 Found 2372 hits with Last Name = 'ge' and Initial = 'y'
Found 2372 hits with Last Name = 'ge' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198754

(CHEMBL3924888)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-16-23(42)17-21(2)24(20)19-28-32(46)39-26(13-7-9-15-37-33(35)36)29(43)40-27(18-22-10-4-3-5-11-22)31(45)38-25(30(44)41-28)12-6-8-14-34/h3-5,10-11,16-17,25-28,42H,6-9,12-15,18-19,34H2,1-2H3,(H,38,45)(H,39,46)(H,40,43)(H,41,44)(H4,35,36,37)/t25-,26+,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198760
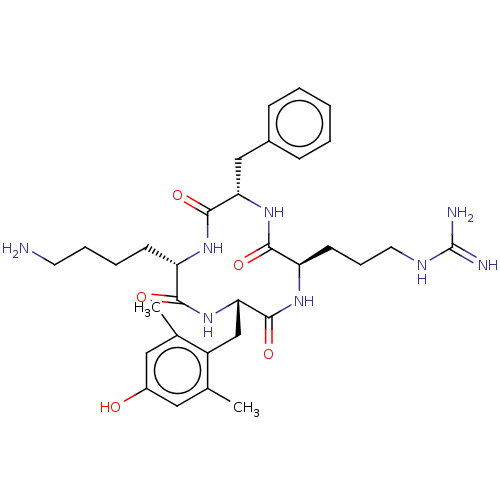
(CHEMBL3897031)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C32H46N8O5/c1-19-15-22(41)16-20(2)23(19)18-27-31(45)38-25(12-8-14-36-32(34)35)29(43)39-26(17-21-9-4-3-5-10-21)30(44)37-24(28(42)40-27)11-6-7-13-33/h3-5,9-10,15-16,24-27,41H,6-8,11-14,17-18,33H2,1-2H3,(H,37,44)(H,38,45)(H,39,43)(H,40,42)(H4,34,35,36)/t24-,25+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198758

(CHEMBL3908315)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)C(CCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C31H44N8O5/c1-18-14-21(40)15-19(2)22(18)17-26-30(44)37-24(11-7-13-35-31(33)34)28(42)38-25(16-20-8-4-3-5-9-20)29(43)36-23(10-6-12-32)27(41)39-26/h3-5,8-9,14-15,23-26,40H,6-7,10-13,16-17,32H2,1-2H3,(H,36,43)(H,37,44)(H,38,42)(H,39,41)(H4,33,34,35)/t23?,24-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50100983
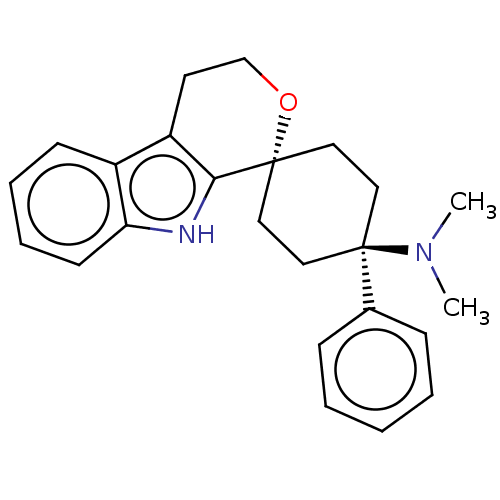
(CHEMBL3326224)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(22.71,-7.97,;18.3,-7.93,;16.96,-7.16,;16.96,-5.62,;15.63,-7.93,;14.86,-9.26,;13.32,-9.27,;12.55,-7.94,;13.32,-6.61,;14.86,-6.6,;11.79,-9.28,;10.25,-9.27,;9.47,-7.94,;10.25,-6.6,;11.79,-6.61,;12.26,-5.14,;11.03,-4.24,;10.87,-2.7,;9.47,-2.07,;8.21,-2.98,;8.37,-4.51,;9.77,-5.14,;16.43,-9.25,;15.68,-10.6,;16.48,-11.91,;18.02,-11.88,;18.76,-10.52,;17.96,-9.21,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198755
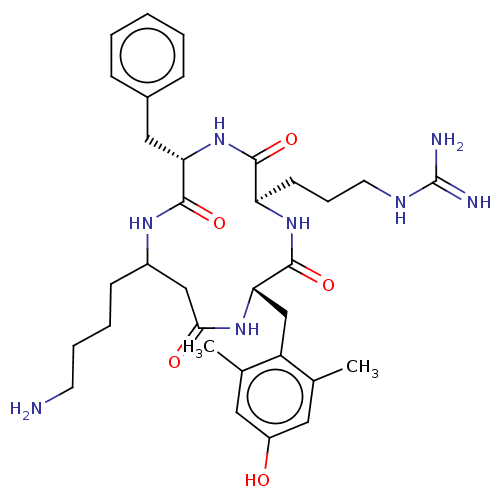
(CHEMBL3979449)Show SMILES Cc1cc(O)cc(C)c1C[C@@H]1NC(=O)CC(CCCCN)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CCCNC(N)=N)NC1=O |r| Show InChI InChI=1S/C33H48N8O5/c1-20-15-24(42)16-21(2)25(20)19-28-32(46)40-26(12-8-14-37-33(35)36)30(44)41-27(17-22-9-4-3-5-10-22)31(45)38-23(11-6-7-13-34)18-29(43)39-28/h3-5,9-10,15-16,23,26-28,42H,6-8,11-14,17-19,34H2,1-2H3,(H,38,45)(H,39,43)(H,40,46)(H,41,44)(H4,35,36,37)/t23?,26-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50198757

(CHEMBL363142)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24?,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs |
J Med Chem 59: 9243-9254 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01200
BindingDB Entry DOI: 10.7270/Q23B623F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50101099

(CHEMBL3326228)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)NCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(19.24,-9.37,;10.32,-12.36,;8.84,-12.77,;7.74,-11.68,;8.44,-14.27,;7.67,-15.59,;6.14,-15.59,;5.38,-14.26,;6.14,-12.93,;7.68,-12.94,;4.47,-15.52,;2.93,-15.35,;2.3,-13.94,;3.21,-12.69,;4.75,-12.85,;5.38,-11.44,;4.23,-10.41,;4.23,-8.85,;2.89,-8.09,;1.56,-8.86,;1.56,-10.41,;2.89,-11.18,;9.98,-14.28,;10.74,-15.62,;12.28,-15.63,;13.06,-14.29,;12.29,-12.95,;10.75,-12.95,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398484
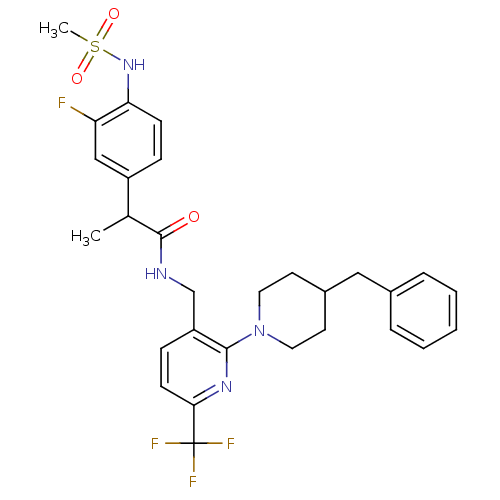
(CHEMBL2177440)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(Cc2ccccc2)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C29H32F4N4O3S/c1-19(22-8-10-25(24(30)17-22)36-41(2,39)40)28(38)34-18-23-9-11-26(29(31,32)33)35-27(23)37-14-12-21(13-15-37)16-20-6-4-3-5-7-20/h3-11,17,19,21,36H,12-16,18H2,1-2H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398553
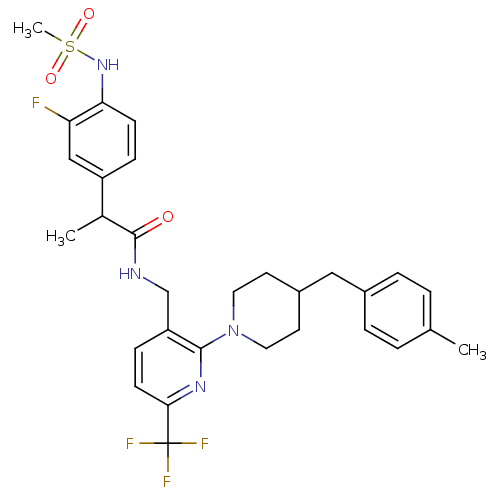
(CHEMBL2177441)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(Cc2ccc(C)cc2)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C30H34F4N4O3S/c1-19-4-6-21(7-5-19)16-22-12-14-38(15-13-22)28-24(9-11-27(36-28)30(32,33)34)18-35-29(39)20(2)23-8-10-26(25(31)17-23)37-42(3,40)41/h4-11,17,20,22,37H,12-16,18H2,1-3H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398552
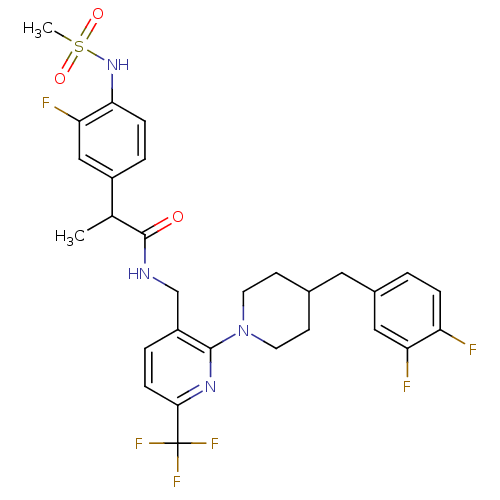
(CHEMBL2178059)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(Cc2ccc(F)c(F)c2)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C29H30F6N4O3S/c1-17(20-4-7-25(24(32)15-20)38-43(2,41)42)28(40)36-16-21-5-8-26(29(33,34)35)37-27(21)39-11-9-18(10-12-39)13-19-3-6-22(30)23(31)14-19/h3-8,14-15,17-18,38H,9-13,16H2,1-2H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049529
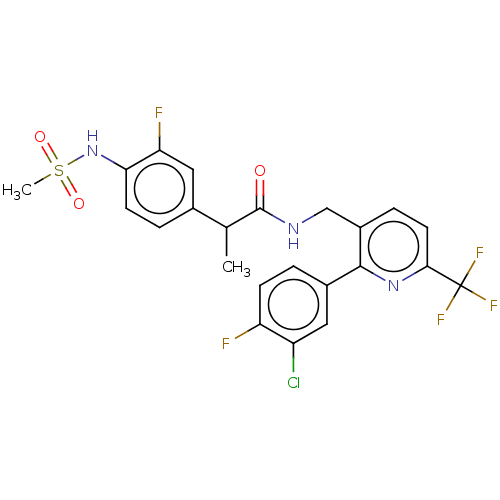
(CHEMBL3317476)Show SMILES CC(C(=O)NCc1ccc(nc1-c1ccc(F)c(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H19ClF5N3O3S/c1-12(13-4-7-19(18(26)10-13)32-36(2,34)35)22(33)30-11-15-5-8-20(23(27,28)29)31-21(15)14-3-6-17(25)16(24)9-14/h3-10,12,32H,11H2,1-2H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398511
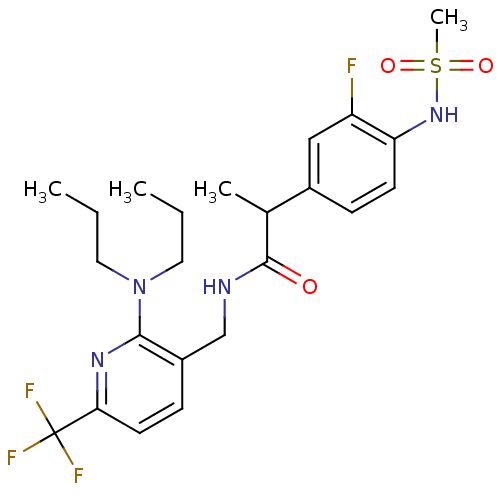
(CHEMBL2178063)Show SMILES CCCN(CCC)c1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C23H30F4N4O3S/c1-5-11-31(12-6-2)21-17(8-10-20(29-21)23(25,26)27)14-28-22(32)15(3)16-7-9-19(18(24)13-16)30-35(4,33)34/h7-10,13,15,30H,5-6,11-12,14H2,1-4H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50101306
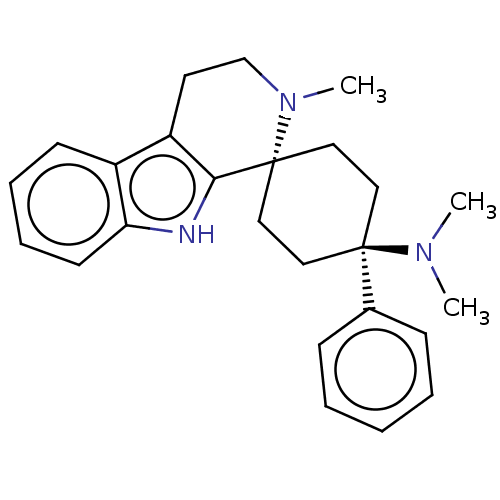
(CHEMBL3326229)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)N(C)CCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(35,-11.75,;25.44,-13.91,;23.96,-14.32,;22.86,-13.23,;23.56,-15.82,;22.79,-17.14,;21.26,-17.14,;20.5,-15.8,;21.26,-14.48,;22.8,-14.49,;19.59,-17.06,;20.22,-18.47,;18.05,-16.9,;17.42,-15.49,;18.33,-14.24,;19.87,-14.4,;20.5,-12.99,;19.35,-11.95,;19.35,-10.4,;18.01,-9.64,;16.68,-10.41,;16.68,-11.95,;18.01,-12.72,;25.1,-15.82,;25.86,-17.16,;27.4,-17.17,;28.18,-15.84,;27.4,-14.5,;25.87,-14.5,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50562972

(CHEMBL4799997)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CCN(C1=O)c1ccc(F)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CAH7 expressed in Escherichia coli BL21 (DE3) by stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02077
BindingDB Entry DOI: 10.7270/Q2C25158 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50100983

(CHEMBL3326224)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(22.71,-7.97,;18.3,-7.93,;16.96,-7.16,;16.96,-5.62,;15.63,-7.93,;14.86,-9.26,;13.32,-9.27,;12.55,-7.94,;13.32,-6.61,;14.86,-6.6,;11.79,-9.28,;10.25,-9.27,;9.47,-7.94,;10.25,-6.6,;11.79,-6.61,;12.26,-5.14,;11.03,-4.24,;10.87,-2.7,;9.47,-2.07,;8.21,-2.98,;8.37,-4.51,;9.77,-5.14,;16.43,-9.25,;15.68,-10.6,;16.48,-11.91,;18.02,-11.88,;18.76,-10.52,;17.96,-9.21,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398506
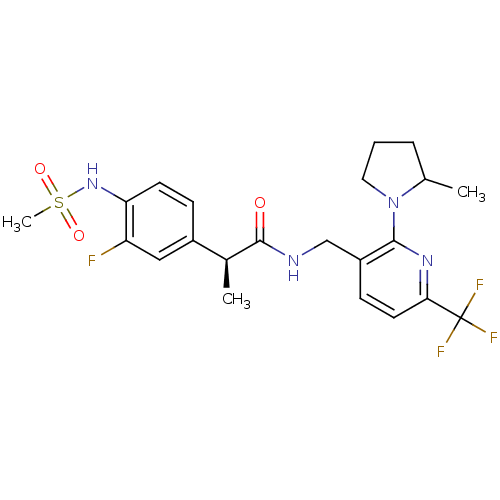
(CHEMBL2178068)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCCC1C)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C22H26F4N4O3S/c1-13-5-4-10-30(13)20-16(7-9-19(28-20)22(24,25)26)12-27-21(31)14(2)15-6-8-18(17(23)11-15)29-34(3,32)33/h6-9,11,13-14,29H,4-5,10,12H2,1-3H3,(H,27,31)/t13?,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049547
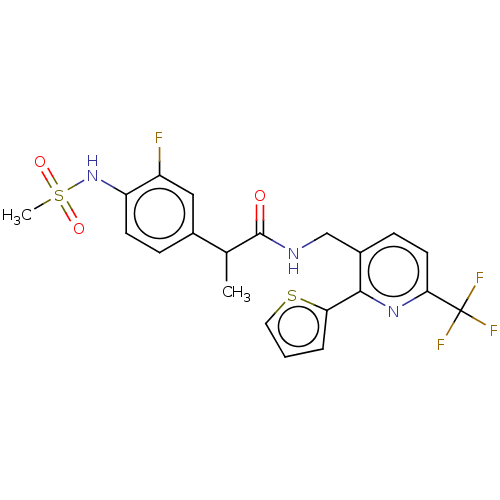
(CHEMBL3317484)Show SMILES CC(C(=O)NCc1ccc(nc1-c1cccs1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19F4N3O3S2/c1-12(13-5-7-16(15(22)10-13)28-33(2,30)31)20(29)26-11-14-6-8-18(21(23,24)25)27-19(14)17-4-3-9-32-17/h3-10,12,28H,11H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50100983

(CHEMBL3326224)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(22.71,-7.97,;18.3,-7.93,;16.96,-7.16,;16.96,-5.62,;15.63,-7.93,;14.86,-9.26,;13.32,-9.27,;12.55,-7.94,;13.32,-6.61,;14.86,-6.6,;11.79,-9.28,;10.25,-9.27,;9.47,-7.94,;10.25,-6.6,;11.79,-6.61,;12.26,-5.14,;11.03,-4.24,;10.87,-2.7,;9.47,-2.07,;8.21,-2.98,;8.37,-4.51,;9.77,-5.14,;16.43,-9.25,;15.68,-10.6,;16.48,-11.91,;18.02,-11.88,;18.76,-10.52,;17.96,-9.21,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50101088
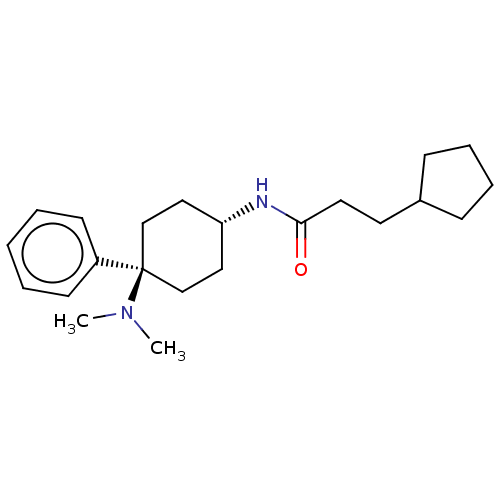
(CHEMBL3326220)Show SMILES Cl.CN(C)[C@]1(CC[C@@H](CC1)NC(=O)CCC1CCCC1)c1ccccc1 |r,wU:4.2,wD:7.9,(15.72,-29.86,;12.69,-28.26,;11.36,-27.5,;11.36,-25.96,;10.03,-28.27,;9.26,-29.6,;7.72,-29.6,;6.95,-28.28,;7.71,-26.95,;9.25,-26.94,;5.41,-28.28,;4.63,-26.95,;5.4,-25.61,;3.1,-26.95,;2.33,-25.61,;.79,-25.61,;-.11,-24.36,;-1.58,-24.83,;-1.58,-26.37,;-.12,-26.85,;10.82,-29.59,;10.08,-30.93,;10.87,-32.25,;12.41,-32.22,;13.16,-30.86,;12.36,-29.55,)| Show InChI InChI=1S/C22H34N2O/c1-24(2)22(19-10-4-3-5-11-19)16-14-20(15-17-22)23-21(25)13-12-18-8-6-7-9-18/h3-5,10-11,18,20H,6-9,12-17H2,1-2H3,(H,23,25)/t20-,22- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398498
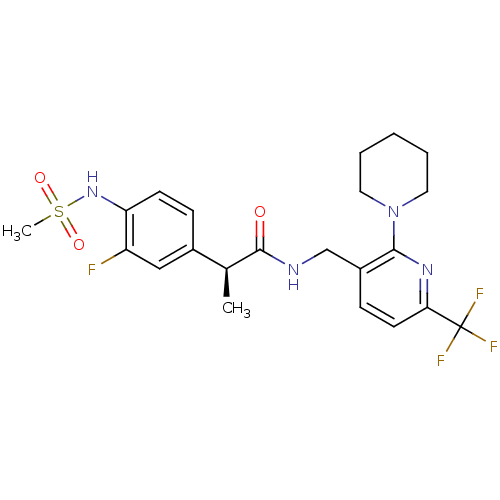
(CHEMBL2177424)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C22H26F4N4O3S/c1-14(15-6-8-18(17(23)12-15)29-34(2,32)33)21(31)27-13-16-7-9-19(22(24,25)26)28-20(16)30-10-4-3-5-11-30/h6-9,12,14,29H,3-5,10-11,13H2,1-2H3,(H,27,31)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398489
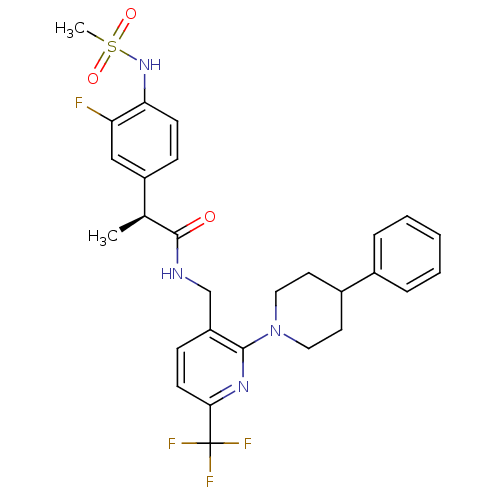
(CHEMBL2177435)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(CC1)c1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C28H30F4N4O3S/c1-18(21-8-10-24(23(29)16-21)35-40(2,38)39)27(37)33-17-22-9-11-25(28(30,31)32)34-26(22)36-14-12-20(13-15-36)19-6-4-3-5-7-19/h3-11,16,18,20,35H,12-15,17H2,1-2H3,(H,33,37)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398493
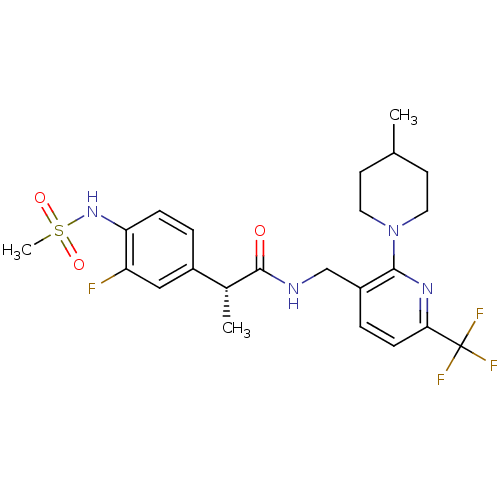
(CHEMBL2177430)Show SMILES C[C@@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398497
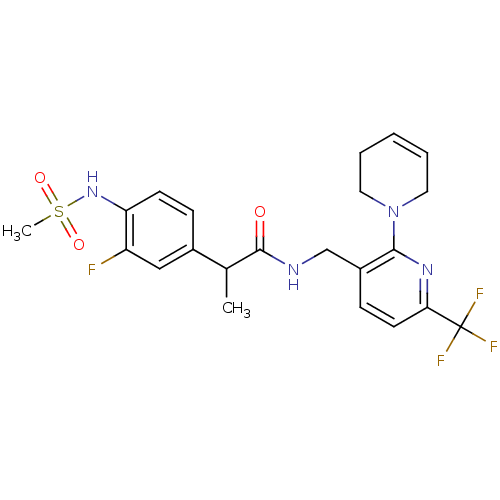
(CHEMBL2177425)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC=CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |c:16| Show InChI InChI=1S/C22H24F4N4O3S/c1-14(15-6-8-18(17(23)12-15)29-34(2,32)33)21(31)27-13-16-7-9-19(22(24,25)26)28-20(16)30-10-4-3-5-11-30/h3-4,6-9,12,14,29H,5,10-11,13H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398491
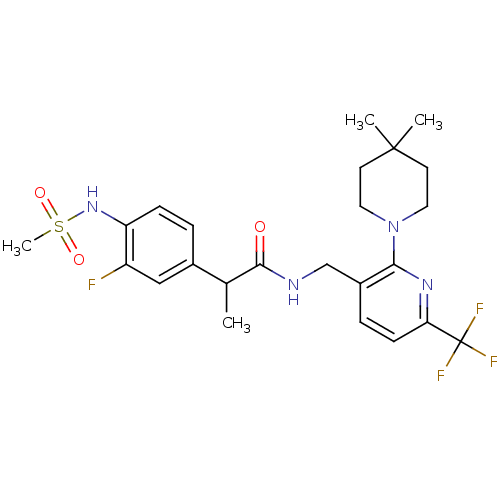
(CHEMBL2177432)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H30F4N4O3S/c1-15(16-5-7-19(18(25)13-16)31-36(4,34)35)22(33)29-14-17-6-8-20(24(26,27)28)30-21(17)32-11-9-23(2,3)10-12-32/h5-8,13,15,31H,9-12,14H2,1-4H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553
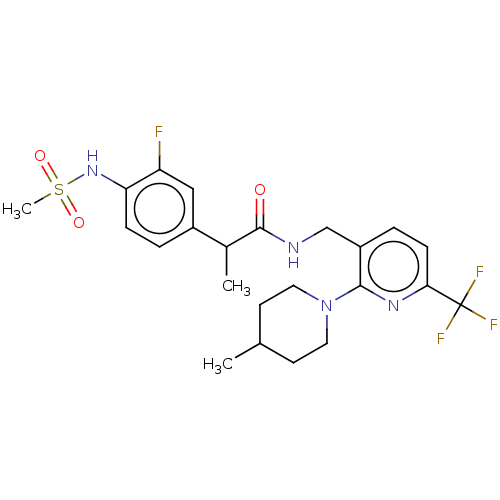
(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50101152

(CHEMBL3326232)Show SMILES OC(=O)CC(O)(CC(O)=O)C(O)=O.CN[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:15.13,wD:18.20,(20.04,-1.08,;21.36,-1.84,;22.69,-1.08,;21.36,-3.37,;20.04,-4.13,;18.72,-3.37,;20.8,-5.45,;22.32,-5.46,;23.08,-6.78,;23.09,-4.13,;19.28,-5.45,;20.04,-6.77,;17.75,-5.44,;8.07,-7.76,;9.17,-8.84,;8.78,-10.34,;8,-11.67,;6.47,-11.66,;5.71,-10.33,;6.47,-9,;8.01,-9.01,;4.8,-11.59,;3.25,-11.43,;2.63,-10.01,;3.53,-8.77,;5.08,-8.93,;5.71,-7.51,;4.56,-6.48,;4.56,-4.92,;3.22,-4.16,;1.88,-4.93,;1.88,-6.48,;3.22,-7.25,;10.31,-10.35,;11.08,-11.69,;12.62,-11.7,;13.4,-10.37,;12.62,-9.02,;11.08,-9.02,)| Show InChI InChI=1S/C23H26N2O.C6H8O7/c1-24-22(17-7-3-2-4-8-17)12-14-23(15-13-22)21-19(11-16-26-23)18-9-5-6-10-20(18)25-21;7-3(8)1-6(13,5(11)12)2-4(9)10/h2-10,24-25H,11-16H2,1H3;13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t22-,23-; | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50101099

(CHEMBL3326228)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)NCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(19.24,-9.37,;10.32,-12.36,;8.84,-12.77,;7.74,-11.68,;8.44,-14.27,;7.67,-15.59,;6.14,-15.59,;5.38,-14.26,;6.14,-12.93,;7.68,-12.94,;4.47,-15.52,;2.93,-15.35,;2.3,-13.94,;3.21,-12.69,;4.75,-12.85,;5.38,-11.44,;4.23,-10.41,;4.23,-8.85,;2.89,-8.09,;1.56,-8.86,;1.56,-10.41,;2.89,-11.18,;9.98,-14.28,;10.74,-15.62,;12.28,-15.63,;13.06,-14.29,;12.29,-12.95,;10.75,-12.95,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049534
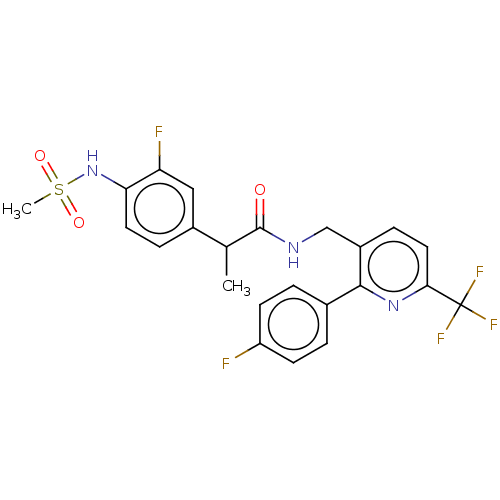
(CHEMBL3319108)Show SMILES CC(C(=O)NCc1ccc(nc1-c1ccc(F)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H20F5N3O3S/c1-13(15-5-9-19(18(25)11-15)31-35(2,33)34)22(32)29-12-16-6-10-20(23(26,27)28)30-21(16)14-3-7-17(24)8-4-14/h3-11,13,31H,12H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398551
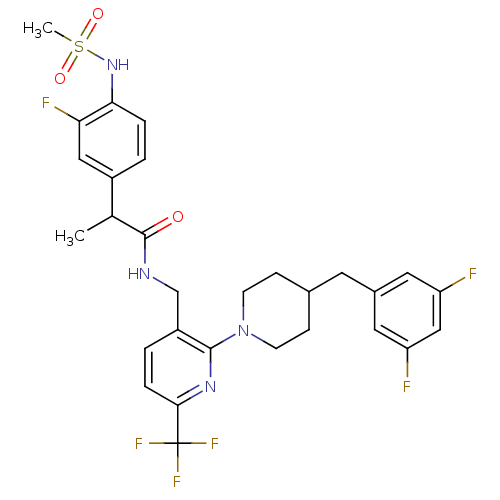
(CHEMBL2177402)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(Cc2cc(F)cc(F)c2)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C29H30F6N4O3S/c1-17(20-3-5-25(24(32)14-20)38-43(2,41)42)28(40)36-16-21-4-6-26(29(33,34)35)37-27(21)39-9-7-18(8-10-39)11-19-12-22(30)15-23(31)13-19/h3-6,12-15,17-18,38H,7-11,16H2,1-2H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50101091
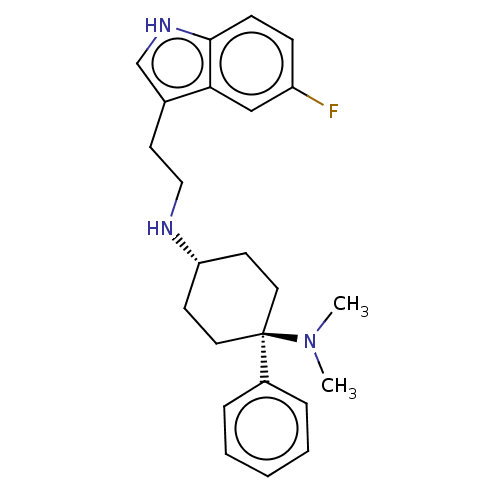
(CHEMBL3326223)Show SMILES Cl.Cl.CN(C)[C@]1(CC[C@@H](CC1)NCCc1c[nH]c2ccc(F)cc12)c1ccccc1 |r,wU:5.2,wD:8.9,(50.15,-23.7,;44.1,-26.45,;47.35,-21.61,;46.01,-20.85,;46.01,-19.3,;44.68,-21.62,;43.91,-22.95,;42.37,-22.95,;41.6,-21.63,;42.36,-20.29,;43.9,-20.29,;40.06,-21.63,;39.26,-22.95,;37.72,-22.91,;36.92,-24.23,;37.52,-25.66,;36.35,-26.66,;35.03,-25.86,;33.57,-26.31,;32.44,-25.27,;32.79,-23.76,;31.66,-22.71,;34.26,-23.31,;35.38,-24.36,;45.47,-22.94,;44.73,-24.28,;45.53,-25.6,;47.07,-25.57,;47.81,-24.21,;47.01,-22.9,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50101096

(CHEMBL3325961)Show SMILES OC(=O)CC(O)(CC(O)=O)C(O)=O.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccc(O)cc12)c1ccccc1 |r,wU:16.14,wD:19.21,(26.19,-30.17,;27.51,-30.93,;28.83,-30.17,;27.51,-32.46,;26.19,-33.22,;24.86,-32.46,;26.94,-34.54,;28.47,-34.55,;29.23,-35.87,;29.24,-33.23,;25.42,-34.54,;26.18,-35.86,;23.89,-34.53,;16.55,-35.59,;17.65,-36.68,;19.13,-36.27,;17.25,-38.18,;16.48,-39.51,;14.95,-39.5,;14.18,-38.17,;14.95,-36.84,;16.49,-36.85,;13.27,-39.43,;11.73,-39.27,;11.1,-37.85,;12.01,-36.6,;13.55,-36.76,;14.18,-35.35,;13.04,-34.31,;13.03,-32.76,;11.69,-31.99,;10.36,-32.76,;9.02,-31.99,;10.36,-34.31,;11.69,-35.08,;18.79,-38.19,;19.55,-39.53,;21.1,-39.54,;21.87,-38.2,;21.1,-36.86,;19.56,-36.86,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049528
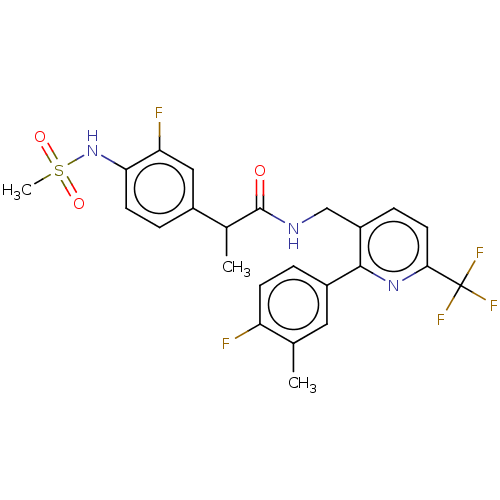
(CHEMBL3317477)Show SMILES CC(C(=O)NCc1ccc(nc1-c1ccc(F)c(C)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H22F5N3O3S/c1-13-10-16(4-7-18(13)25)22-17(6-9-21(31-22)24(27,28)29)12-30-23(33)14(2)15-5-8-20(19(26)11-15)32-36(3,34)35/h4-11,14,32H,12H2,1-3H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398498

(CHEMBL2177424)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C22H26F4N4O3S/c1-14(15-6-8-18(17(23)12-15)29-34(2,32)33)21(31)27-13-16-7-9-19(22(24,25)26)28-20(16)30-10-4-3-5-11-30/h6-9,12,14,29H,3-5,10-11,13H2,1-2H3,(H,27,31)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049530
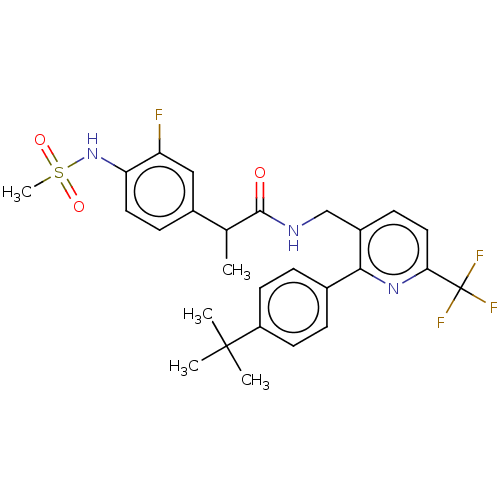
(CHEMBL3319115)Show SMILES CC(C(=O)NCc1ccc(nc1-c1ccc(cc1)C(C)(C)C)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C27H29F4N3O3S/c1-16(18-8-12-22(21(28)14-18)34-38(5,36)37)25(35)32-15-19-9-13-23(27(29,30)31)33-24(19)17-6-10-20(11-7-17)26(2,3)4/h6-14,16,34H,15H2,1-5H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50101088

(CHEMBL3326220)Show SMILES Cl.CN(C)[C@]1(CC[C@@H](CC1)NC(=O)CCC1CCCC1)c1ccccc1 |r,wU:4.2,wD:7.9,(15.72,-29.86,;12.69,-28.26,;11.36,-27.5,;11.36,-25.96,;10.03,-28.27,;9.26,-29.6,;7.72,-29.6,;6.95,-28.28,;7.71,-26.95,;9.25,-26.94,;5.41,-28.28,;4.63,-26.95,;5.4,-25.61,;3.1,-26.95,;2.33,-25.61,;.79,-25.61,;-.11,-24.36,;-1.58,-24.83,;-1.58,-26.37,;-.12,-26.85,;10.82,-29.59,;10.08,-30.93,;10.87,-32.25,;12.41,-32.22,;13.16,-30.86,;12.36,-29.55,)| Show InChI InChI=1S/C22H34N2O/c1-24(2)22(19-10-4-3-5-11-19)16-14-20(15-17-22)23-21(25)13-12-18-8-6-7-9-18/h3-5,10-11,18,20H,6-9,12-17H2,1-2H3,(H,23,25)/t20-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50100991
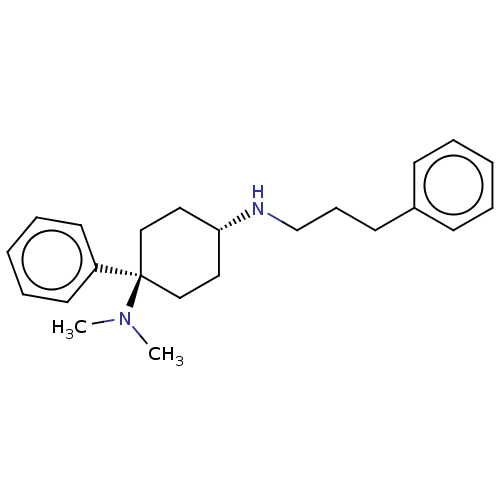
(CHEMBL3325879)Show SMILES Cl.Cl.CN(C)[C@]1(CC[C@@H](CC1)NCCCc1ccccc1)c1ccccc1 |r,wU:5.2,wD:8.9,(20.29,-18.75,;12.97,-20.62,;17.61,-17.09,;16.27,-16.32,;16.27,-14.78,;14.94,-17.09,;14.17,-18.42,;12.63,-18.43,;11.86,-17.1,;12.62,-15.77,;14.17,-15.76,;10.32,-17.1,;9.56,-18.43,;8.02,-18.44,;7.26,-19.78,;5.72,-19.79,;4.95,-18.45,;3.41,-18.46,;2.64,-19.79,;3.42,-21.13,;4.96,-21.12,;15.74,-18.41,;14.99,-19.76,;15.79,-21.08,;17.33,-21.04,;18.07,-19.68,;17.27,-18.37,)| Show InChI InChI=1S/C23H32N2/c1-25(2)23(21-13-7-4-8-14-21)17-15-22(16-18-23)24-19-9-12-20-10-5-3-6-11-20/h3-8,10-11,13-14,22,24H,9,12,15-19H2,1-2H3/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049533
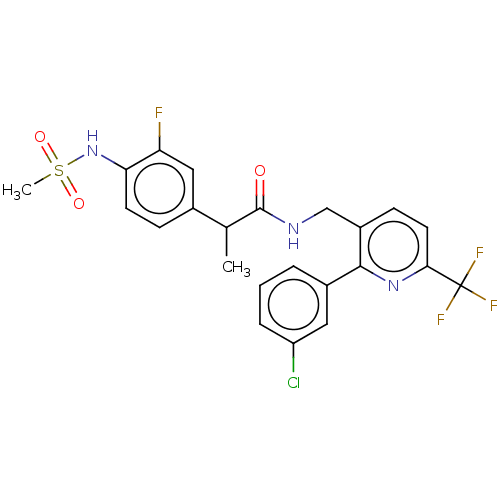
(CHEMBL3319112)Show SMILES CC(C(=O)NCc1ccc(nc1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H20ClF4N3O3S/c1-13(14-6-8-19(18(25)11-14)31-35(2,33)34)22(32)29-12-16-7-9-20(23(26,27)28)30-21(16)15-4-3-5-17(24)10-15/h3-11,13,31H,12H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398549
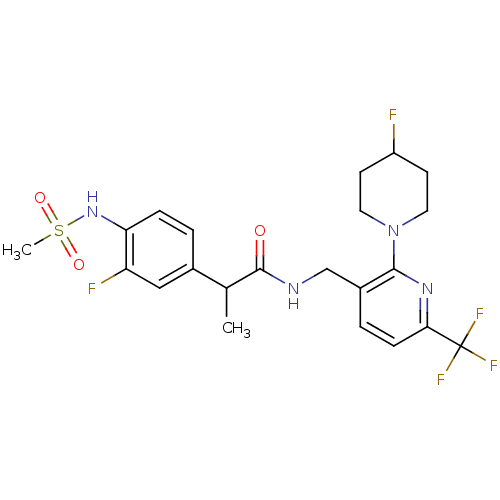
(CHEMBL2177404)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(F)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C22H25F5N4O3S/c1-13(14-3-5-18(17(24)11-14)30-35(2,33)34)21(32)28-12-15-4-6-19(22(25,26)27)29-20(15)31-9-7-16(23)8-10-31/h3-6,11,13,16,30H,7-10,12H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50101306

(CHEMBL3326229)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)N(C)CCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(35,-11.75,;25.44,-13.91,;23.96,-14.32,;22.86,-13.23,;23.56,-15.82,;22.79,-17.14,;21.26,-17.14,;20.5,-15.8,;21.26,-14.48,;22.8,-14.49,;19.59,-17.06,;20.22,-18.47,;18.05,-16.9,;17.42,-15.49,;18.33,-14.24,;19.87,-14.4,;20.5,-12.99,;19.35,-11.95,;19.35,-10.4,;18.01,-9.64,;16.68,-10.41,;16.68,-11.95,;18.01,-12.72,;25.1,-15.82,;25.86,-17.16,;27.4,-17.17,;28.18,-15.84,;27.4,-14.5,;25.87,-14.5,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398510
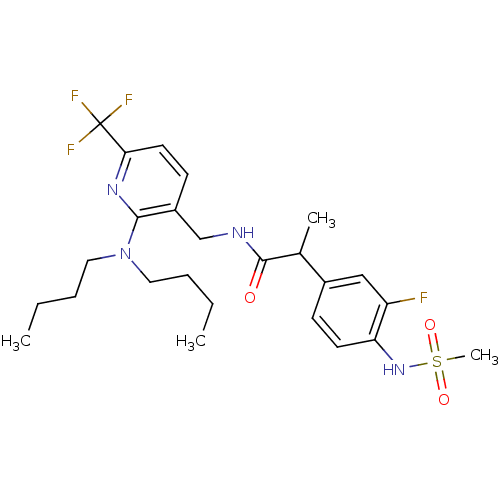
(CHEMBL2178064)Show SMILES CCCCN(CCCC)c1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C25H34F4N4O3S/c1-5-7-13-33(14-8-6-2)23-19(10-12-22(31-23)25(27,28)29)16-30-24(34)17(3)18-9-11-21(20(26)15-18)32-37(4,35)36/h9-12,15,17,32H,5-8,13-14,16H2,1-4H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398548
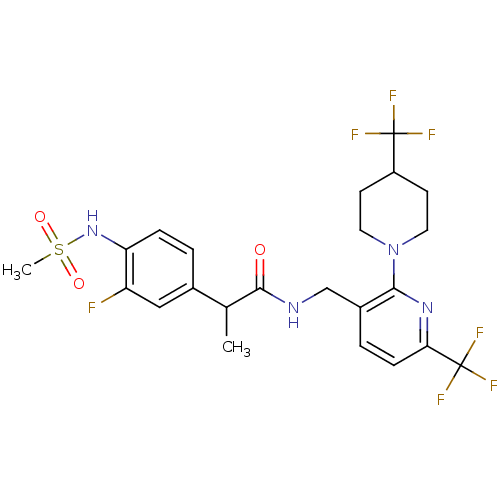
(CHEMBL2177405)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(CC1)C(F)(F)F)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H25F7N4O3S/c1-13(14-3-5-18(17(24)11-14)33-38(2,36)37)21(35)31-12-15-4-6-19(23(28,29)30)32-20(15)34-9-7-16(8-10-34)22(25,26)27/h3-6,11,13,16,33H,7-10,12H2,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049536
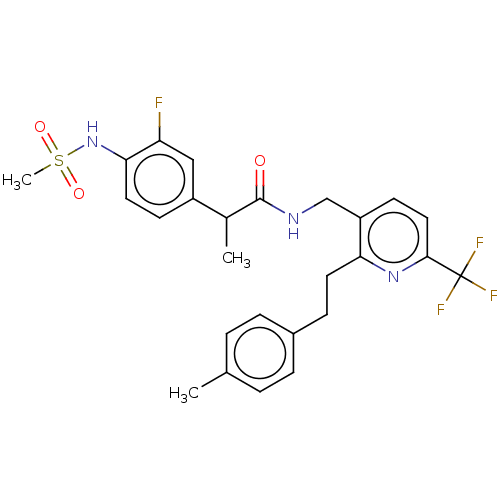
(CHEMBL3317497)Show SMILES CC(C(=O)NCc1ccc(nc1CCc1ccc(C)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C26H27F4N3O3S/c1-16-4-6-18(7-5-16)8-11-22-20(10-13-24(32-22)26(28,29)30)15-31-25(34)17(2)19-9-12-23(21(27)14-19)33-37(3,35)36/h4-7,9-10,12-14,17,33H,8,11,15H2,1-3H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398507
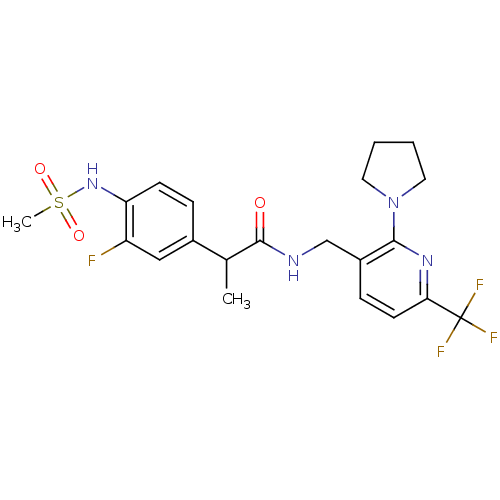
(CHEMBL2178067)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H24F4N4O3S/c1-13(14-5-7-17(16(22)11-14)28-33(2,31)32)20(30)26-12-15-6-8-18(21(23,24)25)27-19(15)29-9-3-4-10-29/h5-8,11,13,28H,3-4,9-10,12H2,1-2H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398488
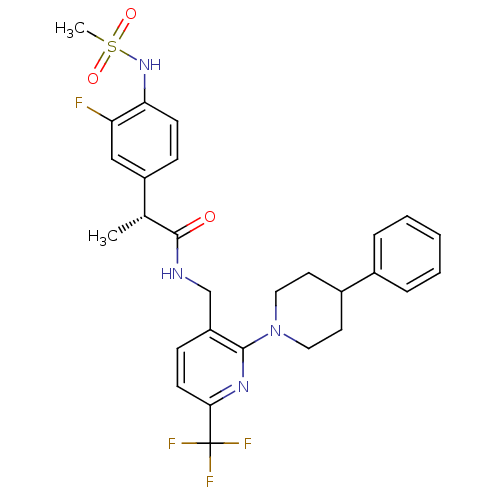
(CHEMBL2177436)Show SMILES C[C@@H](C(=O)NCc1ccc(nc1N1CCC(CC1)c1ccccc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C28H30F4N4O3S/c1-18(21-8-10-24(23(29)16-21)35-40(2,38)39)27(37)33-17-22-9-11-25(28(30,31)32)34-26(22)36-14-12-20(13-15-36)19-6-4-3-5-7-19/h3-11,16,18,20,35H,12-15,17H2,1-2H3,(H,33,37)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin-induced activity by FLIPR assay |
J Med Chem 55: 8392-408 (2012)
Article DOI: 10.1021/jm300780p
BindingDB Entry DOI: 10.7270/Q2TX3GH1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50100983

(CHEMBL3326224)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(22.71,-7.97,;18.3,-7.93,;16.96,-7.16,;16.96,-5.62,;15.63,-7.93,;14.86,-9.26,;13.32,-9.27,;12.55,-7.94,;13.32,-6.61,;14.86,-6.6,;11.79,-9.28,;10.25,-9.27,;9.47,-7.94,;10.25,-6.6,;11.79,-6.61,;12.26,-5.14,;11.03,-4.24,;10.87,-2.7,;9.47,-2.07,;8.21,-2.98,;8.37,-4.51,;9.77,-5.14,;16.43,-9.25,;15.68,-10.6,;16.48,-11.91,;18.02,-11.88,;18.76,-10.52,;17.96,-9.21,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602541
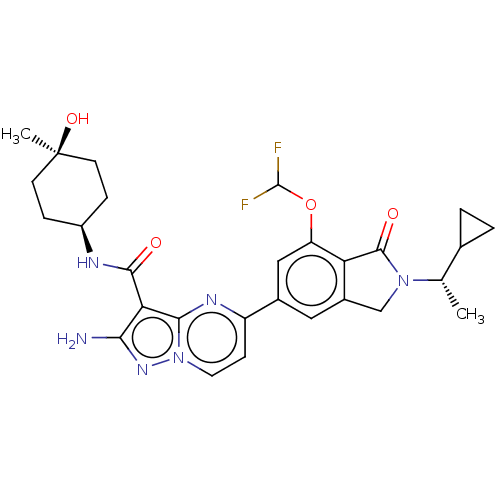
(CHEMBL5209268)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](C)(O)CC3)c2n1 |r,wU:30.32,1.1,33.37,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049549
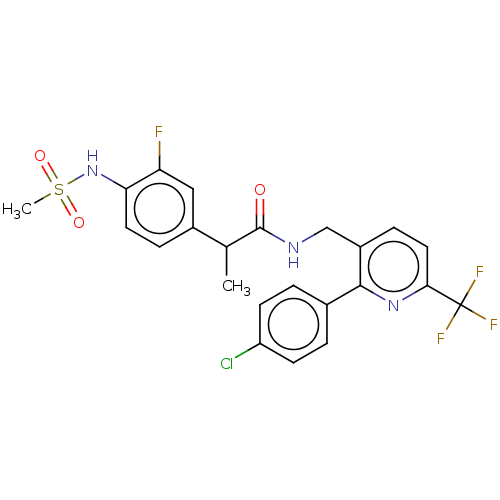
(CHEMBL3319111)Show SMILES CC(C(=O)NCc1ccc(nc1-c1ccc(Cl)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H20ClF4N3O3S/c1-13(15-5-9-19(18(25)11-15)31-35(2,33)34)22(32)29-12-16-6-10-20(23(26,27)28)30-21(16)14-3-7-17(24)8-4-14/h3-11,13,31H,12H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50100993
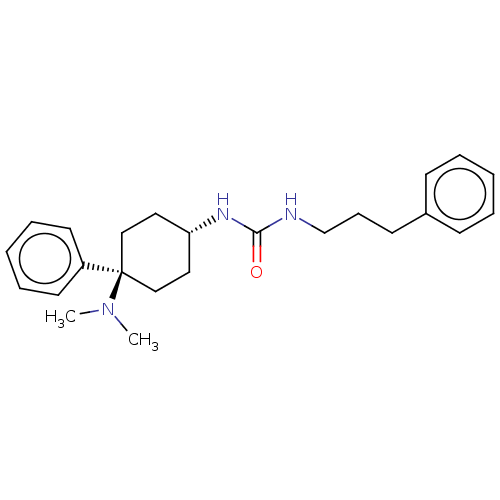
(CHEMBL3325881)Show SMILES Cl.CN(C)[C@]1(CC[C@@H](CC1)NC(=O)NCCCc1ccccc1)c1ccccc1 |r,wU:4.2,wD:7.9,(11.38,-21.45,;17.64,-17.11,;16.3,-16.34,;16.3,-14.8,;14.96,-17.12,;14.2,-18.45,;12.65,-18.46,;11.88,-17.13,;12.65,-15.79,;14.19,-15.79,;10.33,-17.13,;9.57,-18.46,;10.36,-19.8,;8.03,-18.48,;7.27,-19.82,;5.73,-19.82,;4.97,-21.15,;3.43,-21.17,;2.65,-19.84,;1.11,-19.85,;.35,-21.19,;1.14,-22.52,;2.67,-22.5,;15.76,-18.44,;15.02,-19.79,;15.81,-21.11,;17.36,-21.08,;18.1,-19.72,;17.3,-18.4,)| Show InChI InChI=1S/C24H33N3O/c1-27(2)24(21-13-7-4-8-14-21)17-15-22(16-18-24)26-23(28)25-19-9-12-20-10-5-3-6-11-20/h3-8,10-11,13-14,22H,9,12,15-19H2,1-2H3,(H2,25,26,28)/t22-,24- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins |
ACS Med Chem Lett 5: 851-6 (2014)
Article DOI: 10.1021/ml500116x
BindingDB Entry DOI: 10.7270/Q28S4RPM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data