 Found 350 hits with Last Name = 'giuliani' and Initial = 'g'
Found 350 hits with Last Name = 'giuliani' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasoactive intestinal polypeptide receptor 1
(Homo sapiens (Human)) | BDBM50435130
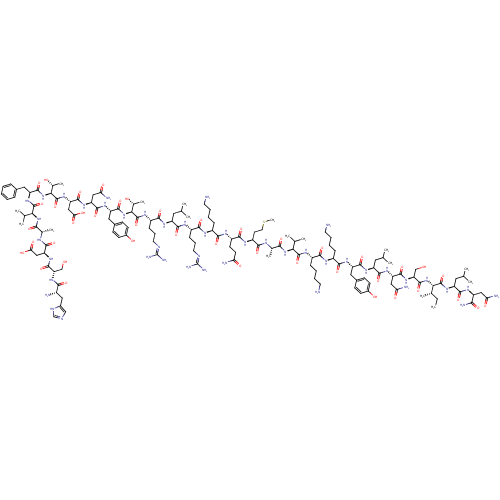
(CHEMBL1893324)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(C)C)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O Show InChI InChI=1S/C147H238N44O42S/c1-18-75(12)115(143(231)182-97(56-72(6)7)131(219)174-94(118(156)206)61-108(153)199)189-140(228)106(68-193)186-135(223)102(63-110(155)201)179-132(220)96(55-71(4)5)176-133(221)98(58-81-37-41-84(196)42-38-81)177-126(214)88(33-23-26-49-149)168-124(212)89(34-24-27-50-150)172-141(229)113(73(8)9)187-119(207)76(13)165-122(210)93(47-53-234-17)171-128(216)92(45-46-107(152)198)170-123(211)87(32-22-25-48-148)167-125(213)90(35-28-51-162-146(157)158)169-130(218)95(54-70(2)3)175-127(215)91(36-29-52-163-147(159)160)173-144(232)116(78(15)194)190-137(225)99(59-82-39-43-85(197)44-40-82)178-134(222)101(62-109(154)200)180-136(224)104(65-112(204)205)184-145(233)117(79(16)195)191-138(226)100(57-80-30-20-19-21-31-80)183-142(230)114(74(10)11)188-120(208)77(14)166-129(217)103(64-111(202)203)181-139(227)105(67-192)185-121(209)86(151)60-83-66-161-69-164-83/h19-21,30-31,37-44,66,69-79,86-106,113-117,192-197H,18,22-29,32-36,45-65,67-68,148-151H2,1-17H3,(H2,152,198)(H2,153,199)(H2,154,200)(H2,155,201)(H2,156,206)(H,161,164)(H,165,210)(H,166,217)(H,167,213)(H,168,212)(H,169,218)(H,170,211)(H,171,216)(H,172,229)(H,173,232)(H,174,219)(H,175,215)(H,176,221)(H,177,214)(H,178,222)(H,179,220)(H,180,224)(H,181,227)(H,182,231)(H,183,230)(H,184,233)(H,185,209)(H,186,223)(H,187,207)(H,188,208)(H,189,228)(H,190,225)(H,191,226)(H,202,203)(H,204,205)(H4,157,158,162)(H4,159,160,163)/t75-,76-,77-,78+,79+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,113-,114-,115-,116-,117-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human VPAC1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50409614
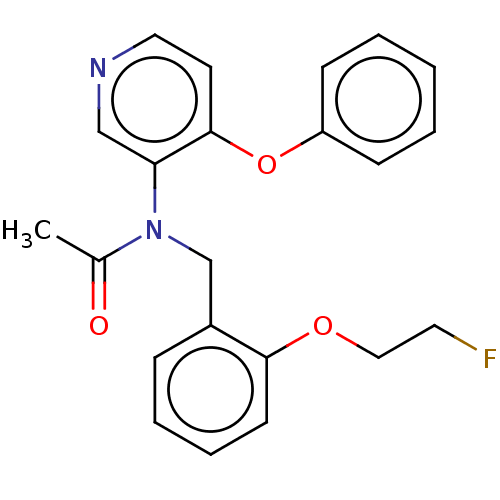
(CHEMBL5288131)Show SMILES OC(=O)C1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C26H35Cl2N3O3/c27-22-4-3-20(13-23(22)28)26(8-5-24(32)31(17-26)14-18-1-2-18)9-12-29-15-21(16-29)30-10-6-19(7-11-30)25(33)34/h3-4,13,18-19,21H,1-2,5-12,14-17H2,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50015490
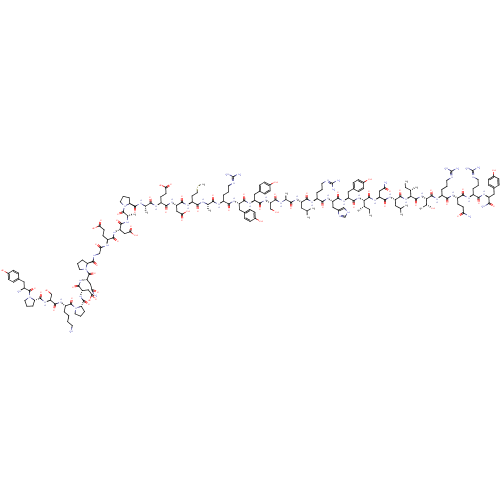
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human neuropeptide Y receptor type 2 by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50015490

(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human neuropeptide Y receptor type 1 by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50335566
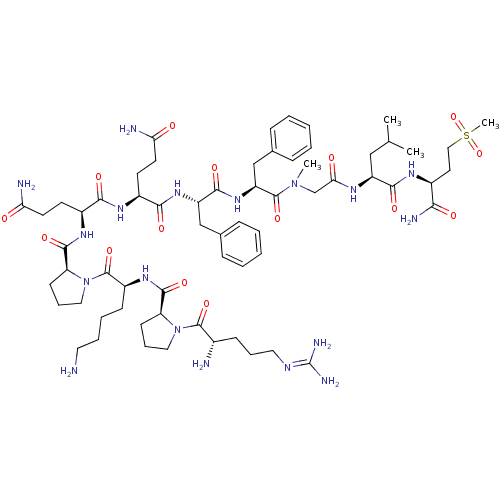
(CHEMBL1651026 | Substance P [Sar9,Met(O2)11])Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]S([#6])(=O)=O)-[#6](-[#7])=O |r| Show InChI InChI=1S/C64H100N18O15S/c1-38(2)34-46(57(89)74-42(54(69)86)28-33-98(4,96)97)73-53(85)37-80(3)62(94)48(36-40-18-9-6-10-19-40)79-58(90)47(35-39-16-7-5-8-17-39)78-56(88)43(24-26-51(67)83)75-55(87)44(25-27-52(68)84)76-59(91)50-23-15-32-82(50)63(95)45(21-11-12-29-65)77-60(92)49-22-14-31-81(49)61(93)41(66)20-13-30-72-64(70)71/h5-10,16-19,38,41-50H,11-15,20-37,65-66H2,1-4H3,(H2,67,83)(H2,68,84)(H2,69,86)(H,73,85)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,88)(H,79,90)(H4,70,71,72)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human NK1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human NTS1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50409613

(CHEMBL5275028)Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCC(=O)CC2)CCC(=O)N(CC2CC2)C1 Show InChI InChI=1S/C25H33Cl2N3O2/c26-22-4-3-19(13-23(22)27)25(8-5-24(32)30(17-25)14-18-1-2-18)9-12-28-15-20(16-28)29-10-6-21(31)7-11-29/h3-4,13,18,20H,1-2,5-12,14-17H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50378616
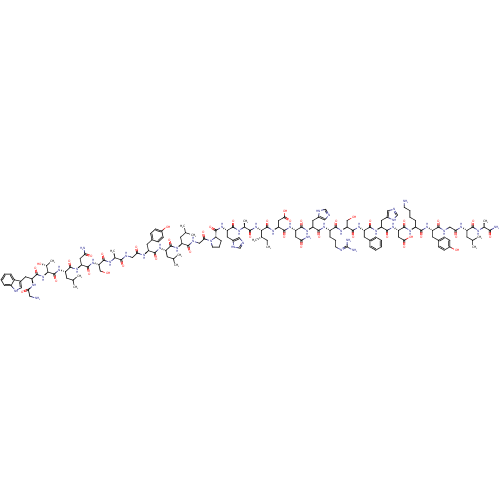
(GALANIN)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(N)=O |r,wU:119.123,135.139,162.167,173.179,200.207,224.233,13.20,42.48,50.61,113.119,93.96,wD:2.2,127.131,145.150,156.161,183.190,191.198,216.224,4.4,8.8,23.23,34.40,66.69,71.76,77.84,85.92,97.112,(19.04,-36.25,;18.27,-34.92,;16.73,-34.92,;15.96,-36.25,;15.96,-33.59,;14.42,-33.59,;13.65,-34.92,;14.42,-36.25,;12.11,-34.92,;11.34,-36.25,;11.34,-33.59,;9.8,-33.59,;9.03,-34.92,;9.03,-32.26,;9.8,-30.92,;9.03,-29.59,;9.66,-28.19,;8.52,-27.16,;7.18,-27.92,;7.5,-29.43,;7.49,-32.26,;6.72,-33.59,;7.49,-34.92,;5.19,-33.67,;4.35,-34.96,;2.86,-34.56,;2.78,-33.03,;4.21,-32.48,;4.62,-30.99,;6.1,-30.59,;3.53,-29.9,;2.04,-30.3,;.95,-29.21,;-.53,-29.61,;1.35,-27.72,;.26,-26.63,;-1.23,-27.03,;-2.31,-25.94,;-1.62,-28.52,;2.83,-27.32,;3.24,-25.83,;2.15,-24.75,;4.72,-25.44,;5.81,-26.53,;5.41,-28.01,;3.93,-28.41,;6.5,-29.11,;5.24,-23.51,;3.83,-22.09,;2.34,-22.49,;4.34,-20.16,;5.83,-19.76,;6.92,-20.85,;8.4,-20.45,;9.5,-21.54,;9.09,-23.02,;10.19,-24.11,;7.61,-23.42,;6.52,-22.33,;2.93,-18.75,;1.44,-19.14,;1.04,-20.63,;.03,-17.72,;-1.46,-18.12,;-1.86,-19.61,;-.77,-20.7,;-3.35,-20.01,;-3.75,-21.5,;-4.43,-18.92,;-5.93,-19.32,;-6.32,-20.81,;-7.01,-18.23,;-8.5,-18.63,;-8.9,-20.12,;-6.61,-16.74,;-7.7,-15.65,;-9.19,-16.05,;-7.3,-14.17,;-8.39,-13.08,;-9.88,-13.47,;-10.97,-12.39,;-10.28,-14.97,;-5.82,-13.77,;-5.42,-12.28,;-6.51,-11.19,;-3.93,-11.88,;-3.53,-10.4,;-4.62,-9.31,;-6.11,-9.71,;-4.22,-7.82,;-2.84,-12.97,;-1.35,-12.57,;-.95,-11.09,;-.27,-13.66,;1.22,-13.27,;2.31,-14.35,;1.91,-15.84,;3.79,-13.96,;4.12,-12.45,;5.58,-11.97,;6.06,-10.51,;7.59,-10.51,;8.07,-11.97,;9.48,-12.6,;9.64,-14.13,;8.39,-15.04,;6.99,-14.41,;6.82,-12.88,;4.89,-15.04,;4.49,-16.53,;3,-16.93,;5.58,-17.62,;7.06,-17.22,;-.66,-15.15,;.43,-16.24,;-2.15,-15.55,;16.73,-32.26,;18.27,-32.26,;15.96,-30.92,;16.73,-29.59,;15.96,-28.26,;14.42,-28.26,;13.65,-29.59,;13.65,-26.93,;18.27,-29.59,;19.04,-28.26,;19.04,-30.92,;20.57,-30.92,;21.35,-32.26,;22.88,-32.26,;23.66,-33.59,;23.66,-30.92,;21.35,-29.59,;22.88,-29.59,;20.57,-28.26,;21.35,-26.93,;20.57,-25.59,;21.35,-24.26,;20.72,-22.86,;21.87,-21.82,;23.2,-22.59,;22.88,-24.09,;22.88,-26.93,;23.66,-28.26,;23.66,-25.59,;25.19,-25.59,;25.97,-26.93,;27.5,-26.93,;28.27,-25.59,;29.81,-25.59,;30.58,-24.26,;32.12,-24.26,;29.81,-22.92,;25.97,-24.26,;27.5,-24.26,;25.19,-22.92,;25.97,-21.59,;27.5,-21.59,;28.27,-20.26,;25.19,-20.26,;25.97,-18.92,;23.66,-20.26,;22.88,-18.92,;21.35,-18.92,;20.57,-17.59,;19.04,-17.59,;18.27,-16.26,;19.04,-14.93,;20.57,-14.93,;21.35,-16.26,;23.66,-17.59,;25.19,-17.59,;22.88,-16.26,;23.66,-14.93,;22.88,-13.59,;23.66,-12.26,;23.03,-10.86,;24.18,-9.82,;25.51,-10.59,;25.19,-12.1,;25.19,-14.93,;25.97,-16.26,;25.97,-13.59,;27.5,-13.59,;28.27,-14.93,;29.81,-14.93,;30.58,-16.26,;30.58,-13.59,;28.27,-12.26,;29.81,-12.26,;27.5,-10.93,;28.27,-9.6,;29.81,-9.6,;30.58,-10.93,;32.12,-10.93,;32.89,-12.26,;34.43,-12.26,;27.5,-8.26,;28.27,-6.92,;25.97,-8.26,;25.19,-6.92,;23.66,-6.92,;22.88,-5.59,;21.35,-5.59,;20.57,-4.26,;21.35,-2.93,;20.57,-1.59,;22.88,-2.93,;23.66,-4.26,;25.97,-5.59,;27.5,-5.59,;25.19,-4.26,;25.97,-2.93,;27.5,-2.93,;28.27,-4.26,;28.27,-1.59,;29.81,-1.59,;30.58,-2.93,;32.12,-2.93,;32.89,-4.26,;32.89,-1.59,;30.58,-.26,;32.12,-.26,;29.81,1.07,;30.58,2.4,;29.81,3.74,;32.12,2.4,;32.89,3.74,;32.89,1.07,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157)/t75-,76-,77-,78-,79+,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,119-,120-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human GAL1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104
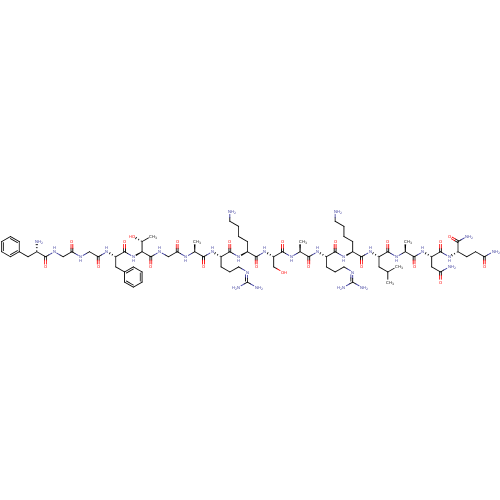
(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human NOP receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM22041
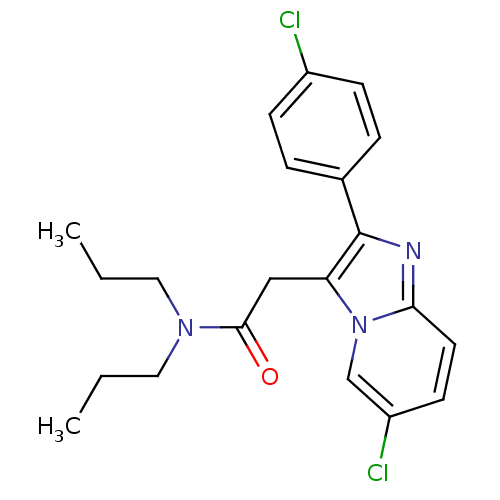
(2-[6-chloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridi...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2ccc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23Cl2N3O/c1-3-11-25(12-4-2)20(27)13-18-21(15-5-7-16(22)8-6-15)24-19-10-9-17(23)14-26(18)19/h5-10,14H,3-4,11-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Dihydrofolate reductase of Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2S receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Translocator protein
(Homo sapiens (Human)) | BDBM50363523

(CHEMBL1944785)Show SMILES CCN(CC)C(=O)[C@H]1CCCc2c1c1c(OC)cccc1n2CCF |r| Show InChI InChI=1S/C20H27FN2O2/c1-4-22(5-2)20(24)14-8-6-9-15-18(14)19-16(23(15)13-12-21)10-7-11-17(19)25-3/h7,10-11,14H,4-6,8-9,12-13H2,1-3H3/t14-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Dihydrofolate reductase of Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Galanin receptor type 2
(Homo sapiens (Human)) | BDBM50378616

(GALANIN)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(N)=O |r,wU:119.123,135.139,162.167,173.179,200.207,224.233,13.20,42.48,50.61,113.119,93.96,wD:2.2,127.131,145.150,156.161,183.190,191.198,216.224,4.4,8.8,23.23,34.40,66.69,71.76,77.84,85.92,97.112,(19.04,-36.25,;18.27,-34.92,;16.73,-34.92,;15.96,-36.25,;15.96,-33.59,;14.42,-33.59,;13.65,-34.92,;14.42,-36.25,;12.11,-34.92,;11.34,-36.25,;11.34,-33.59,;9.8,-33.59,;9.03,-34.92,;9.03,-32.26,;9.8,-30.92,;9.03,-29.59,;9.66,-28.19,;8.52,-27.16,;7.18,-27.92,;7.5,-29.43,;7.49,-32.26,;6.72,-33.59,;7.49,-34.92,;5.19,-33.67,;4.35,-34.96,;2.86,-34.56,;2.78,-33.03,;4.21,-32.48,;4.62,-30.99,;6.1,-30.59,;3.53,-29.9,;2.04,-30.3,;.95,-29.21,;-.53,-29.61,;1.35,-27.72,;.26,-26.63,;-1.23,-27.03,;-2.31,-25.94,;-1.62,-28.52,;2.83,-27.32,;3.24,-25.83,;2.15,-24.75,;4.72,-25.44,;5.81,-26.53,;5.41,-28.01,;3.93,-28.41,;6.5,-29.11,;5.24,-23.51,;3.83,-22.09,;2.34,-22.49,;4.34,-20.16,;5.83,-19.76,;6.92,-20.85,;8.4,-20.45,;9.5,-21.54,;9.09,-23.02,;10.19,-24.11,;7.61,-23.42,;6.52,-22.33,;2.93,-18.75,;1.44,-19.14,;1.04,-20.63,;.03,-17.72,;-1.46,-18.12,;-1.86,-19.61,;-.77,-20.7,;-3.35,-20.01,;-3.75,-21.5,;-4.43,-18.92,;-5.93,-19.32,;-6.32,-20.81,;-7.01,-18.23,;-8.5,-18.63,;-8.9,-20.12,;-6.61,-16.74,;-7.7,-15.65,;-9.19,-16.05,;-7.3,-14.17,;-8.39,-13.08,;-9.88,-13.47,;-10.97,-12.39,;-10.28,-14.97,;-5.82,-13.77,;-5.42,-12.28,;-6.51,-11.19,;-3.93,-11.88,;-3.53,-10.4,;-4.62,-9.31,;-6.11,-9.71,;-4.22,-7.82,;-2.84,-12.97,;-1.35,-12.57,;-.95,-11.09,;-.27,-13.66,;1.22,-13.27,;2.31,-14.35,;1.91,-15.84,;3.79,-13.96,;4.12,-12.45,;5.58,-11.97,;6.06,-10.51,;7.59,-10.51,;8.07,-11.97,;9.48,-12.6,;9.64,-14.13,;8.39,-15.04,;6.99,-14.41,;6.82,-12.88,;4.89,-15.04,;4.49,-16.53,;3,-16.93,;5.58,-17.62,;7.06,-17.22,;-.66,-15.15,;.43,-16.24,;-2.15,-15.55,;16.73,-32.26,;18.27,-32.26,;15.96,-30.92,;16.73,-29.59,;15.96,-28.26,;14.42,-28.26,;13.65,-29.59,;13.65,-26.93,;18.27,-29.59,;19.04,-28.26,;19.04,-30.92,;20.57,-30.92,;21.35,-32.26,;22.88,-32.26,;23.66,-33.59,;23.66,-30.92,;21.35,-29.59,;22.88,-29.59,;20.57,-28.26,;21.35,-26.93,;20.57,-25.59,;21.35,-24.26,;20.72,-22.86,;21.87,-21.82,;23.2,-22.59,;22.88,-24.09,;22.88,-26.93,;23.66,-28.26,;23.66,-25.59,;25.19,-25.59,;25.97,-26.93,;27.5,-26.93,;28.27,-25.59,;29.81,-25.59,;30.58,-24.26,;32.12,-24.26,;29.81,-22.92,;25.97,-24.26,;27.5,-24.26,;25.19,-22.92,;25.97,-21.59,;27.5,-21.59,;28.27,-20.26,;25.19,-20.26,;25.97,-18.92,;23.66,-20.26,;22.88,-18.92,;21.35,-18.92,;20.57,-17.59,;19.04,-17.59,;18.27,-16.26,;19.04,-14.93,;20.57,-14.93,;21.35,-16.26,;23.66,-17.59,;25.19,-17.59,;22.88,-16.26,;23.66,-14.93,;22.88,-13.59,;23.66,-12.26,;23.03,-10.86,;24.18,-9.82,;25.51,-10.59,;25.19,-12.1,;25.19,-14.93,;25.97,-16.26,;25.97,-13.59,;27.5,-13.59,;28.27,-14.93,;29.81,-14.93,;30.58,-16.26,;30.58,-13.59,;28.27,-12.26,;29.81,-12.26,;27.5,-10.93,;28.27,-9.6,;29.81,-9.6,;30.58,-10.93,;32.12,-10.93,;32.89,-12.26,;34.43,-12.26,;27.5,-8.26,;28.27,-6.92,;25.97,-8.26,;25.19,-6.92,;23.66,-6.92,;22.88,-5.59,;21.35,-5.59,;20.57,-4.26,;21.35,-2.93,;20.57,-1.59,;22.88,-2.93,;23.66,-4.26,;25.97,-5.59,;27.5,-5.59,;25.19,-4.26,;25.97,-2.93,;27.5,-2.93,;28.27,-4.26,;28.27,-1.59,;29.81,-1.59,;30.58,-2.93,;32.12,-2.93,;32.89,-4.26,;32.89,-1.59,;30.58,-.26,;32.12,-.26,;29.81,1.07,;30.58,2.4,;29.81,3.74,;32.12,2.4,;32.89,3.74,;32.89,1.07,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157)/t75-,76-,77-,78-,79+,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,119-,120-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human GAL2 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051295
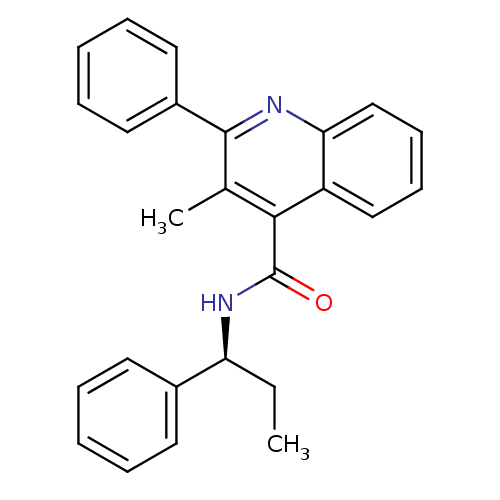
((S)-3-methyl-2-phenyl-N-(1-phenylpropyl)quinoline-...)Show SMILES CC[C@H](NC(=O)c1c(C)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H24N2O/c1-3-22(19-12-6-4-7-13-19)28-26(29)24-18(2)25(20-14-8-5-9-15-20)27-23-17-11-10-16-21(23)24/h4-17,22H,3H2,1-2H3,(H,28,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human NK3 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50045880

(2-[5-Chloro-2-(4-chloro-phenyl)-1H-indol-3-yl]-N,N...)Show SMILES CCCN(CCC)C(=O)Cc1c([nH]c2ccc(Cl)cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H24Cl2N2O/c1-3-11-26(12-4-2)21(27)14-19-18-13-17(24)9-10-20(18)25-22(19)15-5-7-16(23)8-6-15/h5-10,13,25H,3-4,11-12,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM82561
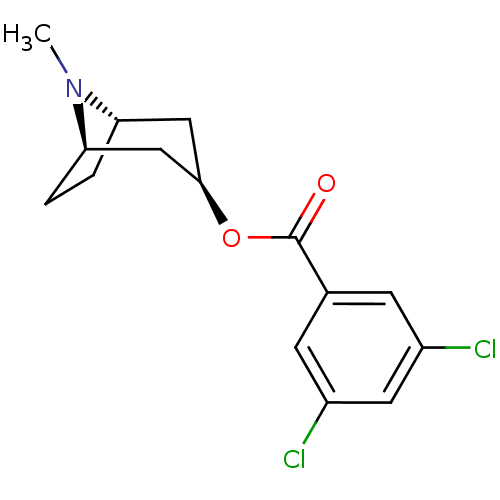
(CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C15H17Cl2NO2/c1-18-12-2-3-13(18)8-14(7-12)20-15(19)9-4-10(16)6-11(17)5-9/h4-6,12-14H,2-3,7-8H2,1H3/t12-,13+,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT3 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human PGI2 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50435131
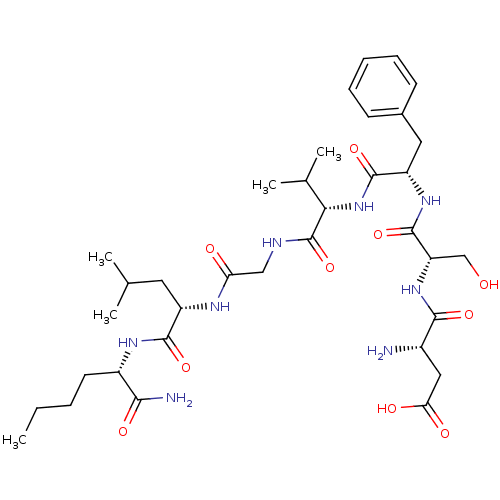
(CHEMBL2390989)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O |r| Show InChI InChI=1S/C35H56N8O10/c1-6-7-13-23(30(37)48)40-32(50)24(14-19(2)3)39-27(45)17-38-35(53)29(20(4)5)43-33(51)25(15-21-11-9-8-10-12-21)41-34(52)26(18-44)42-31(49)22(36)16-28(46)47/h8-12,19-20,22-26,29,44H,6-7,13-18,36H2,1-5H3,(H2,37,48)(H,38,53)(H,39,45)(H,40,50)(H,41,52)(H,42,49)(H,43,51)(H,46,47)/t22-,23-,24-,25-,26-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human NK2 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50243007
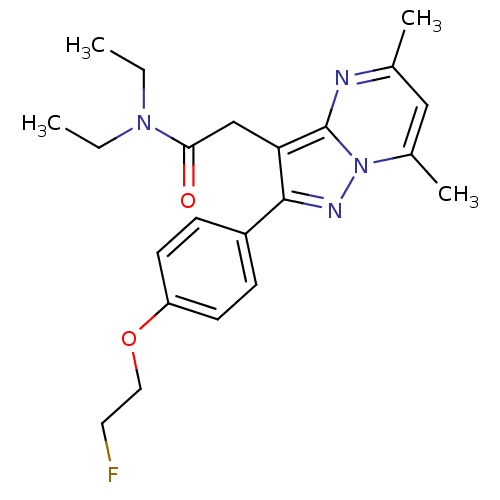
(2-(2-(4-(2-Fluoroethoxy)phenyl)-5,7-dimethylpyrazo...)Show SMILES CCN(CC)C(=O)Cc1c(nn2c(C)cc(C)nc12)-c1ccc(OCCF)cc1 Show InChI InChI=1S/C22H27FN4O2/c1-5-26(6-2)20(28)14-19-21(17-7-9-18(10-8-17)29-12-11-23)25-27-16(4)13-15(3)24-22(19)27/h7-10,13H,5-6,11-12,14H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50435129
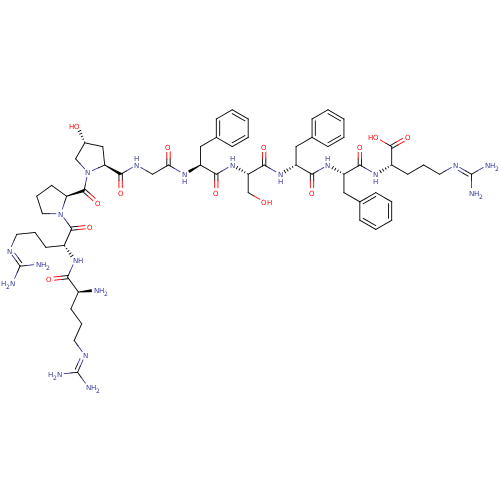
(CHEMBL2392354)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C60H87N19O13/c61-39(20-10-24-68-58(62)63)49(83)73-40(21-11-25-69-59(64)65)55(89)78-27-13-23-46(78)56(90)79-33-38(81)31-47(79)54(88)71-32-48(82)72-42(28-35-14-4-1-5-15-35)50(84)77-45(34-80)53(87)76-44(30-37-18-8-3-9-19-37)52(86)75-43(29-36-16-6-2-7-17-36)51(85)74-41(57(91)92)22-12-26-70-60(66)67/h1-9,14-19,38-47,80-81H,10-13,20-34,61H2,(H,71,88)(H,72,82)(H,73,83)(H,74,85)(H,75,86)(H,76,87)(H,77,84)(H,91,92)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t38-,39+,40-,41+,42+,43+,44-,45+,46+,47+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B2 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50119657

(8-Butyl-7-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN1N(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)C(=O)C2(CCCC2)CC1=O Show InChI InChI=1S/C26H30N6O2/c1-2-3-16-31-23(33)17-26(14-6-7-15-26)25(34)32(31)18-19-10-12-20(13-11-19)21-8-4-5-9-22(21)24-27-29-30-28-24/h4-5,8-13H,2-3,6-7,14-18H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1,Ile8-Ang2 from AT1 receptor in Wistar rat hepatic membrane |
J Med Chem 51: 2137-46 (2008)
Article DOI: 10.1021/jm7011563
BindingDB Entry DOI: 10.7270/Q20P10X0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50056415
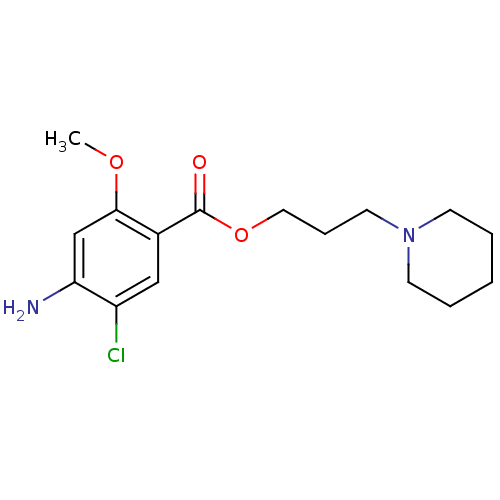
(2-piperidinopropyl 4-amino-5-chloro-2-methoxybenzo...)Show InChI InChI=1S/C16H23ClN2O3/c1-21-15-11-14(18)13(17)10-12(15)16(20)22-9-5-8-19-6-3-2-4-7-19/h10-11H,2-9,18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
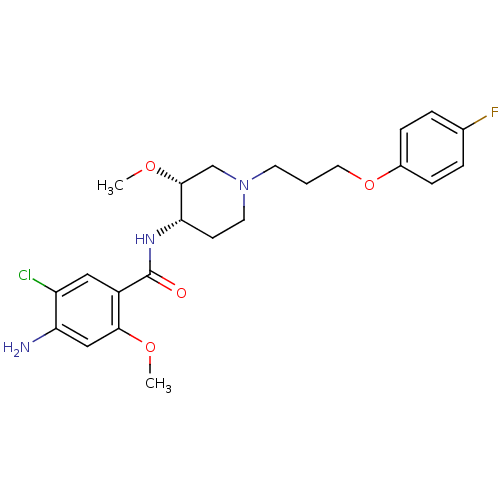
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50388686
(CHEMBL74656)Show SMILES CO[C@@H]1CN(CCCOc2ccc(F)cc2)CC[C@@H]1NC(=O)c1cc(Cl)c(N)cc1OC |r| Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29)/t20-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50089990
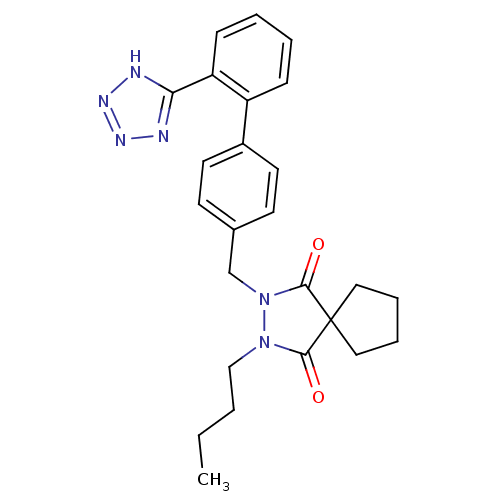
(2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN1N(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)C(=O)C2(CCCC2)C1=O Show InChI InChI=1S/C25H28N6O2/c1-2-3-16-30-23(32)25(14-6-7-15-25)24(33)31(30)17-18-10-12-19(13-11-18)20-8-4-5-9-21(20)22-26-28-29-27-22/h4-5,8-13H,2-3,6-7,14-17H2,1H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1,Ile8-Ang2 from AT1 receptor in Wistar rat hepatic membrane |
J Med Chem 51: 2137-46 (2008)
Article DOI: 10.1021/jm7011563
BindingDB Entry DOI: 10.7270/Q20P10X0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50019529
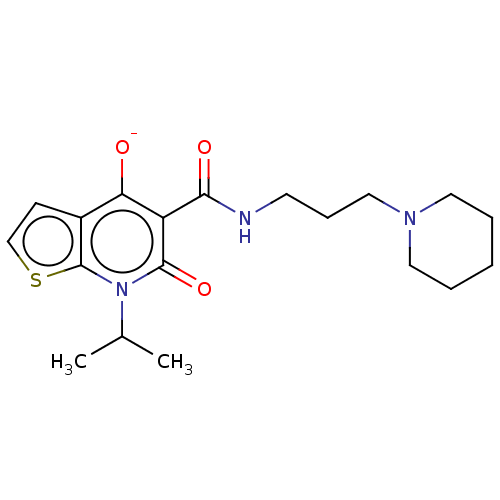
(CHEMBL3291085)Show SMILES [K+].CC(C)n1c2sccc2c([O-])c(C(=O)NCCCN2CCCCC2)c1=O Show InChI InChI=1S/C19H27N3O3S.K/c1-13(2)22-18(25)15(16(23)14-7-12-26-19(14)22)17(24)20-8-6-11-21-9-4-3-5-10-21;/h7,12-13,23H,3-6,8-11H2,1-2H3,(H,20,24);/q;+1/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT4R (unknown origin) |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019523
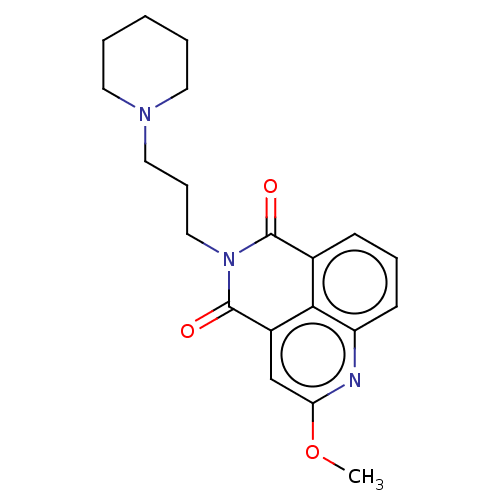
(CHEMBL3291078)Show SMILES COc1cc2C(=O)N(CCCN3CCCCC3)C(=O)c3cccc(n1)c23 Show InChI InChI=1S/C20H23N3O3/c1-26-17-13-15-18-14(7-5-8-16(18)21-17)19(24)23(20(15)25)12-6-11-22-9-3-2-4-10-22/h5,7-8,13H,2-4,6,9-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019520
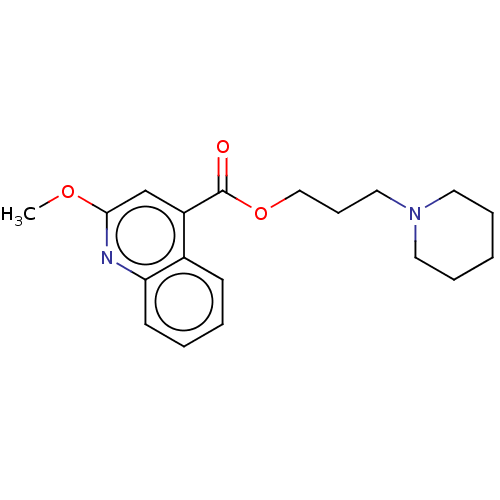
(CHEMBL3291075)Show InChI InChI=1S/C19H24N2O3/c1-23-18-14-16(15-8-3-4-9-17(15)20-18)19(22)24-13-7-12-21-10-5-2-6-11-21/h3-4,8-9,14H,2,5-7,10-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM50119658
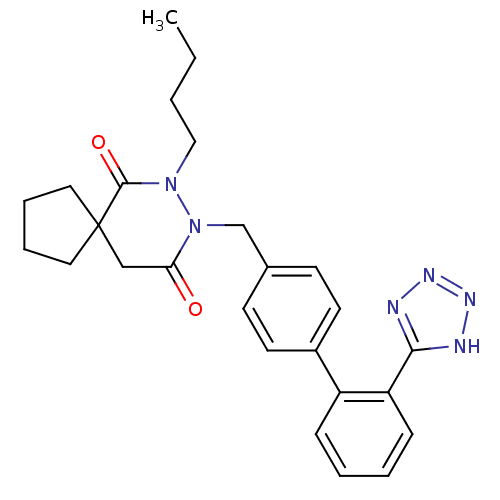
(7-Butyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCN1N(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)C(=O)CC2(CCCC2)C1=O Show InChI InChI=1S/C26H30N6O2/c1-2-3-16-31-25(34)26(14-6-7-15-26)17-23(33)32(31)18-19-10-12-20(13-11-19)21-8-4-5-9-22(21)24-27-29-30-28-24/h4-5,8-13H,2-3,6-7,14-18H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1,Ile8-Ang2 from AT1 receptor in Wistar rat hepatic membrane |
J Med Chem 51: 2137-46 (2008)
Article DOI: 10.1021/jm7011563
BindingDB Entry DOI: 10.7270/Q20P10X0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019525
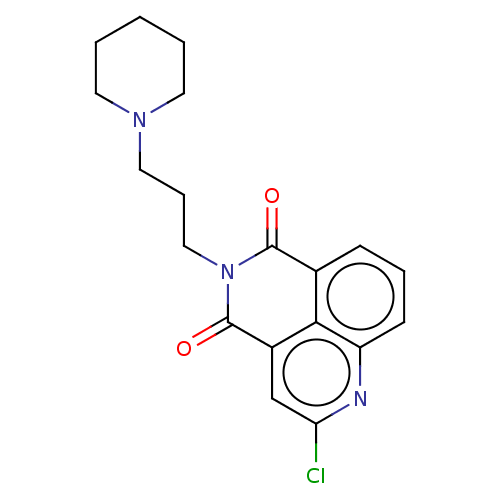
(CHEMBL3291080)Show SMILES Clc1cc2C(=O)N(CCCN3CCCCC3)C(=O)c3cccc(n1)c23 Show InChI InChI=1S/C19H20ClN3O2/c20-16-12-14-17-13(6-4-7-15(17)21-16)18(24)23(19(14)25)11-5-10-22-8-2-1-3-9-22/h4,6-7,12H,1-3,5,8-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50403559
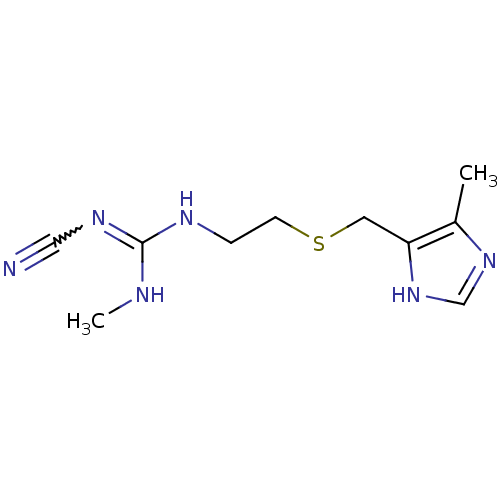
(Brumetadina | CIMETIDINE)Show InChI InChI=1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H2 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019522
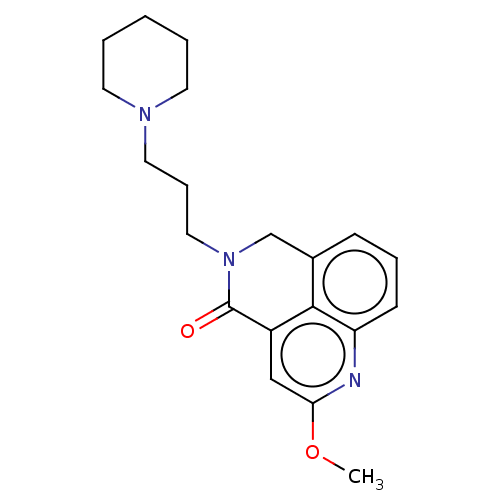
(CHEMBL3291077)Show InChI InChI=1S/C20H25N3O2/c1-25-18-13-16-19-15(7-5-8-17(19)21-18)14-23(20(16)24)12-6-11-22-9-3-2-4-10-22/h5,7-8,13H,2-4,6,9-12,14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 283 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019528

(CHEMBL3291083)Show SMILES O=C1N(CCCN2CCCCC2)C(=O)c2cc(nc3cccc1c23)N1CCCCC1 Show InChI InChI=1S/C24H30N4O2/c29-23-18-9-7-10-20-22(18)19(17-21(25-20)27-14-5-2-6-15-27)24(30)28(23)16-8-13-26-11-3-1-4-12-26/h7,9-10,17H,1-6,8,11-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 344 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019524
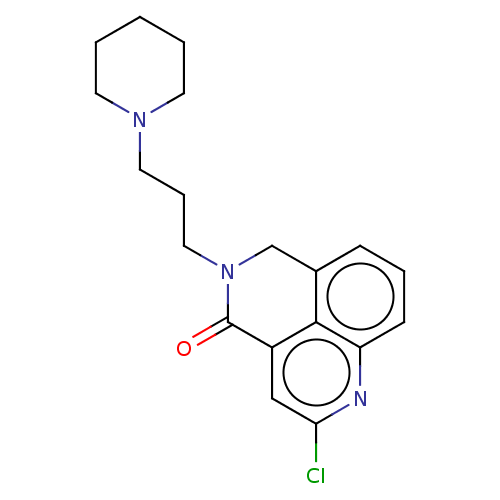
(CHEMBL3291079)Show InChI InChI=1S/C19H22ClN3O/c20-17-12-15-18-14(6-4-7-16(18)21-17)13-23(19(15)24)11-5-10-22-8-2-1-3-9-22/h4,6-7,12H,1-3,5,8-11,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 563 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019527

(CHEMBL3291082)Show SMILES O=C1N(CCCN2CCCCC2)C(=O)c2cc(nc3cccc1c23)N1CCCC1 Show InChI InChI=1S/C23H28N4O2/c28-22-17-8-6-9-19-21(17)18(16-20(24-19)26-13-4-5-14-26)23(29)27(22)15-7-12-25-10-2-1-3-11-25/h6,8-9,16H,1-5,7,10-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019521

(CHEMBL3291076)Show InChI InChI=1S/C19H25N3O2/c1-24-18-14-16(15-8-3-4-9-17(15)21-18)19(23)20-10-7-13-22-11-5-2-6-12-22/h3-4,8-9,14H,2,5-7,10-13H2,1H3,(H,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50019526
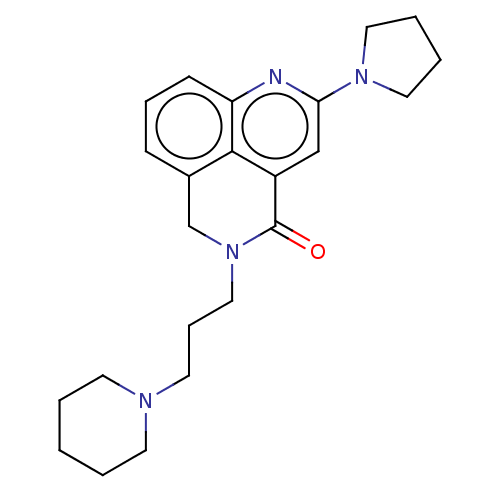
(CHEMBL3291081)Show SMILES O=C1N(CCCN2CCCCC2)Cc2cccc3nc(cc1c23)N1CCCC1 Show InChI InChI=1S/C23H30N4O/c28-23-19-16-21(26-13-4-5-14-26)24-20-9-6-8-18(22(19)20)17-27(23)15-7-12-25-10-2-1-3-11-25/h6,8-9,16H,1-5,7,10-15,17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from Dunkin-Hartley guinea pig brain striatum 5HT4R after 30 mins |
Eur J Med Chem 82: 36-46 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.015
BindingDB Entry DOI: 10.7270/Q21Z45ZQ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140783
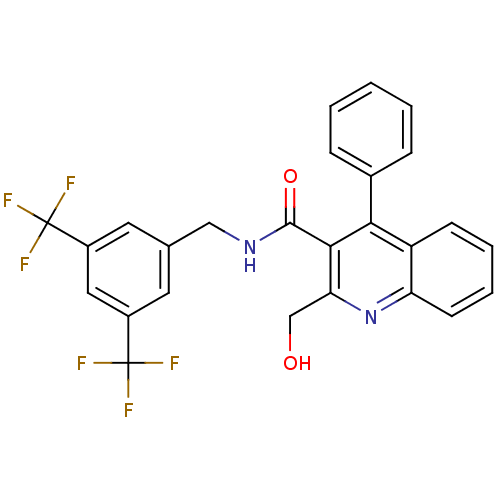
(2-Hydroxymethyl-4-phenyl-quinoline-3-carboxylic ac...)Show SMILES OCc1nc2ccccc2c(-c2ccccc2)c1C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H18F6N2O2/c27-25(28,29)17-10-15(11-18(12-17)26(30,31)32)13-33-24(36)23-21(14-35)34-20-9-5-4-8-19(20)22(23)16-6-2-1-3-7-16/h1-12,35H,13-14H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.000100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140770
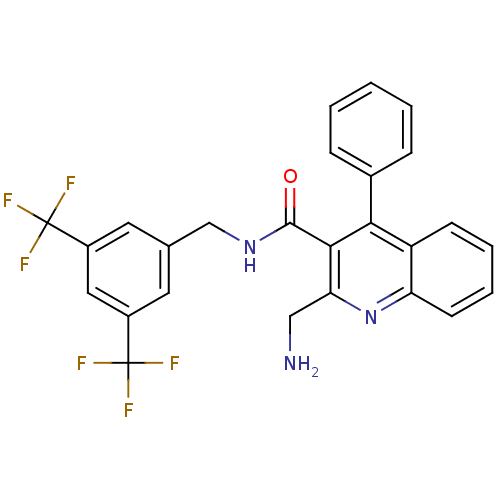
(2-Aminomethyl-4-phenyl-quinoline-3-carboxylic acid...)Show SMILES NCc1nc2ccccc2c(-c2ccccc2)c1C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H19F6N3O/c27-25(28,29)17-10-15(11-18(12-17)26(30,31)32)14-34-24(36)23-21(13-33)35-20-9-5-4-8-19(20)22(23)16-6-2-1-3-7-16/h1-12H,13-14,33H2,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140773

(2-Bromomethyl-4-phenyl-quinoline-3-carboxylic acid...)Show SMILES FC(F)(F)c1cc(CNC(=O)c2c(CBr)nc3ccccc3c2-c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C26H17BrF6N2O/c27-13-21-23(22(16-6-2-1-3-7-16)19-8-4-5-9-20(19)35-21)24(36)34-14-15-10-17(25(28,29)30)12-18(11-15)26(31,32)33/h1-12H,13-14H2,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140785

(2-Methylaminomethyl-4-phenyl-quinoline-3-carboxyli...)Show SMILES CNCc1nc2ccccc2c(-c2ccccc2)c1C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H21F6N3O/c1-34-15-22-24(23(17-7-3-2-4-8-17)20-9-5-6-10-21(20)36-22)25(37)35-14-16-11-18(26(28,29)30)13-19(12-16)27(31,32)33/h2-13,34H,14-15H2,1H3,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140786
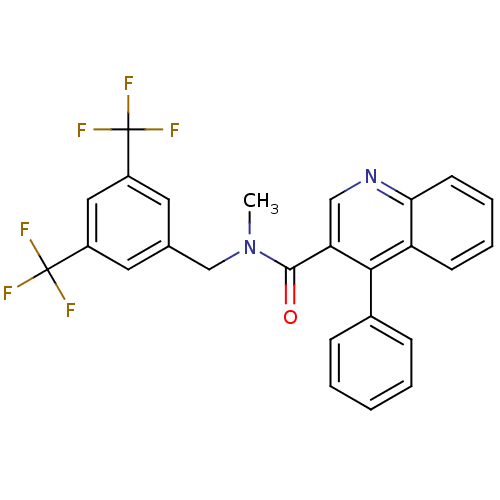
(4-Phenyl-quinoline-3-carboxylic acid (3,5-bis-trif...)Show SMILES CN(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(=O)c1cnc2ccccc2c1-c1ccccc1 Show InChI InChI=1S/C26H18F6N2O/c1-34(15-16-11-18(25(27,28)29)13-19(12-16)26(30,31)32)24(35)21-14-33-22-10-6-5-9-20(22)23(21)17-7-3-2-4-8-17/h2-14H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140772
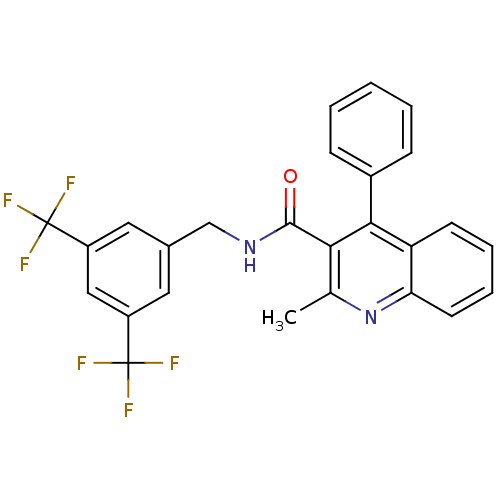
(2-Methyl-4-phenyl-quinoline-3-carboxylic acid 3,5-...)Show SMILES Cc1nc2ccccc2c(-c2ccccc2)c1C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H18F6N2O/c1-15-22(23(17-7-3-2-4-8-17)20-9-5-6-10-21(20)34-15)24(35)33-14-16-11-18(25(27,28)29)13-19(12-16)26(30,31)32/h2-13H,14H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50418331

(SPERGUALIN)Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CC[C@@H](C[C@H]1Cc1ccc(Cl)cc1)NC(=O)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C31H24ClF6N3O2/c32-22-7-5-18(6-8-22)13-24-17-23(40-28(42)26-9-11-39-27-4-2-1-3-25(26)27)10-12-41(24)29(43)19-14-20(30(33,34)35)16-21(15-19)31(36,37)38/h1-9,11,14-16,23-24H,10,12-13,17H2,(H,40,42)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human NK1 receptor |
Bioorg Med Chem 19: 2242-51 (2011)
Article DOI: 10.1016/j.bmc.2011.02.031
BindingDB Entry DOI: 10.7270/Q2QJ7J8H |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand |
J Med Chem 47: 1315-8 (2004)
Article DOI: 10.1021/jm034219a
BindingDB Entry DOI: 10.7270/Q2F76C0W |
More data for this
Ligand-Target Pair | |
Vasoactive intestinal polypeptide receptor 1
(Homo sapiens (Human)) | BDBM50435130

(CHEMBL1893324)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(C)C)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O Show InChI InChI=1S/C147H238N44O42S/c1-18-75(12)115(143(231)182-97(56-72(6)7)131(219)174-94(118(156)206)61-108(153)199)189-140(228)106(68-193)186-135(223)102(63-110(155)201)179-132(220)96(55-71(4)5)176-133(221)98(58-81-37-41-84(196)42-38-81)177-126(214)88(33-23-26-49-149)168-124(212)89(34-24-27-50-150)172-141(229)113(73(8)9)187-119(207)76(13)165-122(210)93(47-53-234-17)171-128(216)92(45-46-107(152)198)170-123(211)87(32-22-25-48-148)167-125(213)90(35-28-51-162-146(157)158)169-130(218)95(54-70(2)3)175-127(215)91(36-29-52-163-147(159)160)173-144(232)116(78(15)194)190-137(225)99(59-82-39-43-85(197)44-40-82)178-134(222)101(62-109(154)200)180-136(224)104(65-112(204)205)184-145(233)117(79(16)195)191-138(226)100(57-80-30-20-19-21-31-80)183-142(230)114(74(10)11)188-120(208)77(14)166-129(217)103(64-111(202)203)181-139(227)105(67-192)185-121(209)86(151)60-83-66-161-69-164-83/h19-21,30-31,37-44,66,69-79,86-106,113-117,192-197H,18,22-29,32-36,45-65,67-68,148-151H2,1-17H3,(H2,152,198)(H2,153,199)(H2,154,200)(H2,155,201)(H2,156,206)(H,161,164)(H,165,210)(H,166,217)(H,167,213)(H,168,212)(H,169,218)(H,170,211)(H,171,216)(H,172,229)(H,173,232)(H,174,219)(H,175,215)(H,176,221)(H,177,214)(H,178,222)(H,179,220)(H,180,224)(H,181,227)(H,182,231)(H,183,230)(H,184,233)(H,185,209)(H,186,223)(H,187,207)(H,188,208)(H,189,228)(H,190,225)(H,191,226)(H,202,203)(H,204,205)(H4,157,158,162)(H4,159,160,163)/t75-,76-,77-,78+,79+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,113-,114-,115-,116-,117-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human VPAC1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50355784

(CHEMBL1911623)Show SMILES CN(C(=O)Cn1c2nn(-c3ccccc3)c(=O)c2c(C)c2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H21ClN4O2/c1-17-21-10-6-7-11-22(21)30(16-23(32)29(2)19-14-12-18(27)13-15-19)25-24(17)26(33)31(28-25)20-8-4-3-5-9-20/h3-15H,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat brain homogenates after 90 mins by liquid scintillation spectrometry |
J Med Chem 54: 7165-75 (2011)
Article DOI: 10.1021/jm200770f
BindingDB Entry DOI: 10.7270/Q2MG7PWM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50355785
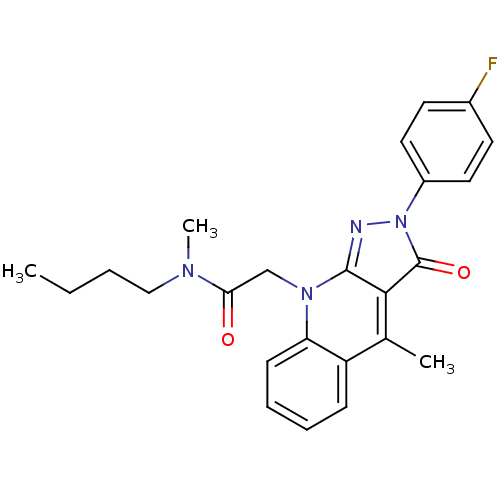
(CHEMBL1911624)Show SMILES CCCCN(C)C(=O)Cn1c2nn(-c3ccc(F)cc3)c(=O)c2c(C)c2ccccc12 Show InChI InChI=1S/C24H25FN4O2/c1-4-5-14-27(3)21(30)15-28-20-9-7-6-8-19(20)16(2)22-23(28)26-29(24(22)31)18-12-10-17(25)11-13-18/h6-13H,4-5,14-15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat brain homogenates after 90 mins by liquid scintillation spectrometry |
J Med Chem 54: 7165-75 (2011)
Article DOI: 10.1021/jm200770f
BindingDB Entry DOI: 10.7270/Q2MG7PWM |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50355781
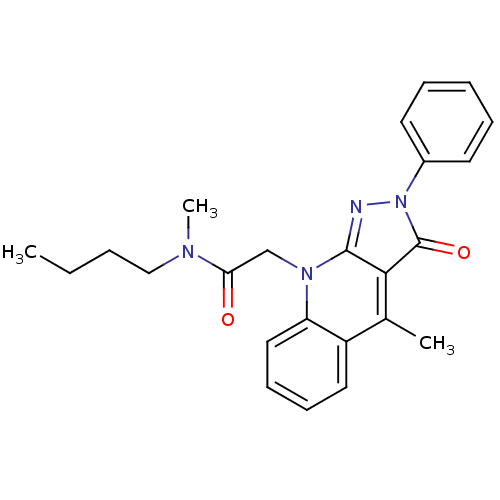
(CHEMBL1911620)Show SMILES CCCCN(C)C(=O)Cn1c2nn(-c3ccccc3)c(=O)c2c(C)c2ccccc12 Show InChI InChI=1S/C24H26N4O2/c1-4-5-15-26(3)21(29)16-27-20-14-10-9-13-19(20)17(2)22-23(27)25-28(24(22)30)18-11-7-6-8-12-18/h6-14H,4-5,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO in Sprague-Dawley rat brain homogenates after 90 mins by liquid scintillation spectrometry |
J Med Chem 54: 7165-75 (2011)
Article DOI: 10.1021/jm200770f
BindingDB Entry DOI: 10.7270/Q2MG7PWM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































