-37(35-28)17-7-11-21-9-2-1-3-10-21/h1-6,8-10,12-16,18,20H,7,11,17,19H2,(H,32,34,35,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2b receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330628
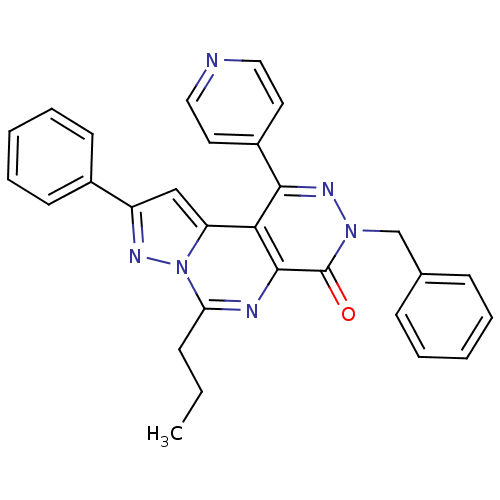
(3-Benzyl-9-phenyl-6-n-propyl-1-pyridin-4-yl-pyrazo...)Show SMILES CCCc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C29H24N6O/c1-2-9-25-31-28-26(24-18-23(32-35(24)25)21-12-7-4-8-13-21)27(22-14-16-30-17-15-22)33-34(29(28)36)19-20-10-5-3-6-11-20/h3-8,10-18H,2,9,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330630
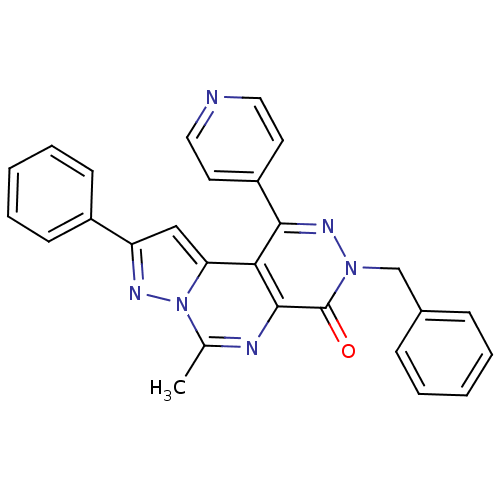
(3-Benzyl-6-methyl-9-phenyl-1-pyridin-4-yl-pyrazolo...)Show SMILES Cc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C27H20N6O/c1-18-29-26-24(23-16-22(30-33(18)23)20-10-6-3-7-11-20)25(21-12-14-28-15-13-21)31-32(27(26)34)17-19-8-4-2-5-9-19/h2-16H,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330626
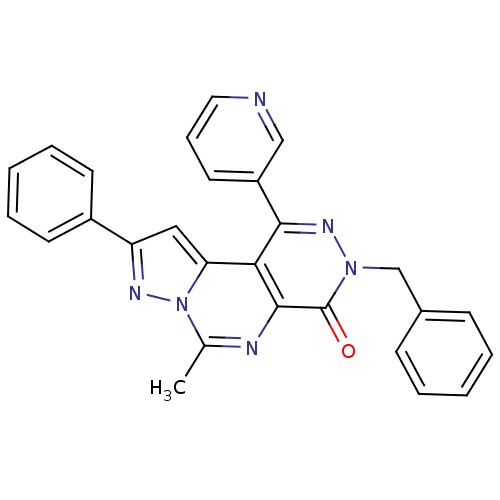
(3-Benzyl-6-methyl-9-phenyl-1-pyridin-3-yl-pyrazolo...)Show SMILES Cc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2cccnc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C27H20N6O/c1-18-29-26-24(23-15-22(30-33(18)23)20-11-6-3-7-12-20)25(21-13-8-14-28-16-21)31-32(27(26)34)17-19-9-4-2-5-10-19/h2-16H,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2b receptor expressed in HEK293 cells after 30 mins |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50330632

(3-Benzyl-9-phenyl-1-pyridin-4-yl-pyrazolo[1',5':1,...)Show SMILES O=c1n(Cc2ccccc2)nc(-c2ccncc2)c2c3cc(nn3cnc12)-c1ccccc1 Show InChI InChI=1S/C26H18N6O/c33-26-25-23(22-15-21(29-32(22)17-28-25)19-9-5-2-6-10-19)24(20-11-13-27-14-12-20)30-31(26)16-18-7-3-1-4-8-18/h1-15,17H,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330629

(3-Benzyl-6-ethyl-9-phenyl-1-pyridin-4-yl-pyrazolo[...)Show SMILES CCc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C28H22N6O/c1-2-24-30-27-25(23-17-22(31-34(23)24)20-11-7-4-8-12-20)26(21-13-15-29-16-14-21)32-33(28(27)35)18-19-9-5-3-6-10-19/h3-17H,2,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330623

(3-Benzyl-1-methyl-9-thiophen-3-yl-pyrazolo[1',5':1...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2ncn3nc(cc3c12)-c1ccsc1 Show InChI InChI=1S/C20H15N5OS/c1-13-18-17-9-16(15-7-8-27-11-15)23-25(17)12-21-19(18)20(26)24(22-13)10-14-5-3-2-4-6-14/h2-9,11-12H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330638
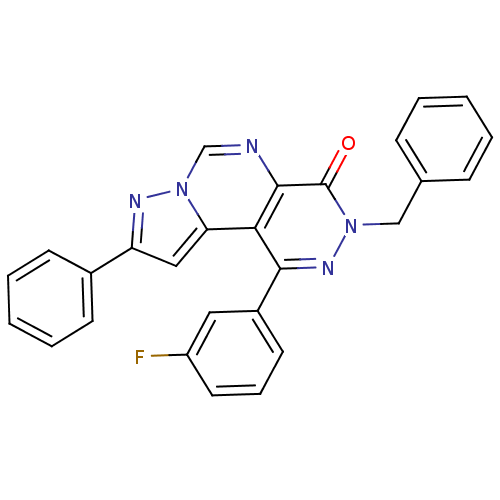
(3-Benzyl-1-(3-fluorophenyl)-9-phenylpyrazolo[1',5'...)Show SMILES Fc1cccc(c1)-c1nn(Cc2ccccc2)c(=O)c2ncn3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C27H18FN5O/c28-21-13-7-12-20(14-21)25-24-23-15-22(19-10-5-2-6-11-19)30-33(23)17-29-26(24)27(34)32(31-25)16-18-8-3-1-4-9-18/h1-15,17H,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50193584

(3-benzyl-1-methyl-9-phenylpyrazolo[1',5':1,6]pyrim...)Show SMILES Cc1nn(Cc2ccccc2)c(=O)c2ncn3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C22H17N5O/c1-15-20-19-12-18(17-10-6-3-7-11-17)25-27(19)14-23-21(20)22(28)26(24-15)13-16-8-4-2-5-9-16/h2-12,14H,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330639
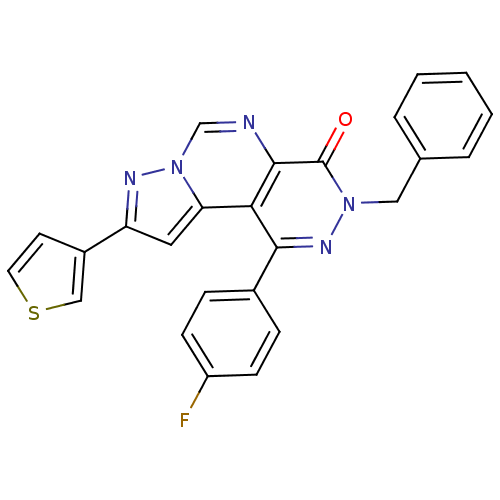
(3-Benzyl-1-(4-fluorophenyl)-9-thiophen-3-yl-pyrazo...)Show SMILES Fc1ccc(cc1)-c1nn(Cc2ccccc2)c(=O)c2ncn3nc(cc3c12)-c1ccsc1 Show InChI InChI=1S/C25H16FN5OS/c26-19-8-6-17(7-9-19)23-22-21-12-20(18-10-11-33-14-18)28-31(21)15-27-24(22)25(32)30(29-23)13-16-4-2-1-3-5-16/h1-12,14-15H,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330625
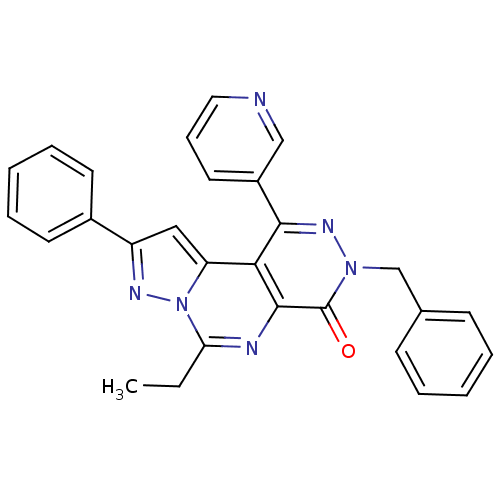
(3-Benzyl-6-ethyl-9-phenyl-1-pyridin-3-yl-pyrazolo[...)Show SMILES CCc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2cccnc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C28H22N6O/c1-2-24-30-27-25(23-16-22(31-34(23)24)20-12-7-4-8-13-20)26(21-14-9-15-29-17-21)32-33(28(27)35)18-19-10-5-3-6-11-19/h3-17H,2,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330637

(3-Benzyl-1-(3-fluorophenyl)-9-thiophen-3-yl-pyrazo...)Show SMILES Fc1cccc(c1)-c1nn(Cc2ccccc2)c(=O)c2ncn3nc(cc3c12)-c1ccsc1 Show InChI InChI=1S/C25H16FN5OS/c26-19-8-4-7-17(11-19)23-22-21-12-20(18-9-10-33-14-18)28-31(21)15-27-24(22)25(32)30(29-23)13-16-5-2-1-3-6-16/h1-12,14-15H,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330627

(3-Benzyl-6-isopropyl-9-phenyl-1-pyridin-4-yl-pyraz...)Show SMILES CC(C)c1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C29H24N6O/c1-19(2)28-31-27-25(24-17-23(32-35(24)28)21-11-7-4-8-12-21)26(22-13-15-30-16-14-22)33-34(29(27)36)18-20-9-5-3-6-10-20/h3-17,19H,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330640

(3-Benzyl-1-(4-fluorophenyl)-9-phenylpyrazolo[1',5'...)Show SMILES Fc1ccc(cc1)-c1nn(Cc2ccccc2)c(=O)c2ncn3nc(cc3c12)-c1ccccc1 Show InChI InChI=1S/C27H18FN5O/c28-21-13-11-20(12-14-21)25-24-23-15-22(19-9-5-2-6-10-19)30-33(23)17-29-26(24)27(34)32(31-25)16-18-7-3-1-4-8-18/h1-15,17H,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330624

(3-Benzyl-6-methyl-9-phenyl-1-pyridin-4-ylpyrazolo[...)Show SMILES Cc1nc2c(c(nn(Cc3ccccc3)c2=S)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C27H20N6S/c1-18-29-26-24(23-16-22(30-33(18)23)20-10-6-3-7-11-20)25(21-12-14-28-15-13-21)31-32(27(26)34)17-19-8-4-2-5-9-19/h2-16H,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Bos taurus) | BDBM50133368

(CHEMBL335950 | N-(2-(furan-2-yl)-8-(3-phenylpropyl...)Show SMILES O=C(Cc1cccc2ccccc12)Nc1nc2nn(CCCc3ccccc3)cc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C31H25N7O2/c39-27(19-23-14-6-13-22-12-4-5-15-24(22)23)32-31-34-28-25(30-33-29(36-38(30)31)26-16-8-18-40-26)20-37(35-28)17-7-11-21-9-2-1-3-10-21/h1-6,8-10,12-16,18,20H,7,11,17,19H2,(H,32,34,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to bovine adenosine A3 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330622
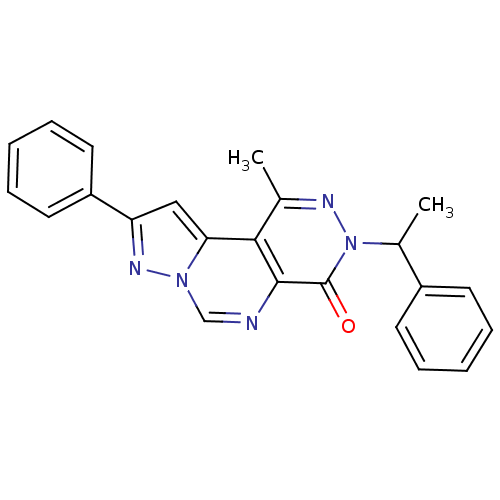
(1-Methyl-9-phenyl-3-(1-phenylethyl)pyrazolo[1',5':...)Show SMILES CC(c1ccccc1)n1nc(C)c2c3cc(nn3cnc2c1=O)-c1ccccc1 Show InChI InChI=1S/C23H19N5O/c1-15-21-20-13-19(18-11-7-4-8-12-18)26-27(20)14-24-22(21)23(29)28(25-15)16(2)17-9-5-3-6-10-17/h3-14,16H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 475 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50330630

(3-Benzyl-6-methyl-9-phenyl-1-pyridin-4-yl-pyrazolo...)Show SMILES Cc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C27H20N6O/c1-18-29-26-24(23-16-22(30-33(18)23)20-10-6-3-7-11-20)25(21-12-14-28-15-13-21)31-32(27(26)34)17-19-8-4-2-5-9-19/h2-16H,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330634

(3-Benzyl-1,9-diphenyl-6-n-propylpyrazolo[1',5':1,6...)Show SMILES CCCc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccccc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C30H25N5O/c1-2-12-26-31-29-27(25-19-24(32-35(25)26)22-15-8-4-9-16-22)28(23-17-10-5-11-18-23)33-34(30(29)36)20-21-13-6-3-7-14-21/h3-11,13-19H,2,12,20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 564 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50330624

(3-Benzyl-6-methyl-9-phenyl-1-pyridin-4-ylpyrazolo[...)Show SMILES Cc1nc2c(c(nn(Cc3ccccc3)c2=S)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C27H20N6S/c1-18-29-26-24(23-16-22(30-33(18)23)20-10-6-3-7-11-20)25(21-12-14-28-15-13-21)31-32(27(26)34)17-19-8-4-2-5-9-19/h2-16H,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50330628

(3-Benzyl-9-phenyl-6-n-propyl-1-pyridin-4-yl-pyrazo...)Show SMILES CCCc1nc2c(c(nn(Cc3ccccc3)c2=O)-c2ccncc2)c2cc(nn12)-c1ccccc1 Show InChI InChI=1S/C29H24N6O/c1-2-9-25-31-28-26(24-18-23(32-35(24)25)21-12-7-4-8-13-21)27(22-14-16-30-17-15-22)33-34(29(28)36)19-20-10-5-3-6-11-20/h3-8,10-18H,2,9,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 773 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50330631

(3-Benzyl-9-phenyl-1-pyridin-3-yl-pyrazolo[1',5':1,...)Show SMILES O=c1n(Cc2ccccc2)nc(-c2cccnc2)c2c3cc(nn3cnc12)-c1ccccc1 Show InChI InChI=1S/C26H18N6O/c33-26-25-23(22-14-21(29-32(22)17-28-25)19-10-5-2-6-11-19)24(20-12-7-13-27-15-20)30-31(26)16-18-8-3-1-4-9-18/h1-15,17H,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 805 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2a receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Bos taurus) | BDBM50102282

(3,10-Diphenyl-10H-benzo[4,5]imidazo[2,1-c][1,2,4]t...)Show SMILES O=c1c(nnc2n(-c3ccccc3)c3ccccc3n12)-c1ccccc1 Show InChI InChI=1S/C21H14N4O/c26-20-19(15-9-3-1-4-10-15)22-23-21-24(16-11-5-2-6-12-16)17-13-7-8-14-18(17)25(20)21/h1-14H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to bovine adenosine A3 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50133368

(CHEMBL335950 | N-(2-(furan-2-yl)-8-(3-phenylpropyl...)Show SMILES O=C(Cc1cccc2ccccc12)Nc1nc2nn(CCCc3ccccc3)cc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C31H25N7O2/c39-27(19-23-14-6-13-22-12-4-5-15-24(22)23)32-31-34-28-25(30-33-29(36-38(30)31)26-16-8-18-40-26)20-37(35-28)17-7-11-21-9-2-1-3-10-21/h1-6,8-10,12-16,18,20H,7,11,17,19H2,(H,32,34,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50094037

(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human adenosine A3 receptor expressed in HeLa cells after 180 mins |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50330618
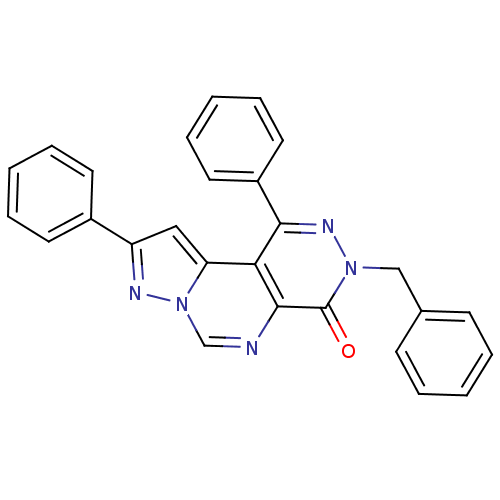
(3-Benzyl-1,9-diphenylpyrazolo[1',5':1,6]pyrimido[4...)Show SMILES O=c1n(Cc2ccccc2)nc(-c2ccccc2)c2c3cc(nn3cnc12)-c1ccccc1 Show InChI InChI=1S/C27H19N5O/c33-27-26-24(23-16-22(29-32(23)18-28-26)20-12-6-2-7-13-20)25(21-14-8-3-9-15-21)30-31(27)17-19-10-4-1-5-11-19/h1-16,18H,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 18: 7890-9 (2010)
Article DOI: 10.1016/j.bmc.2010.09.043
BindingDB Entry DOI: 10.7270/Q2V12510 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data