 Found 277 hits with Last Name = 'grenier' and Initial = 'l'
Found 277 hits with Last Name = 'grenier' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50069984

((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)B(O)O Show InChI InChI=1S/C25H42BN3O6/c1-16(2)12-20(24(31)29-22(26(33)34)14-18(5)6)27-23(30)21(13-17(3)4)28-25(32)35-15-19-10-8-7-9-11-19/h7-11,16-18,20-22,33-34H,12-15H2,1-6H3,(H,27,30)(H,28,32)(H,29,31)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cathepsin B |
Bioorg Med Chem Lett 8: 333-8 (1999)
BindingDB Entry DOI: 10.7270/Q2RV0MVQ |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Chymotrypsinogen |
Bioorg Med Chem Lett 8: 333-8 (1999)
BindingDB Entry DOI: 10.7270/Q2RV0MVQ |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin G |
Bioorg Med Chem Lett 8: 333-8 (1999)
BindingDB Entry DOI: 10.7270/Q2RV0MVQ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
Bioorg Med Chem Lett 8: 333-8 (1999)
BindingDB Entry DOI: 10.7270/Q2RV0MVQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 8: 333-8 (1999)
BindingDB Entry DOI: 10.7270/Q2RV0MVQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228710
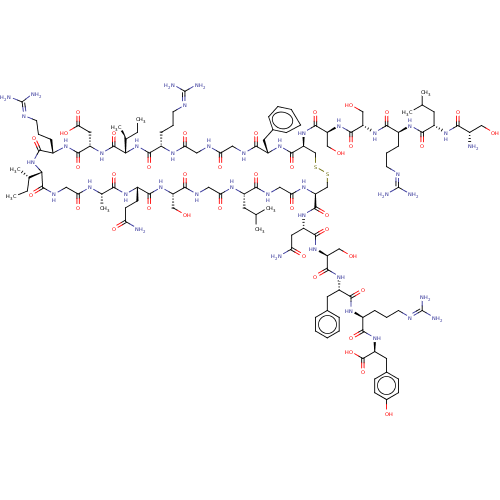
(CHEMBL3349899)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C122H193N41O38S2/c1-10-61(7)95-116(198)142-49-90(173)143-63(9)97(179)147-73(34-35-87(124)170)104(186)157-81(53-165)101(183)141-50-92(175)145-74(40-59(3)4)99(181)140-51-93(176)146-85(114(196)154-78(45-88(125)171)109(191)159-82(54-166)111(193)153-77(43-65-24-16-13-17-25-65)108(190)149-70(27-19-37-135-120(128)129)102(184)156-80(118(200)201)44-66-30-32-67(169)33-31-66)57-202-203-58-86(161-113(195)84(56-168)160-112(194)83(55-167)158-103(185)71(28-20-38-136-121(130)131)148-107(189)75(41-60(5)6)151-98(180)68(123)52-164)115(197)152-76(42-64-22-14-12-15-23-64)100(182)139-47-89(172)138-48-91(174)144-69(26-18-36-134-119(126)127)105(187)163-96(62(8)11-2)117(199)155-79(46-94(177)178)110(192)150-72(106(188)162-95)29-21-39-137-122(132)133/h12-17,22-25,30-33,59-63,68-86,95-96,164-169H,10-11,18-21,26-29,34-58,123H2,1-9H3,(H2,124,170)(H2,125,171)(H,138,172)(H,139,182)(H,140,181)(H,141,183)(H,142,198)(H,143,173)(H,144,174)(H,145,175)(H,146,176)(H,147,179)(H,148,189)(H,149,190)(H,150,192)(H,151,180)(H,152,197)(H,153,193)(H,154,196)(H,155,199)(H,156,184)(H,157,186)(H,158,185)(H,159,191)(H,160,194)(H,161,195)(H,162,188)(H,163,187)(H,177,178)(H,200,201)(H4,126,127,134)(H4,128,129,135)(H4,130,131,136)(H4,132,133,137)/t61-,62-,63-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,95-,96-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228710

(CHEMBL3349899)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C122H193N41O38S2/c1-10-61(7)95-116(198)142-49-90(173)143-63(9)97(179)147-73(34-35-87(124)170)104(186)157-81(53-165)101(183)141-50-92(175)145-74(40-59(3)4)99(181)140-51-93(176)146-85(114(196)154-78(45-88(125)171)109(191)159-82(54-166)111(193)153-77(43-65-24-16-13-17-25-65)108(190)149-70(27-19-37-135-120(128)129)102(184)156-80(118(200)201)44-66-30-32-67(169)33-31-66)57-202-203-58-86(161-113(195)84(56-168)160-112(194)83(55-167)158-103(185)71(28-20-38-136-121(130)131)148-107(189)75(41-60(5)6)151-98(180)68(123)52-164)115(197)152-76(42-64-22-14-12-15-23-64)100(182)139-47-89(172)138-48-91(174)144-69(26-18-36-134-119(126)127)105(187)163-96(62(8)11-2)117(199)155-79(46-94(177)178)110(192)150-72(106(188)162-95)29-21-39-137-122(132)133/h12-17,22-25,30-33,59-63,68-86,95-96,164-169H,10-11,18-21,26-29,34-58,123H2,1-9H3,(H2,124,170)(H2,125,171)(H,138,172)(H,139,182)(H,140,181)(H,141,183)(H,142,198)(H,143,173)(H,144,174)(H,145,175)(H,146,176)(H,147,179)(H,148,189)(H,149,190)(H,150,192)(H,151,180)(H,152,197)(H,153,193)(H,154,196)(H,155,199)(H,156,184)(H,157,186)(H,158,185)(H,159,191)(H,160,194)(H,161,195)(H,162,188)(H,163,187)(H,177,178)(H,200,201)(H4,126,127,134)(H4,128,129,135)(H4,130,131,136)(H4,132,133,137)/t61-,62-,63-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,95-,96-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013340
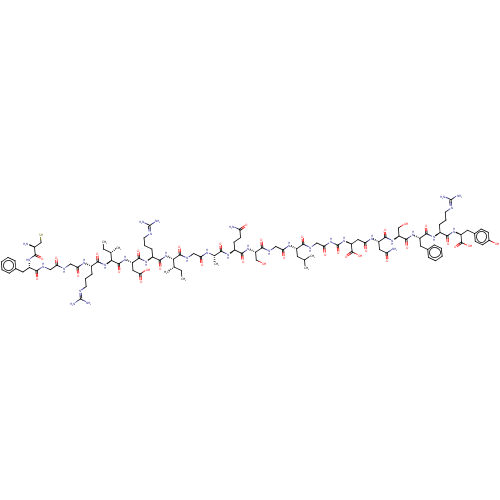
(CHEMBL3349626)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H158N34O33S/c1-8-51(5)81(136-91(159)61(25-18-34-115-102(111)112)127-94(162)67(41-80(149)150)130-97(165)82(52(6)9-2)137-90(158)59(23-16-32-113-100(107)108)122-77(146)43-116-75(144)42-117-86(154)64(128-84(152)58(104)49-171)36-54-19-12-10-13-20-54)96(164)120-44-76(145)121-53(7)83(151)125-62(30-31-72(105)141)89(157)132-70(47-138)87(155)119-45-78(147)124-63(35-50(3)4)85(153)118-46-79(148)135-103(170)134-69(99(168)169)40-74(143)123-66(39-73(106)142)93(161)133-71(48-139)95(163)129-65(37-55-21-14-11-15-22-55)92(160)126-60(24-17-33-114-101(109)110)88(156)131-68(98(166)167)38-56-26-28-57(140)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,138-140,171H,8-9,16-18,23-25,30-49,104H2,1-7H3,(H2,105,141)(H2,106,142)(H,116,144)(H,117,154)(H,118,153)(H,119,155)(H,120,164)(H,121,145)(H,122,146)(H,123,143)(H,124,147)(H,125,151)(H,126,160)(H,127,162)(H,128,152)(H,129,163)(H,130,165)(H,131,156)(H,132,157)(H,133,161)(H,136,159)(H,137,158)(H,149,150)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,148,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013343
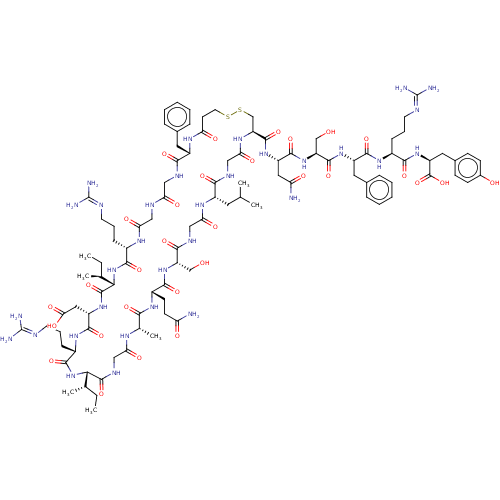
(CHEMBL3349621 | deamino [Mpr105,Cys121] r-ANF (99-...)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H154N32O30S2/c1-8-52(5)81-96(160)117-45-76(141)118-54(7)83(147)123-62(30-31-72(102)137)88(152)130-69(48-134)86(150)116-46-78(143)121-63(37-51(3)4)84(148)115-47-79(144)122-71(95(159)127-66(41-73(103)138)92(156)131-70(49-135)94(158)126-65(39-56-21-14-11-15-22-56)91(155)124-60(24-17-34-111-100(106)107)87(151)129-68(98(162)163)40-57-26-28-58(136)29-27-57)50-165-164-36-32-74(139)120-64(38-55-19-12-10-13-20-55)85(149)114-43-75(140)113-44-77(142)119-59(23-16-33-110-99(104)105)89(153)133-82(53(6)9-2)97(161)128-67(42-80(145)146)93(157)125-61(90(154)132-81)25-18-35-112-101(108)109/h10-15,19-22,26-29,51-54,59-71,81-82,134-136H,8-9,16-18,23-25,30-50H2,1-7H3,(H2,102,137)(H2,103,138)(H,113,140)(H,114,149)(H,115,148)(H,116,150)(H,117,160)(H,118,141)(H,119,142)(H,120,139)(H,121,143)(H,122,144)(H,123,147)(H,124,155)(H,125,157)(H,126,158)(H,127,159)(H,128,161)(H,129,151)(H,130,152)(H,131,156)(H,132,154)(H,133,153)(H,145,146)(H,162,163)(H4,104,105,110)(H4,106,107,111)(H4,108,109,112)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013341
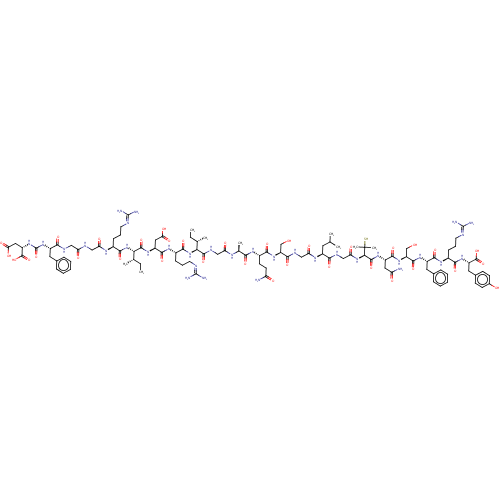
(CHEMBL3349629)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)C([#6])([#6])[#16] |r| Show InChI InChI=1S/C105H161N33O34S/c1-10-52(5)81(137-91(160)61(27-20-36-116-103(112)113)127-94(163)67(42-79(149)150)129-97(166)82(53(6)11-2)138-90(159)59(25-18-34-114-101(108)109)123-76(146)45-117-74(144)44-118-86(155)64(38-55-21-14-12-15-22-55)134-104(172)135-69(100(170)171)43-80(151)152)96(165)121-46-75(145)122-54(7)84(153)125-62(32-33-72(106)142)89(158)132-70(49-139)87(156)120-47-77(147)124-63(37-51(3)4)85(154)119-48-78(148)136-83(105(8,9)173)98(167)130-66(41-73(107)143)93(162)133-71(50-140)95(164)128-65(39-56-23-16-13-17-24-56)92(161)126-60(26-19-35-115-102(110)111)88(157)131-68(99(168)169)40-57-28-30-58(141)31-29-57/h12-17,21-24,28-31,51-54,59-71,81-83,139-141,173H,10-11,18-20,25-27,32-50H2,1-9H3,(H2,106,142)(H2,107,143)(H,117,144)(H,118,155)(H,119,154)(H,120,156)(H,121,165)(H,122,145)(H,123,146)(H,124,147)(H,125,153)(H,126,161)(H,127,163)(H,128,164)(H,129,166)(H,130,167)(H,131,157)(H,132,158)(H,133,162)(H,136,148)(H,137,160)(H,138,159)(H,149,150)(H,151,152)(H,168,169)(H,170,171)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,135,172)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-,83+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013338
(CHEMBL413659 | r-ANF (103-126)(Atrial Natriuretic ...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,75-,84-,85-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013342

(CHEMBL3349625)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#8]-[#6])-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C104H159N33O34S/c1-9-52(5)82(136-91(158)61(26-19-35-115-103(111)112)127-94(161)67(41-80(148)149)130-98(165)83(53(6)10-2)137-90(157)59(24-17-33-113-101(107)108)122-77(145)44-116-75(143)43-117-86(153)64(37-55-20-13-11-14-21-55)134-104(170)135-69(100(168)169)42-81(150)171-8)97(164)120-45-76(144)121-54(7)84(151)125-62(31-32-73(105)141)89(156)132-70(48-138)87(154)119-46-78(146)123-63(36-51(3)4)85(152)118-47-79(147)124-72(50-172)96(163)129-66(40-74(106)142)93(160)133-71(49-139)95(162)128-65(38-56-22-15-12-16-23-56)92(159)126-60(25-18-34-114-102(109)110)88(155)131-68(99(166)167)39-57-27-29-58(140)30-28-57/h11-16,20-23,27-30,51-54,59-72,82-83,138-140,172H,9-10,17-19,24-26,31-50H2,1-8H3,(H2,105,141)(H2,106,142)(H,116,143)(H,117,153)(H,118,152)(H,119,154)(H,120,164)(H,121,144)(H,122,145)(H,123,146)(H,124,147)(H,125,151)(H,126,159)(H,127,161)(H,128,162)(H,129,163)(H,130,165)(H,131,155)(H,132,156)(H,133,160)(H,136,158)(H,137,157)(H,148,149)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,170)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,72-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228711

(CHEMBL3349900)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H155N33O30S2/c1-8-51(5)80-96(161)118-43-75(141)119-53(7)82(147)123-62(30-31-72(103)138)88(153)131-69(46-135)86(151)117-44-77(143)121-63(35-50(3)4)84(149)116-45-78(144)122-71(95(160)128-66(39-73(104)139)92(157)132-70(47-136)94(159)127-65(37-55-21-14-11-15-22-55)91(156)124-60(24-17-33-112-100(107)108)87(152)130-68(98(163)164)38-56-26-28-57(137)29-27-56)49-166-165-48-58(102)83(148)126-64(36-54-19-12-10-13-20-54)85(150)115-41-74(140)114-42-76(142)120-59(23-16-32-111-99(105)106)89(154)134-81(52(6)9-2)97(162)129-67(40-79(145)146)93(158)125-61(90(155)133-80)25-18-34-113-101(109)110/h10-15,19-22,26-29,50-53,58-71,80-81,135-137H,8-9,16-18,23-25,30-49,102H2,1-7H3,(H2,103,138)(H2,104,139)(H,114,140)(H,115,150)(H,116,149)(H,117,151)(H,118,161)(H,119,141)(H,120,142)(H,121,143)(H,122,144)(H,123,147)(H,124,156)(H,125,158)(H,126,148)(H,127,159)(H,128,160)(H,129,162)(H,130,152)(H,131,153)(H,132,157)(H,133,155)(H,134,154)(H,145,146)(H,163,164)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,80-,81-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013344
(CHEMBL3349628)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H157N33O34S/c1-8-51(5)81(135-90(158)60(25-18-34-114-102(110)111)126-93(161)66(40-79(147)148)129-97(165)82(52(6)9-2)136-89(157)58(23-16-32-112-100(106)107)121-76(144)43-115-74(142)42-116-85(153)63(36-54-19-12-10-13-20-54)133-103(170)134-68(99(168)169)41-80(149)150)96(164)119-44-75(143)120-53(7)83(151)124-61(30-31-72(104)140)88(156)131-69(47-137)86(154)118-45-77(145)122-62(35-50(3)4)84(152)117-46-78(146)123-71(49-171)95(163)128-65(39-73(105)141)92(160)132-70(48-138)94(162)127-64(37-55-21-14-11-15-22-55)91(159)125-59(24-17-33-113-101(108)109)87(155)130-67(98(166)167)38-56-26-28-57(139)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,137-139,171H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,104,140)(H2,105,141)(H,115,142)(H,116,153)(H,117,152)(H,118,154)(H,119,164)(H,120,143)(H,121,144)(H,122,145)(H,123,146)(H,124,151)(H,125,159)(H,126,161)(H,127,162)(H,128,163)(H,129,165)(H,130,155)(H,131,156)(H,132,160)(H,135,158)(H,136,157)(H,147,148)(H,149,150)(H,166,167)(H,168,169)(H4,106,107,112)(H4,108,109,113)(H4,110,111,114)(H2,133,134,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50228712
(CHEMBL3350078)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H154N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,40,42,50-53,58-67,70-71,82-83,140-142H,8-9,16-18,23-25,30-39,41,43-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/b68-40+,69-42+/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,70-,71-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on mouse fibroblasts (NIH 3T3) cells (Atrionatriu... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033458

((S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Benzyl-3-phen...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)C(Cc1ccccc1)Cc1ccccc1)C(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C44H62N6O10/c1-26(2)21-34(44(59)60)47-41(56)33(25-36(52)53)45-40(55)32(24-35(51)50-19-13-14-20-50)46-42(57)37(27(3)4)49-43(58)38(28(5)6)48-39(54)31(22-29-15-9-7-10-16-29)23-30-17-11-8-12-18-30/h7-12,15-18,26-28,31-34,37-38H,13-14,19-25H2,1-6H3,(H,45,55)(H,46,57)(H,47,56)(H,48,54)(H,49,58)(H,52,53)(H,59,60)/t32-,33-,34-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Mus musculus) | BDBM50013339

(CHEMBL3349627)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H158N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,50-53,58-71,82-83,140-142H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,82-,83-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites onmouse fibroblasts (NIH 3T3) cells (Atrionatriur... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033457

(1-[(S)-((S)-1-Carboxy-3-methyl-butylcarbamoyl)-((S...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCc1ccccc1)C(C)C)C(C)C)C1(CCCC1)C(O)=O)C(O)=O Show InChI InChI=1S/C41H62N6O10/c1-24(2)22-29(39(54)55)43-38(53)34(41(40(56)57)18-10-11-19-41)46-35(50)28(23-31(49)47-20-12-13-21-47)42-36(51)33(26(5)6)45-37(52)32(25(3)4)44-30(48)17-16-27-14-8-7-9-15-27/h7-9,14-15,24-26,28-29,32-34H,10-13,16-23H2,1-6H3,(H,42,51)(H,43,53)(H,44,48)(H,45,52)(H,46,50)(H,54,55)(H,56,57)/t28-,29-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013343

(CHEMBL3349621 | deamino [Mpr105,Cys121] r-ANF (99-...)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H154N32O30S2/c1-8-52(5)81-96(160)117-45-76(141)118-54(7)83(147)123-62(30-31-72(102)137)88(152)130-69(48-134)86(150)116-46-78(143)121-63(37-51(3)4)84(148)115-47-79(144)122-71(95(159)127-66(41-73(103)138)92(156)131-70(49-135)94(158)126-65(39-56-21-14-11-15-22-56)91(155)124-60(24-17-34-111-100(106)107)87(151)129-68(98(162)163)40-57-26-28-58(136)29-27-57)50-165-164-36-32-74(139)120-64(38-55-19-12-10-13-20-55)85(149)114-43-75(140)113-44-77(142)119-59(23-16-33-110-99(104)105)89(153)133-82(53(6)9-2)97(161)128-67(42-80(145)146)93(157)125-61(90(154)132-81)25-18-35-112-101(108)109/h10-15,19-22,26-29,51-54,59-71,81-82,134-136H,8-9,16-18,23-25,30-50H2,1-7H3,(H2,102,137)(H2,103,138)(H,113,140)(H,114,149)(H,115,148)(H,116,150)(H,117,160)(H,118,141)(H,119,142)(H,120,139)(H,121,143)(H,122,144)(H,123,147)(H,124,155)(H,125,157)(H,126,158)(H,127,159)(H,128,161)(H,129,151)(H,130,152)(H,131,156)(H,132,154)(H,133,153)(H,145,146)(H,162,163)(H4,104,105,110)(H4,106,107,111)(H4,108,109,112)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50192880
(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in C5a-stimulated mouse RAW264.7 cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013338

(CHEMBL413659 | r-ANF (103-126)(Atrial Natriuretic ...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CO)[C@@H](C)CC Show InChI InChI=1S/C107H165N35O34S2/c1-8-53(5)84-102(173)124-43-79(151)125-55(7)86(157)129-64(30-31-76(109)148)92(163)138-71(47-144)90(161)123-44-81(153)127-65(35-52(3)4)88(159)122-45-82(154)128-74(100(171)134-68(39-77(110)149)96(167)139-73(49-146)98(169)133-67(37-57-21-14-11-15-22-57)95(166)130-62(24-17-33-118-106(113)114)91(162)136-70(104(175)176)38-58-26-28-59(147)29-27-58)50-177-178-51-75(140-99(170)72(48-145)137-87(158)60(108)46-143)101(172)132-66(36-56-19-12-10-13-20-56)89(160)121-41-78(150)120-42-80(152)126-61(23-16-32-117-105(111)112)93(164)142-85(54(6)9-2)103(174)135-69(40-83(155)156)97(168)131-63(94(165)141-84)25-18-34-119-107(115)116/h10-15,19-22,26-29,52-55,60-75,84-85,143-147H,8-9,16-18,23-25,30-51,108H2,1-7H3,(H2,109,148)(H2,110,149)(H,120,150)(H,121,160)(H,122,159)(H,123,161)(H,124,173)(H,125,151)(H,126,152)(H,127,153)(H,128,154)(H,129,157)(H,130,166)(H,131,168)(H,132,172)(H,133,169)(H,134,171)(H,135,174)(H,136,162)(H,137,158)(H,138,163)(H,139,167)(H,140,170)(H,141,165)(H,142,164)(H,155,156)(H,175,176)(H4,111,112,117)(H4,113,114,118)(H4,115,116,119)/t53-,54-,55-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71+,72-,73-,74-,75-,84-,85-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228711

(CHEMBL3349900)Show SMILES [H][C@]1([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)[C@@]([H])([#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6]1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)[#6@@H](-[#6])-[#6]-[#6])[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C101H155N33O30S2/c1-8-51(5)80-96(161)118-43-75(141)119-53(7)82(147)123-62(30-31-72(103)138)88(153)131-69(46-135)86(151)117-44-77(143)121-63(35-50(3)4)84(149)116-45-78(144)122-71(95(160)128-66(39-73(104)139)92(157)132-70(47-136)94(159)127-65(37-55-21-14-11-15-22-55)91(156)124-60(24-17-33-112-100(107)108)87(152)130-68(98(163)164)38-56-26-28-57(137)29-27-56)49-166-165-48-58(102)83(148)126-64(36-54-19-12-10-13-20-54)85(150)115-41-74(140)114-42-76(142)120-59(23-16-32-111-99(105)106)89(154)134-81(52(6)9-2)97(162)129-67(40-79(145)146)93(158)125-61(90(155)133-80)25-18-34-113-101(109)110/h10-15,19-22,26-29,50-53,58-71,80-81,135-137H,8-9,16-18,23-25,30-49,102H2,1-7H3,(H2,103,138)(H2,104,139)(H,114,140)(H,115,150)(H,116,149)(H,117,151)(H,118,161)(H,119,141)(H,120,142)(H,121,143)(H,122,144)(H,123,147)(H,124,156)(H,125,158)(H,126,148)(H,127,159)(H,128,160)(H,129,162)(H,130,152)(H,131,153)(H,132,157)(H,133,155)(H,134,154)(H,145,146)(H,163,164)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,80-,81-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033463
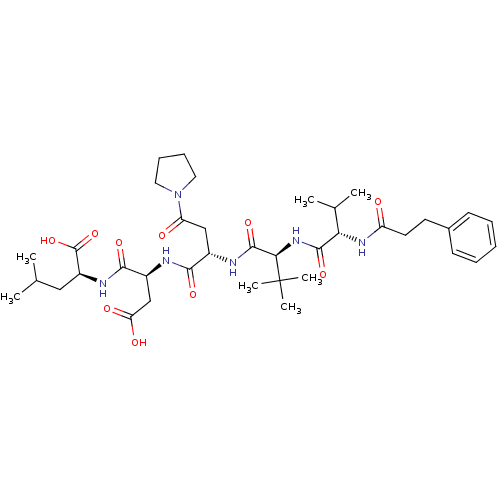
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3,3-dimethyl-2-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCc1ccccc1)C(C)C)C(C)(C)C)C(O)=O Show InChI InChI=1S/C38H58N6O10/c1-22(2)19-27(37(53)54)41-34(50)26(21-30(47)48)39-33(49)25(20-29(46)44-17-11-12-18-44)40-36(52)32(38(5,6)7)43-35(51)31(23(3)4)42-28(45)16-15-24-13-9-8-10-14-24/h8-10,13-14,22-23,25-27,31-32H,11-12,15-21H2,1-7H3,(H,39,49)(H,40,52)(H,41,50)(H,42,45)(H,43,51)(H,47,48)(H,53,54)/t25-,26-,27-,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033465

((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)(C)C)C(O)=O Show InChI InChI=1S/C38H58N6O10/c1-22(2)31(42-28(45)16-15-24-13-9-8-10-14-24)36(52)43-32(23(3)4)35(51)40-25(19-29(46)44-17-11-12-18-44)33(49)39-26(20-30(47)48)34(50)41-27(37(53)54)21-38(5,6)7/h8-10,13-14,22-23,25-27,31-32H,11-12,15-21H2,1-7H3,(H,39,49)(H,40,51)(H,41,50)(H,42,45)(H,43,52)(H,47,48)(H,53,54)/t25-,26-,27-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013344

(CHEMBL3349628)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H157N33O34S/c1-8-51(5)81(135-90(158)60(25-18-34-114-102(110)111)126-93(161)66(40-79(147)148)129-97(165)82(52(6)9-2)136-89(157)58(23-16-32-112-100(106)107)121-76(144)43-115-74(142)42-116-85(153)63(36-54-19-12-10-13-20-54)133-103(170)134-68(99(168)169)41-80(149)150)96(164)119-44-75(143)120-53(7)83(151)124-61(30-31-72(104)140)88(156)131-69(47-137)86(154)118-45-77(145)122-62(35-50(3)4)84(152)117-46-78(146)123-71(49-171)95(163)128-65(39-73(105)141)92(160)132-70(48-138)94(162)127-64(37-55-21-14-11-15-22-55)91(159)125-59(24-17-33-113-101(108)109)87(155)130-67(98(166)167)38-56-26-28-57(139)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,137-139,171H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,104,140)(H2,105,141)(H,115,142)(H,116,153)(H,117,152)(H,118,154)(H,119,164)(H,120,143)(H,121,144)(H,122,145)(H,123,146)(H,124,151)(H,125,159)(H,126,161)(H,127,162)(H,128,163)(H,129,165)(H,130,155)(H,131,156)(H,132,160)(H,135,158)(H,136,157)(H,147,148)(H,149,150)(H,166,167)(H,168,169)(H4,106,107,112)(H4,108,109,113)(H4,110,111,114)(H2,133,134,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69+,70-,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033453
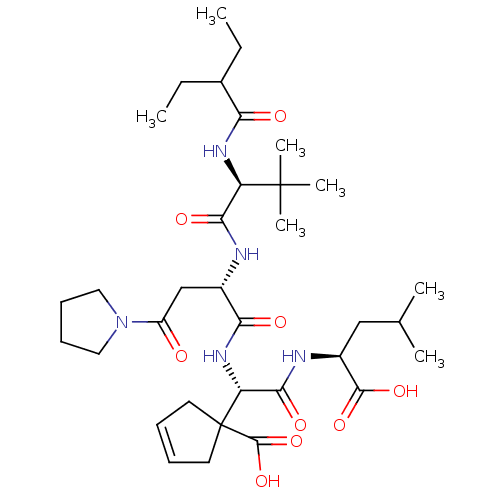
(1-((S)-((S)-1-Carboxy-3-methyl-butylcarbamoyl)-{(S...)Show SMILES CCC(CC)C(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C1(CC=CC1)C(O)=O)C(C)(C)C |c:39| Show InChI InChI=1S/C34H55N5O9/c1-8-21(9-2)27(41)37-25(33(5,6)7)29(43)35-22(19-24(40)39-16-12-13-17-39)28(42)38-26(34(32(47)48)14-10-11-15-34)30(44)36-23(31(45)46)18-20(3)4/h10-11,20-23,25-26H,8-9,12-19H2,1-7H3,(H,35,43)(H,36,44)(H,37,41)(H,38,42)(H,45,46)(H,47,48)/t22-,23-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033470

((S)-2-{(S)-3-Carboxy-2-[(S)-2-((S)-3-methyl-2-{(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)CCc1ccccc1)C(C)C)C(O)=O Show InChI InChI=1S/C38H58N6O10/c1-22(2)19-28(38(53)54)41-35(50)27(21-31(47)48)39-34(49)26(20-30(46)44-17-11-12-18-44)40-36(51)32(23(3)4)42-37(52)33(24(5)6)43(7)29(45)16-15-25-13-9-8-10-14-25/h8-10,13-14,22-24,26-28,32-33H,11-12,15-21H2,1-7H3,(H,39,49)(H,40,51)(H,41,50)(H,42,52)(H,47,48)(H,53,54)/t26-,27-,28-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013340

(CHEMBL3349626)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16])-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C103H158N34O33S/c1-8-51(5)81(136-91(159)61(25-18-34-115-102(111)112)127-94(162)67(41-80(149)150)130-97(165)82(52(6)9-2)137-90(158)59(23-16-32-113-100(107)108)122-77(146)43-116-75(144)42-117-86(154)64(128-84(152)58(104)49-171)36-54-19-12-10-13-20-54)96(164)120-44-76(145)121-53(7)83(151)125-62(30-31-72(105)141)89(157)132-70(47-138)87(155)119-45-78(147)124-63(35-50(3)4)85(153)118-46-79(148)135-103(170)134-69(99(168)169)40-74(143)123-66(39-73(106)142)93(161)133-71(48-139)95(163)129-65(37-55-21-14-11-15-22-55)92(160)126-60(24-17-33-114-101(109)110)88(156)131-68(98(166)167)38-56-26-28-57(140)29-27-56/h10-15,19-22,26-29,50-53,58-71,81-82,138-140,171H,8-9,16-18,23-25,30-49,104H2,1-7H3,(H2,105,141)(H2,106,142)(H,116,144)(H,117,154)(H,118,153)(H,119,155)(H,120,164)(H,121,145)(H,122,146)(H,123,143)(H,124,147)(H,125,151)(H,126,160)(H,127,162)(H,128,152)(H,129,163)(H,130,165)(H,131,156)(H,132,157)(H,133,161)(H,136,159)(H,137,158)(H,149,150)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,148,170)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013341

(CHEMBL3349629)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)C([#6])([#6])[#16] |r| Show InChI InChI=1S/C105H161N33O34S/c1-10-52(5)81(137-91(160)61(27-20-36-116-103(112)113)127-94(163)67(42-79(149)150)129-97(166)82(53(6)11-2)138-90(159)59(25-18-34-114-101(108)109)123-76(146)45-117-74(144)44-118-86(155)64(38-55-21-14-12-15-22-55)134-104(172)135-69(100(170)171)43-80(151)152)96(165)121-46-75(145)122-54(7)84(153)125-62(32-33-72(106)142)89(158)132-70(49-139)87(156)120-47-77(147)124-63(37-51(3)4)85(154)119-48-78(148)136-83(105(8,9)173)98(167)130-66(41-73(107)143)93(162)133-71(50-140)95(164)128-65(39-56-23-16-13-17-24-56)92(161)126-60(26-19-35-115-102(110)111)88(157)131-68(99(168)169)40-57-28-30-58(141)31-29-57/h12-17,21-24,28-31,51-54,59-71,81-83,139-141,173H,10-11,18-20,25-27,32-50H2,1-9H3,(H2,106,142)(H2,107,143)(H,117,144)(H,118,155)(H,119,154)(H,120,156)(H,121,165)(H,122,145)(H,123,146)(H,124,147)(H,125,153)(H,126,161)(H,127,163)(H,128,164)(H,129,166)(H,130,167)(H,131,157)(H,132,158)(H,133,162)(H,136,148)(H,137,160)(H,138,159)(H,149,150)(H,151,152)(H,168,169)(H,170,171)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,135,172)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,81-,82-,83+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013342

(CHEMBL3349625)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#8]-[#6])-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#16])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C104H159N33O34S/c1-9-52(5)82(136-91(158)61(26-19-35-115-103(111)112)127-94(161)67(41-80(148)149)130-98(165)83(53(6)10-2)137-90(157)59(24-17-33-113-101(107)108)122-77(145)44-116-75(143)43-117-86(153)64(37-55-20-13-11-14-21-55)134-104(170)135-69(100(168)169)42-81(150)171-8)97(164)120-45-76(144)121-54(7)84(151)125-62(31-32-73(105)141)89(156)132-70(48-138)87(154)119-46-78(146)123-63(36-51(3)4)85(152)118-47-79(147)124-72(50-172)96(163)129-66(40-74(106)142)93(160)133-71(49-139)95(162)128-65(38-56-22-15-12-16-23-56)92(159)126-60(25-18-34-114-102(109)110)88(155)131-68(99(166)167)39-57-27-29-58(140)30-28-57/h11-16,20-23,27-30,51-54,59-72,82-83,138-140,172H,9-10,17-19,24-26,31-50H2,1-8H3,(H2,105,141)(H2,106,142)(H,116,143)(H,117,153)(H,118,152)(H,119,154)(H,120,164)(H,121,144)(H,122,145)(H,123,146)(H,124,147)(H,125,151)(H,126,159)(H,127,161)(H,128,162)(H,129,163)(H,130,165)(H,131,155)(H,132,156)(H,133,160)(H,136,158)(H,137,157)(H,148,149)(H,166,167)(H,168,169)(H4,107,108,113)(H4,109,110,114)(H4,111,112,115)(H2,134,135,170)/t52-,53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,72-,82-,83-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192889
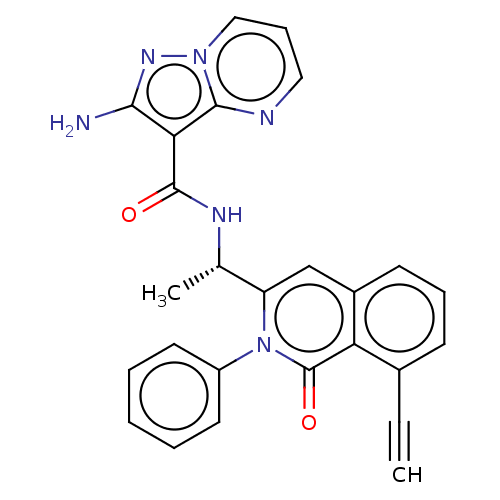
(CHEMBL3975359)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20N6O2/c1-3-17-9-7-10-18-15-20(32(26(34)21(17)18)19-11-5-4-6-12-19)16(2)29-25(33)22-23(27)30-31-14-8-13-28-24(22)31/h1,4-16H,2H3,(H2,27,30)(H,29,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192880

(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033477

((S)-2-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(2-Benzyl-3-phe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)C(Cc1ccccc1)Cc1ccccc1)C(C)C)C(O)=O Show InChI InChI=1S/C41H58N6O10/c1-23(2)18-31(41(56)57)45-37(52)30(22-33(49)50)43-36(51)29(21-32(42)48)44-38(53)34(24(3)4)46-39(54)35(25(5)6)47(7)40(55)28(19-26-14-10-8-11-15-26)20-27-16-12-9-13-17-27/h8-17,23-25,28-31,34-35H,18-22H2,1-7H3,(H2,42,48)(H,43,51)(H,44,53)(H,45,52)(H,46,54)(H,49,50)(H,56,57)/t29-,30-,31-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033456

((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[(S)-2-cycloh...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCc1ccccc1)C1CCCCC1)C(C)C)C(O)=O Show InChI InChI=1S/C40H60N6O10/c1-24(2)21-30(40(55)56)43-37(52)29(23-33(49)50)41-36(51)28(22-32(48)46-19-11-12-20-46)42-38(53)34(25(3)4)45-39(54)35(27-15-9-6-10-16-27)44-31(47)18-17-26-13-7-5-8-14-26/h5,7-8,13-14,24-25,27-30,34-35H,6,9-12,15-23H2,1-4H3,(H,41,51)(H,42,53)(H,43,52)(H,44,47)(H,45,54)(H,49,50)(H,55,56)/t28-,29-,30-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033462
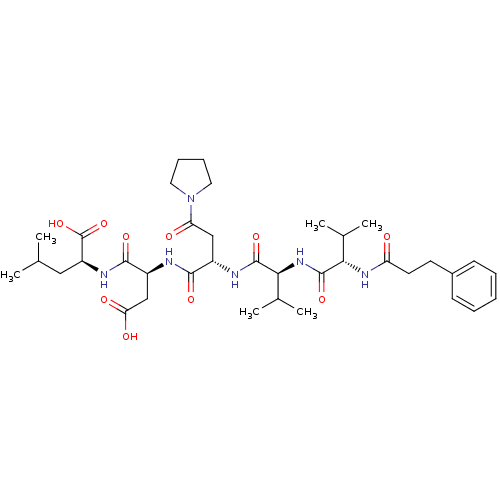
((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCc1ccccc1)C(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C37H56N6O10/c1-21(2)18-27(37(52)53)40-34(49)26(20-30(46)47)38-33(48)25(19-29(45)43-16-10-11-17-43)39-35(50)32(23(5)6)42-36(51)31(22(3)4)41-28(44)15-14-24-12-8-7-9-13-24/h7-9,12-13,21-23,25-27,31-32H,10-11,14-20H2,1-6H3,(H,38,48)(H,39,50)(H,40,49)(H,41,44)(H,42,51)(H,46,47)(H,52,53)/t25-,26-,27-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192882
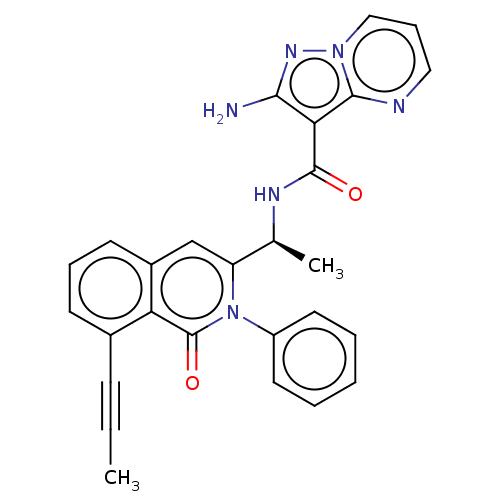
(CHEMBL3937119)Show SMILES CC#Cc1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C27H22N6O2/c1-3-9-18-10-7-11-19-16-21(33(27(35)22(18)19)20-12-5-4-6-13-20)17(2)30-26(34)23-24(28)31-32-15-8-14-29-25(23)32/h4-8,10-17H,1-2H3,(H2,28,31)(H,30,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50013339

(CHEMBL3349627)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H158N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,50-53,58-71,82-83,140-142H,8-9,16-18,23-25,30-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70+,71-,82-,83-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033461
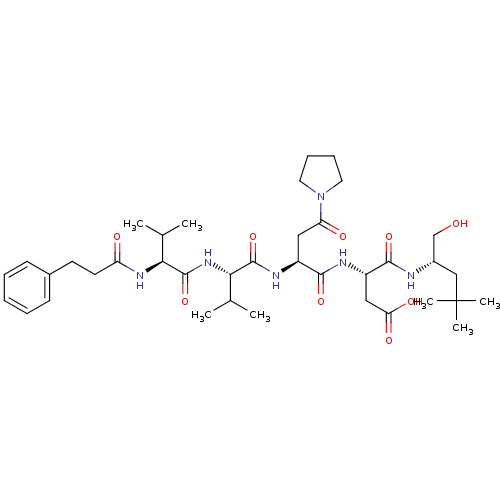
((S)-N-((S)-1-Hydroxymethyl-3,3-dimethyl-butyl)-3-(...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](CO)CC(C)(C)C Show InChI InChI=1S/C38H60N6O9/c1-23(2)32(42-29(46)16-15-25-13-9-8-10-14-25)37(53)43-33(24(3)4)36(52)41-27(19-30(47)44-17-11-12-18-44)35(51)40-28(20-31(48)49)34(50)39-26(22-45)21-38(5,6)7/h8-10,13-14,23-24,26-28,32-33,45H,11-12,15-22H2,1-7H3,(H,39,50)(H,40,51)(H,41,52)(H,42,46)(H,43,53)(H,48,49)/t26-,27-,28-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033449

((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[(S)-3,3-dime...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(=O)N1CCCC1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCc1ccccc1)C(C)(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C38H58N6O10/c1-22(2)19-27(37(53)54)41-34(50)26(21-30(47)48)39-33(49)25(20-29(46)44-17-11-12-18-44)40-35(51)31(23(3)4)43-36(52)32(38(5,6)7)42-28(45)16-15-24-13-9-8-10-14-24/h8-10,13-14,22-23,25-27,31-32H,11-12,15-21H2,1-7H3,(H,39,49)(H,40,51)(H,41,50)(H,42,45)(H,43,52)(H,47,48)(H,53,54)/t25-,26-,27-,31-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192899
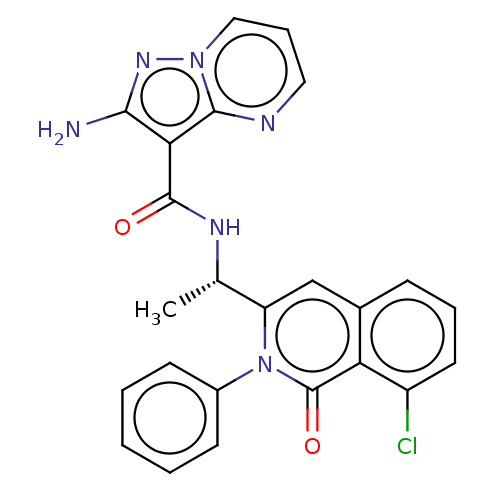
(CHEMBL3963736)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19ClN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50033455

((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(2-ethyl-buty...)Show SMILES CCC(CC)C(=O)N[C@H](C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C(O)=O)C(C)(C)C Show InChI InChI=1S/C32H55N5O9/c1-10-19(11-2)25(39)35-23(31(5,6)7)27(41)33-20(17-22(38)37-14-12-13-15-37)26(40)36-24(32(8,9)30(45)46)28(42)34-21(29(43)44)16-18(3)4/h18-21,23-24H,10-17H2,1-9H3,(H,33,41)(H,34,42)(H,35,39)(H,36,40)(H,43,44)(H,45,46)/t20-,21-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192883
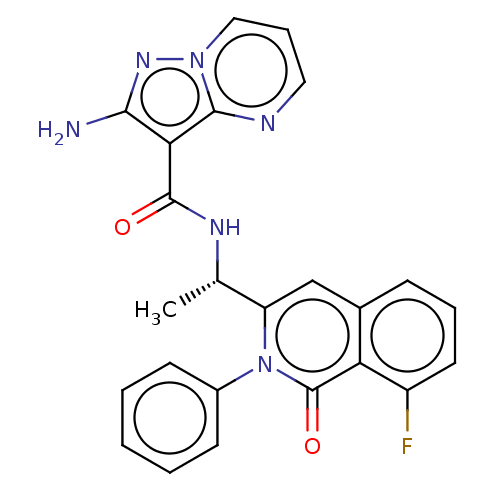
(CHEMBL3976330)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19FN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 2
(Bos taurus) | BDBM50228712

(CHEMBL3350078)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](-[#8])=O)-[#6](-[#8])=O)-[#6@@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](=O)-[#7]\[#6](=[#6]\[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C105H154N34O37/c1-8-51(5)82(138-91(162)60(25-18-34-116-103(112)113)128-94(165)66(41-80(151)152)130-97(168)83(52(6)9-2)139-90(161)58(23-16-32-114-101(108)109)123-77(148)44-117-75(146)43-118-86(157)63(36-54-19-12-10-13-20-54)134-104(175)136-69(100(173)174)42-81(153)154)96(167)121-45-76(147)122-53(7)84(155)126-61(30-31-72(106)143)89(160)132-70(48-140)87(158)120-46-78(149)125-62(35-50(3)4)85(156)119-47-79(150)137-105(176)135-68(99(171)172)40-74(145)124-65(39-73(107)144)93(164)133-71(49-141)95(166)129-64(37-55-21-14-11-15-22-55)92(163)127-59(24-17-33-115-102(110)111)88(159)131-67(98(169)170)38-56-26-28-57(142)29-27-56/h10-15,19-22,26-29,40,42,50-53,58-67,70-71,82-83,140-142H,8-9,16-18,23-25,30-39,41,43-49H2,1-7H3,(H2,106,143)(H2,107,144)(H,117,146)(H,118,157)(H,119,156)(H,120,158)(H,121,167)(H,122,147)(H,123,148)(H,124,145)(H,125,149)(H,126,155)(H,127,163)(H,128,165)(H,129,166)(H,130,168)(H,131,159)(H,132,160)(H,133,164)(H,138,162)(H,139,161)(H,151,152)(H,153,154)(H,169,170)(H,171,172)(H,173,174)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116)(H2,134,136,175)(H2,135,137,150,176)/b68-40+,69-42+/t51-,52-,53-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,70-,71-,82-,83-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio Mega Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity was determined based on the displacement of [125]r-ANF (99-126) from binding sites on bovine adrenal zona glomerulosa cell membranes... |
J Med Chem 33: 661-7 (1990)
BindingDB Entry DOI: 10.7270/Q2251JS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192901
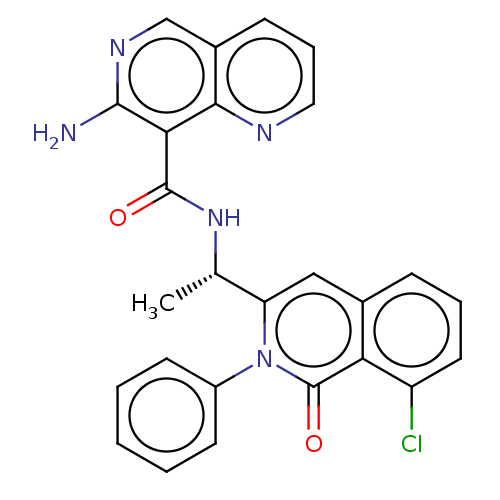
(CHEMBL3911278)Show SMILES C[C@H](NC(=O)c1c(N)ncc2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20ClN5O2/c1-15(31-25(33)22-23-17(8-6-12-29-23)14-30-24(22)28)20-13-16-7-5-11-19(27)21(16)26(34)32(20)18-9-3-2-4-10-18/h2-15H,1H3,(H2,28,30)(H,31,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit/subunit M2
(Homo sapiens (Human)) | BDBM50369049
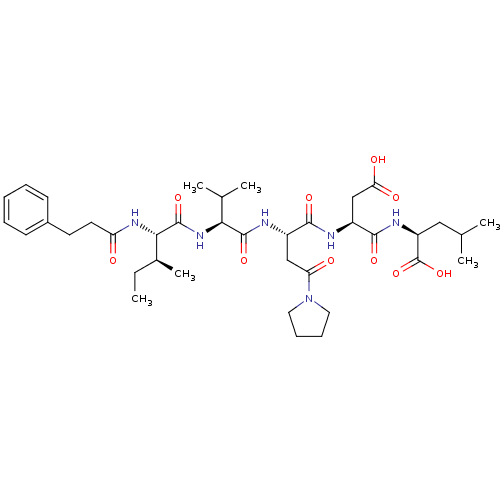
(CHEMBL1790855)Show SMILES CC[C@H](C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)N1CCCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H58N6O10/c1-7-24(6)33(42-29(45)16-15-25-13-9-8-10-14-25)37(52)43-32(23(4)5)36(51)40-26(20-30(46)44-17-11-12-18-44)34(49)39-27(21-31(47)48)35(50)41-28(38(53)54)19-22(2)3/h8-10,13-14,22-24,26-28,32-33H,7,11-12,15-21H2,1-6H3,(H,39,49)(H,40,51)(H,41,50)(H,42,45)(H,43,52)(H,47,48)(H,53,54)/t24-,26-,27-,28-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against HSV ribonucleotide reductase |
J Med Chem 38: 3617-23 (1995)
BindingDB Entry DOI: 10.7270/Q21V5FMW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192885
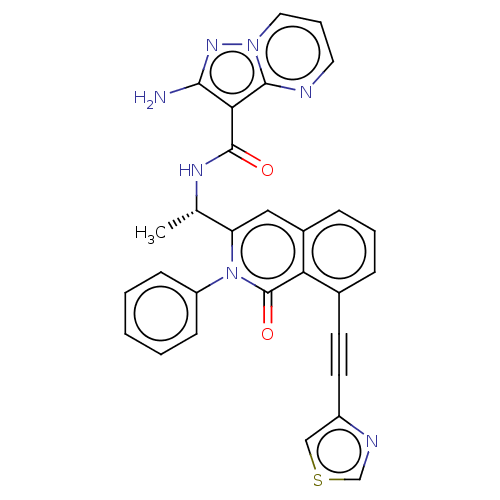
(CHEMBL3923629 | US10329299, Compound 54 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cscn3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C29H21N7O2S/c1-18(33-28(37)25-26(30)34-35-14-6-13-31-27(25)35)23-15-20-8-5-7-19(11-12-21-16-39-17-32-21)24(20)29(38)36(23)22-9-3-2-4-10-22/h2-10,13-18H,1H3,(H2,30,34)(H,33,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50130245
(6,8-Dichloro-7-cyclopropylmethoxy-9H-beta-carbolin...)Show InChI InChI=1S/C15H12Cl2N2O/c16-11-5-10-9-3-4-18-6-12(9)19-14(10)13(17)15(11)20-7-8-1-2-8/h3-6,8,18H,1-2,7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against IkappaB kinase(IKK) isolated from HeLa cells activated with recombinant MEEK1 in an ELISA phosphorylaton assay. |
Bioorg Med Chem Lett 13: 2419-22 (2003)
BindingDB Entry DOI: 10.7270/Q2PK0FJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192881
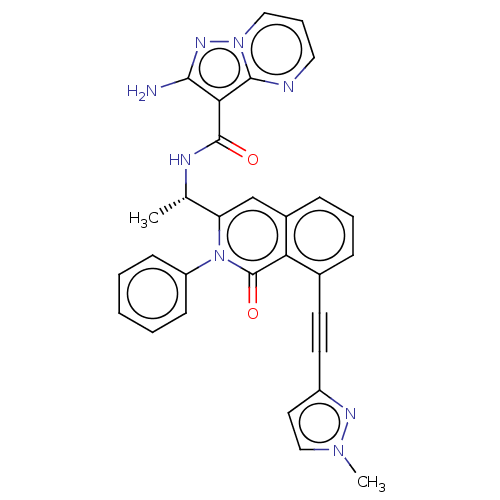
(CHEMBL3914602 | US10329299, Compound 30 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3ccn(C)n3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(33-29(39)26-27(31)35-37-16-7-15-32-28(26)37)24-18-21-9-6-8-20(12-13-22-14-17-36(2)34-22)25(21)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,33,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50130248
(CHEMBL79004 | N-(6-Chloro-9H-beta-carbolin-8-yl)-n...)Show InChI InChI=1S/C17H11ClN4O/c18-11-6-13-12-3-5-20-9-15(12)21-16(13)14(7-11)22-17(23)10-2-1-4-19-8-10/h1-9,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against IkappaB kinase(IKK) isolated from HeLa cells activated with recombinant MEEK1 in an ELISA phosphorylaton assay. |
Bioorg Med Chem Lett 13: 2419-22 (2003)
BindingDB Entry DOI: 10.7270/Q2PK0FJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192888
(CHEMBL3947903)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H22N6O2/c1-15-8-6-9-17-14-19(31(25(33)20(15)17)18-10-4-3-5-11-18)16(2)28-24(32)21-22(26)29-30-13-7-12-27-23(21)30/h3-14,16H,1-2H3,(H2,26,29)(H,28,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































