

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27614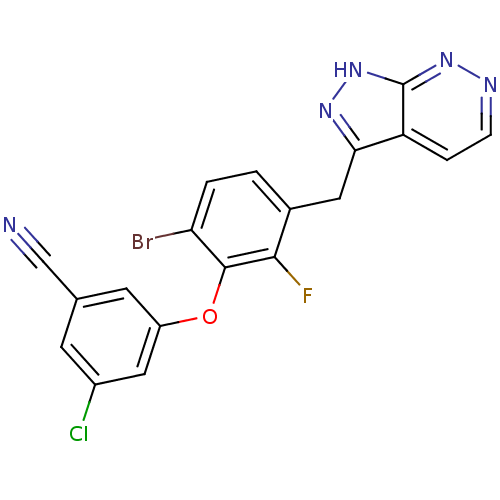 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | n/a | n/a | 3 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | >25 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27614 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318447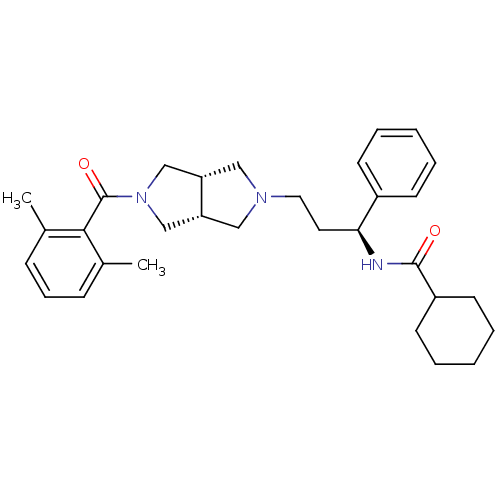 (CHEMBL1096764 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318446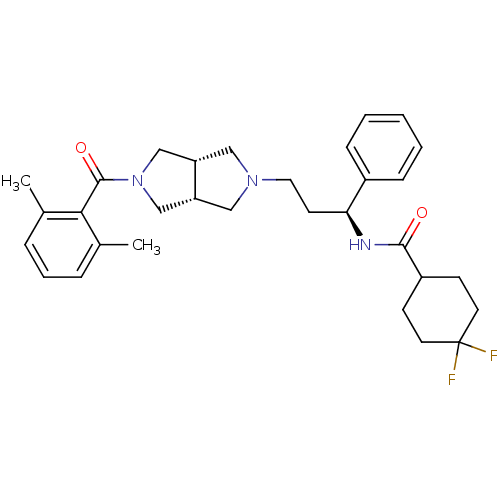 (CHEMBL1096765 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 2 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50115528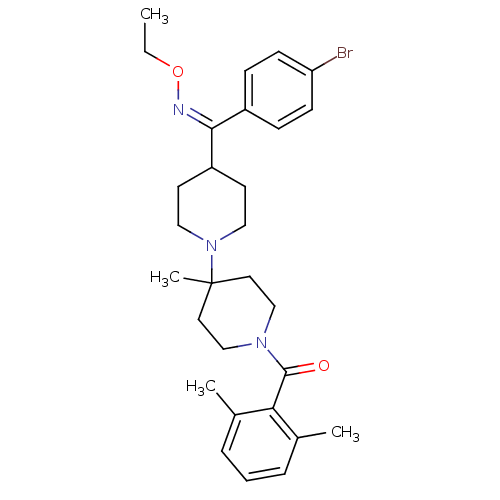 ((Z)-(4-((4-bromophenyl)(ethoxyimino)methyl)-4'-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318439 (CHEMBL1097815 | N-((S)-3-((3aR,6aS)-5-(4-fluoro-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318451 (CHEMBL1096445 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499493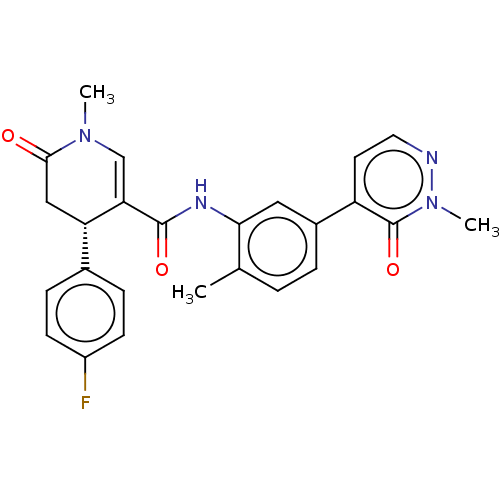 (CHEMBL3739648) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310731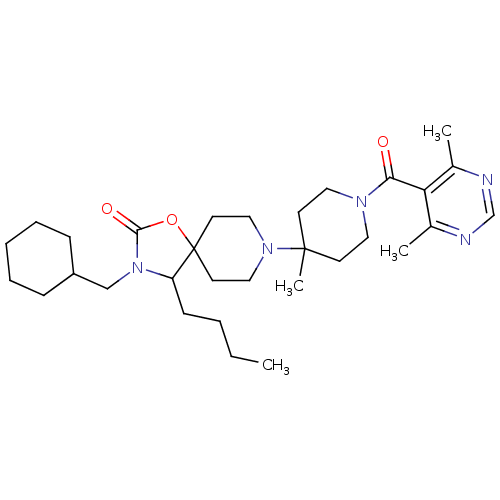 (4-butyl-3-(cyclohexylmethyl)-8-(1-(4,6-dimethylpyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318436 (CHEMBL1097169 | N-((S)-3-((3aR,6aS)-5-(2,4-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318448 (CHEMBL1096768 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27612 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318445 (CHEMBL1096766 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499492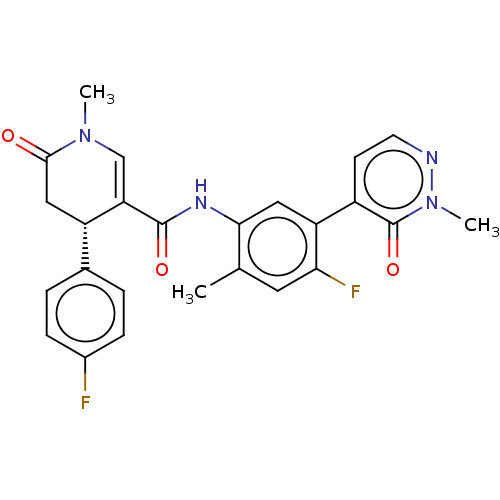 (CHEMBL3740684) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | 18 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318427 (2-(3,3-difluorocyclobutyl)-N-((S)-3-((3aR,6aS)-5-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318441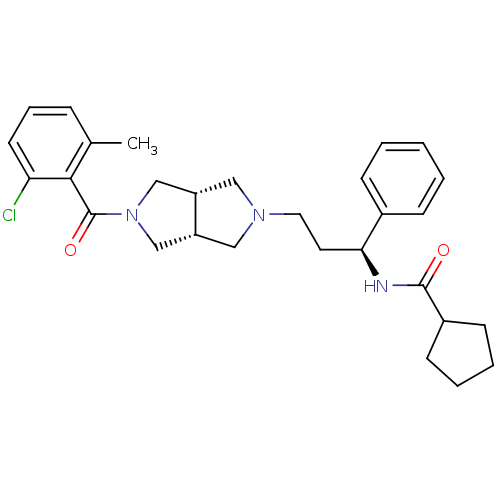 (CHEMBL1096102 | N-((S)-3-((3aR,6aS)-5-(2-chloro-6-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499491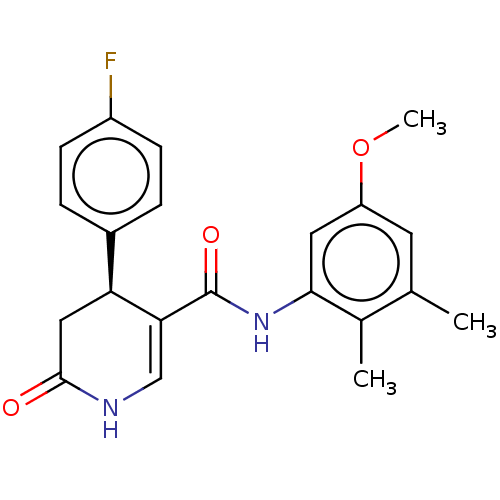 (CHEMBL3741668) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499501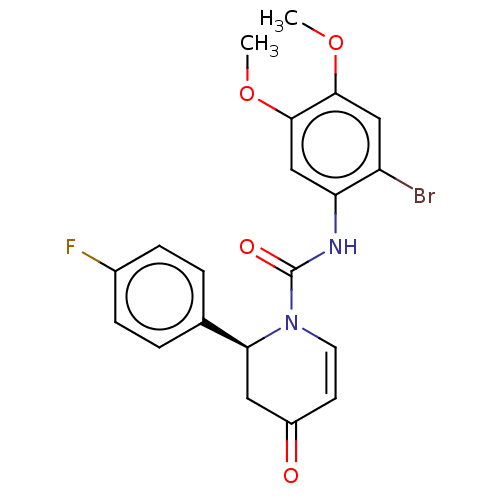 (CHEMBL3741934) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499502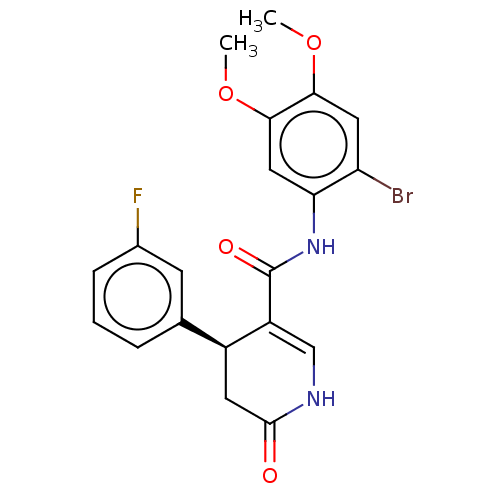 (CHEMBL3740237) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499504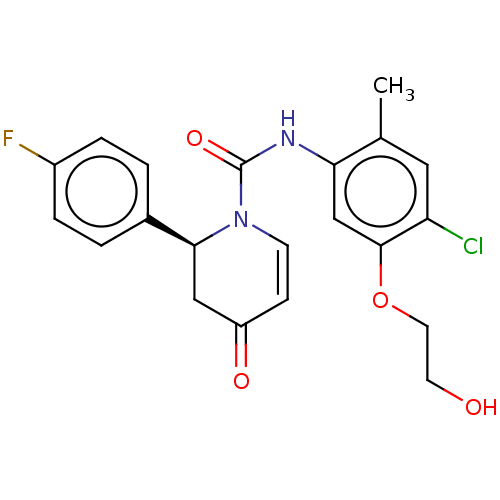 (CHEMBL3741412) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310730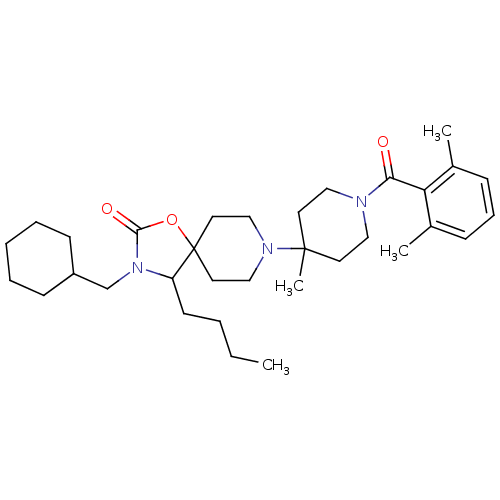 (4-butyl-3-(cyclohexylmethyl)-8-(1-(2,6-dimethylben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310747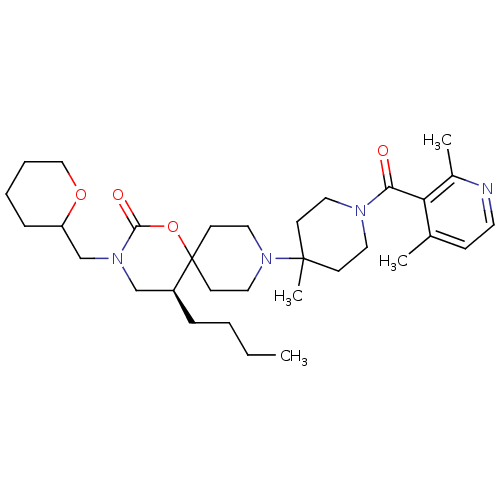 ((5S)-5-butyl-9-(1-(2,4-dimethylnicotinoyl)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318449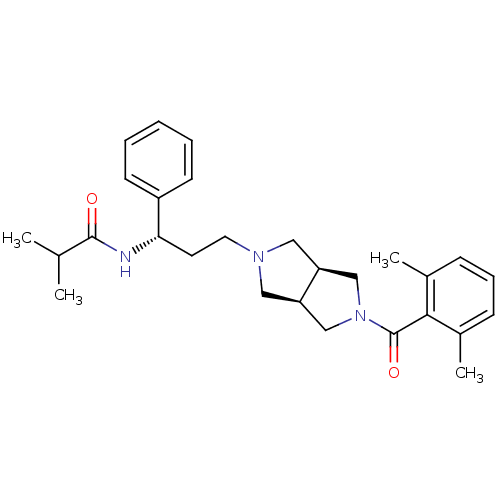 (CHEMBL1096447 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 97 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310744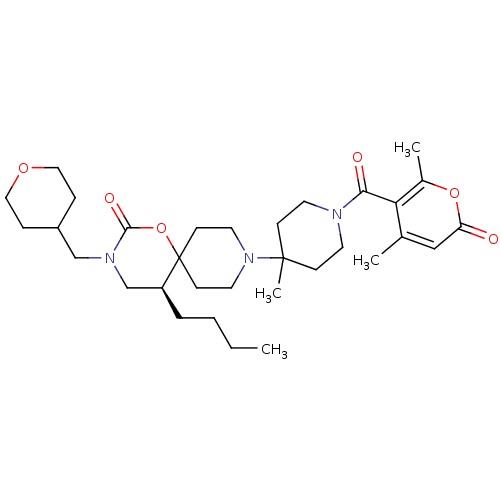 ((S)-5-butyl-9-(1-(4,6-dimethyl-2-oxo-2H-pyran-5-ca...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318428 (2-(4,4-difluorocyclohexyl)-N-((S)-3-((3aR,6aS)-5-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318432 (CHEMBL1095827 | N-((S)-3-((3aR,6aS)-5-(6-cyano-2,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499508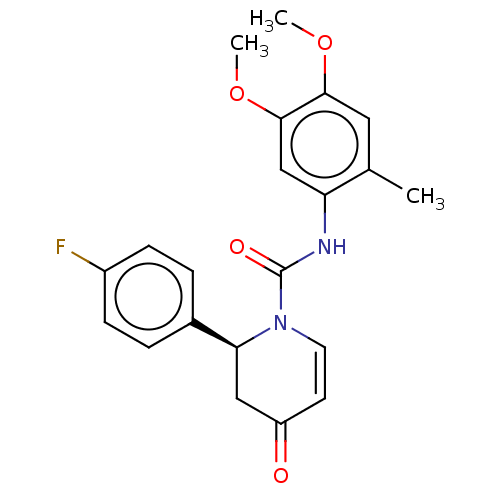 (CHEMBL3740363) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human P2X7R expressed in BzATP-stimulated human 1321N1 cells incubated for 20 mins followed by BzATP stimulation measured ever... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318429 (2-cyclopentyl-N-((S)-3-((3aR,6aS)-5-(4,6-dimethylp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499492 (CHEMBL3740684) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | >100 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318434 (CHEMBL1095517 | N-((S)-3-((3aR,6aS)-5-(4,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318440 (CHEMBL1097515 | N-((S)-3-((3aR,6aS)-5-(2,6-dichlor...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482300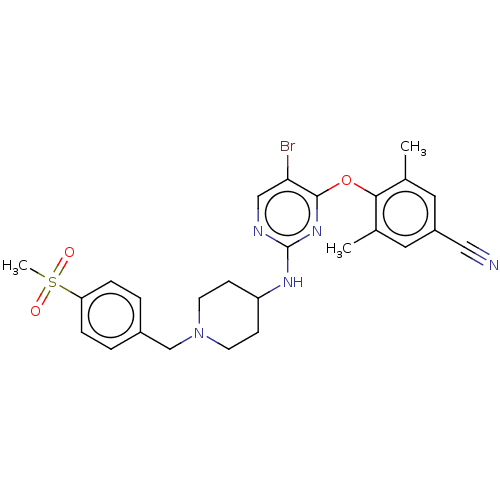 (CHEMBL1170386) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27609 (3-{[4-bromo-3-(3-chloro-5-cyanophenoxy)-2-fluoroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | 53 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318443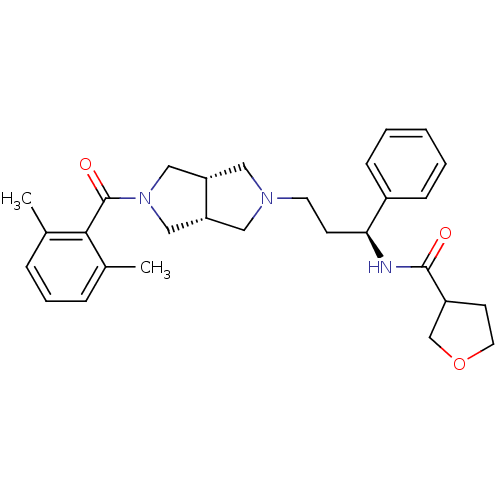 (CHEMBL1098151 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318450 (CHEMBL1096446 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482304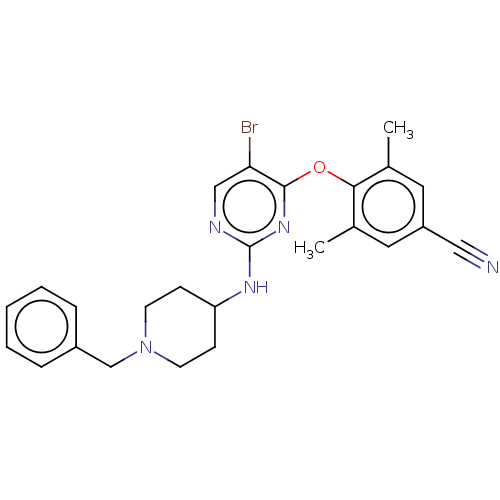 (CHEMBL1170190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499488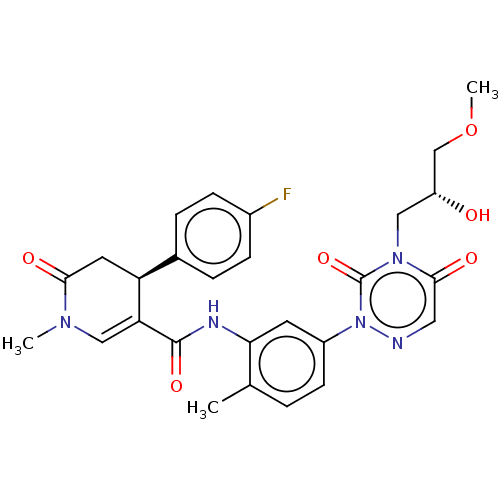 (CHEMBL3739741) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50499495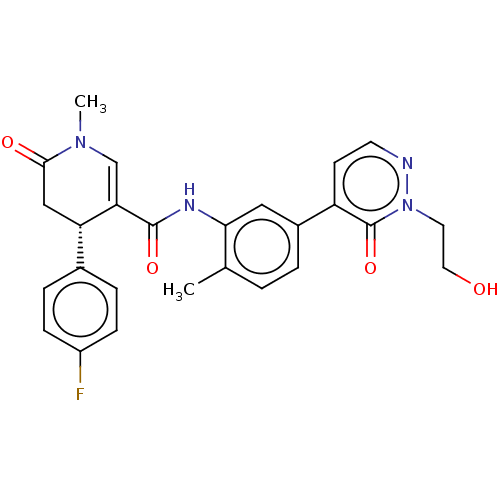 (CHEMBL3740645) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in human whole blood assessed as inhibition of LPS-induced IL-1beta production incubated for 30 mins followed by ATP add... | J Med Chem 58: 8413-26 (2015) Article DOI: 10.1021/acs.jmedchem.5b00365 BindingDB Entry DOI: 10.7270/Q2QZ2DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310726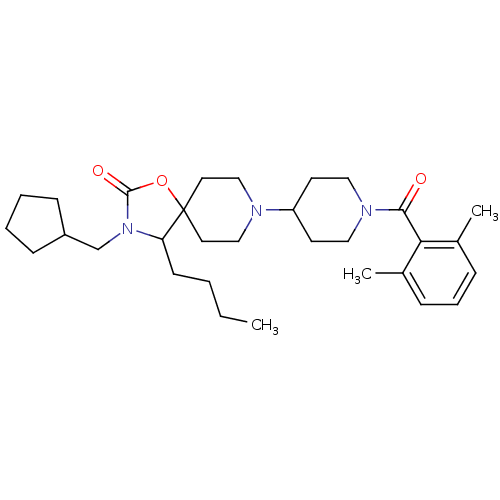 (4-butyl-3-(cyclopentylmethyl)-8-(1-(2,6-dimethylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318444 (CHEMBL1098150 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50482308 (CHEMBL1169643) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50318437 (CHEMBL1096486 | N-((S)-3-((3aR,6aS)-5-(3,5-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay | Bioorg Med Chem Lett 20: 3116-9 (2010) Article DOI: 10.1016/j.bmcl.2010.03.095 BindingDB Entry DOI: 10.7270/Q27S7PQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50482304 (CHEMBL1170190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA heteropolymeric assay | Bioorg Med Chem Lett 20: 4215-8 (2010) Article DOI: 10.1016/j.bmcl.2010.05.040 BindingDB Entry DOI: 10.7270/Q21Z478K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 305 total ) | Next | Last >> |