 Found 65 hits with Last Name = 'hayakawa' and Initial = 'y'
Found 65 hits with Last Name = 'hayakawa' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241817
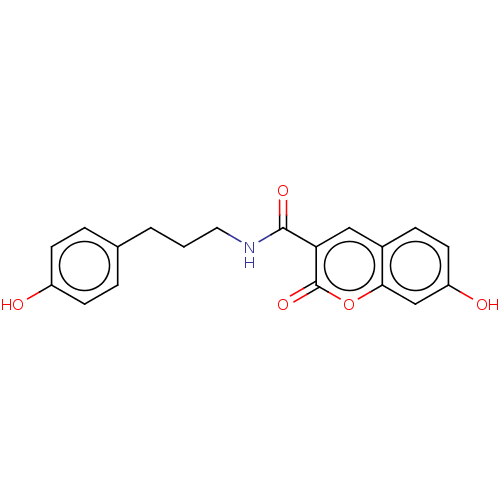
(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human AKR1B10 in presence of geraniol as substrate by Lineweaver-Burk plot method |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081804
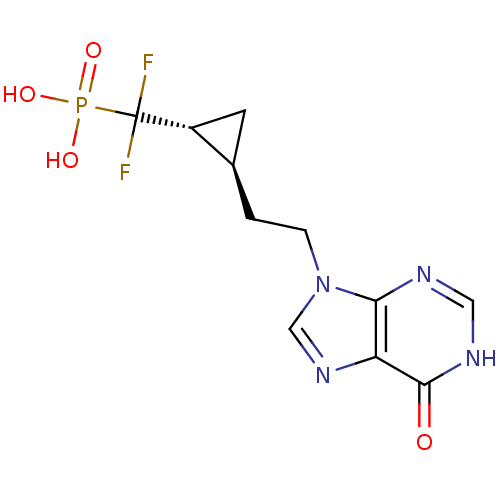
((Difluoro-{(1R,2S)-2-[2-(6-oxo-1,6-dihydro-purin-9...)Show SMILES OP(O)(=O)C(F)(F)[C@@H]1C[C@H]1CCn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H13F2N4O4P/c12-11(13,22(19,20)21)7-3-6(7)1-2-17-5-16-8-9(17)14-4-15-10(8)18/h4-7H,1-3H2,(H,14,15,18)(H2,19,20,21)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081803
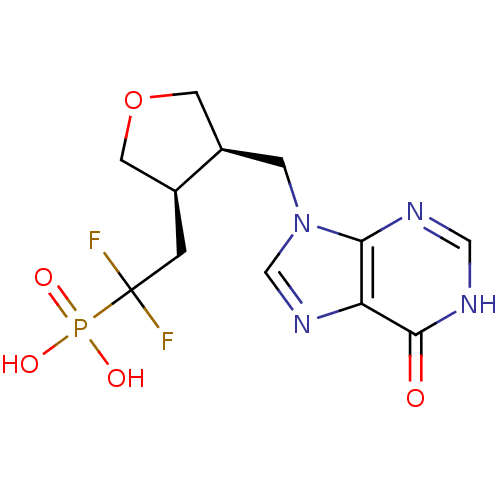
(CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081807
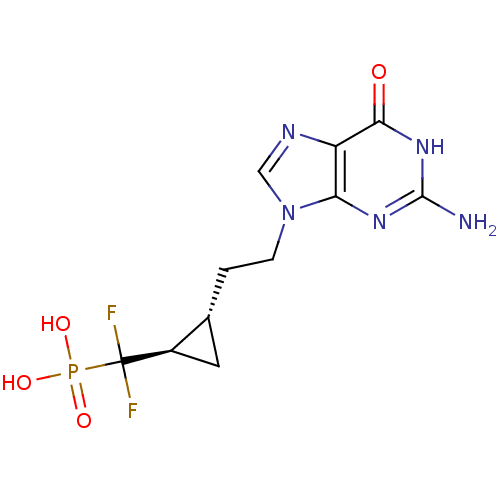
(({(1R,2S)-2-[2-(2-Amino-6-oxo-1,6-dihydro-purin-9-...)Show SMILES Nc1nc2n(CC[C@@H]3C[C@H]3C(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H14F2N5O4P/c12-11(13,23(20,21)22)6-3-5(6)1-2-18-4-15-7-8(18)16-10(14)17-9(7)19/h4-6H,1-3H2,(H2,20,21,22)(H3,14,16,17,19)/t5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214705
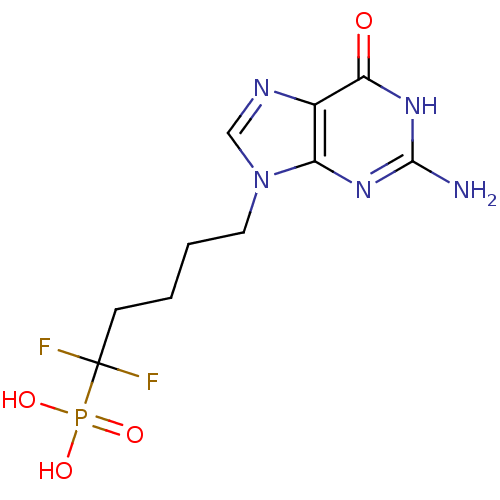
(5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...)Show SMILES Nc1nc2n(CCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C10H14F2N5O4P/c11-10(12,22(19,20)21)3-1-2-4-17-5-14-6-7(17)15-9(13)16-8(6)18/h5H,1-4H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241828
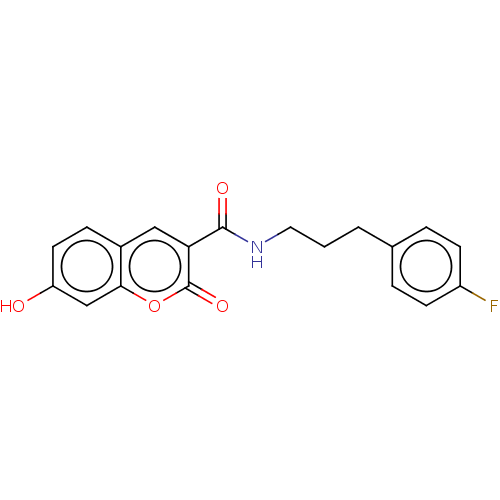
(CHEMBL4089817)Show InChI InChI=1S/C19H16FNO4/c20-14-6-3-12(4-7-14)2-1-9-21-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,22H,1-2,9H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241817

(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50442489

(CHEMBL2440417)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-29-20-11-8-18(9-12-20)26-24-21(13-17-7-10-19(27)14-22(17)30-24)23(28)25-15-16-5-3-2-4-6-16/h2-14,27H,15H2,1H3,(H,25,28)/b26-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1B10 (unknown origin) |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241818
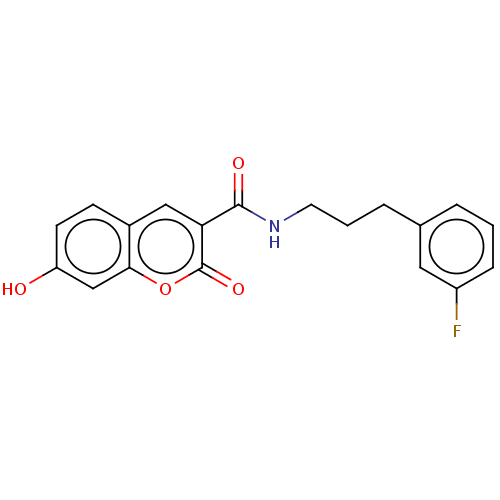
(CHEMBL4060049)Show InChI InChI=1S/C19H16FNO4/c20-14-5-1-3-12(9-14)4-2-8-21-18(23)16-10-13-6-7-15(22)11-17(13)25-19(16)24/h1,3,5-7,9-11,22H,2,4,8H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241816

(CHEMBL4098452)Show InChI InChI=1S/C19H17NO5/c21-14-8-7-13-10-15(19(24)25-17(13)11-14)18(23)20-9-3-5-12-4-1-2-6-16(12)22/h1-2,4,6-8,10-11,21-22H,3,5,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241822

(CHEMBL4062779)Show InChI InChI=1S/C20H19NO4/c1-13-4-6-14(7-5-13)3-2-10-21-19(23)17-11-15-8-9-16(22)12-18(15)25-20(17)24/h4-9,11-12,22H,2-3,10H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241826
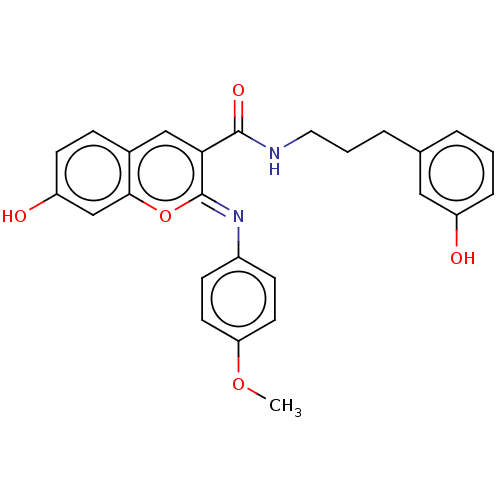
(CHEMBL4089816)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCCCc1cccc(O)c1 Show InChI InChI=1S/C26H24N2O5/c1-32-22-11-8-19(9-12-22)28-26-23(15-18-7-10-21(30)16-24(18)33-26)25(31)27-13-3-5-17-4-2-6-20(29)14-17/h2,4,6-12,14-16,29-30H,3,5,13H2,1H3,(H,27,31)/b28-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241821

(CHEMBL4068704)Show SMILES Oc1ccc2cc(C(=O)NCCCc3cc(F)cc(F)c3)c(=O)oc2c1 Show InChI InChI=1S/C19H15F2NO4/c20-13-6-11(7-14(21)9-13)2-1-5-22-18(24)16-8-12-3-4-15(23)10-17(12)26-19(16)25/h3-4,6-10,23H,1-2,5H2,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241827

(CHEMBL4071421)Show InChI InChI=1S/C19H17NO5/c21-14-5-1-3-12(9-14)4-2-8-20-18(23)16-10-13-6-7-15(22)11-17(13)25-19(16)24/h1,3,5-7,9-11,21-22H,2,4,8H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16314
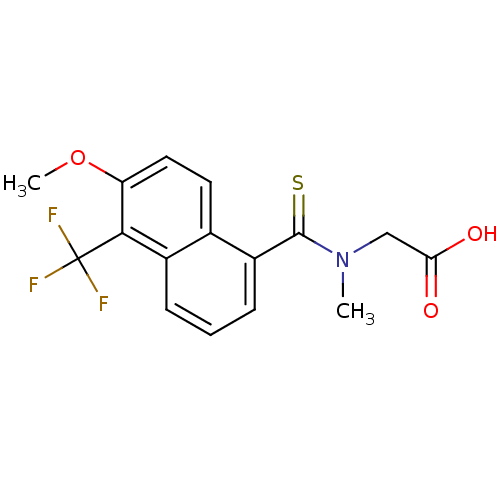
(2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...)Show InChI InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1B1 assessed as decrease in glyceraldehyde reduction |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241823

(CHEMBL4084456)Show InChI InChI=1S/C20H19NO5/c1-25-16-8-4-13(5-9-16)3-2-10-21-19(23)17-11-14-6-7-15(22)12-18(14)26-20(17)24/h4-9,11-12,22H,2-3,10H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241829
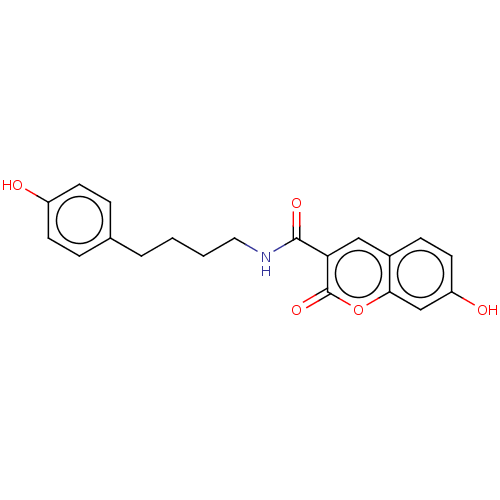
(CHEMBL4071420)Show InChI InChI=1S/C20H19NO5/c22-15-7-4-13(5-8-15)3-1-2-10-21-19(24)17-11-14-6-9-16(23)12-18(14)26-20(17)25/h4-9,11-12,22-23H,1-3,10H2,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241826

(CHEMBL4089816)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCCCc1cccc(O)c1 Show InChI InChI=1S/C26H24N2O5/c1-32-22-11-8-19(9-12-22)28-26-23(15-18-7-10-21(30)16-24(18)33-26)25(31)27-13-3-5-17-4-2-6-20(29)14-17/h2,4,6-12,14-16,29-30H,3,5,13H2,1H3,(H,27,31)/b28-26- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241830
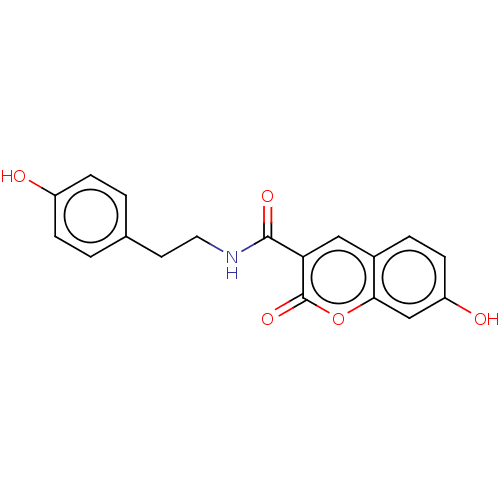
(CHEMBL3318218)Show InChI InChI=1S/C18H15NO5/c20-13-4-1-11(2-5-13)7-8-19-17(22)15-9-12-3-6-14(21)10-16(12)24-18(15)23/h1-6,9-10,20-21H,7-8H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B10 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50442489

(CHEMBL2440417)Show SMILES COc1ccc(cc1)\N=c1/oc2cc(O)ccc2cc1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-29-20-11-8-18(9-12-20)26-24-21(13-17-7-10-19(27)14-22(17)30-24)23(28)25-15-16-5-3-2-4-6-16/h2-14,27H,15H2,1H3,(H,25,28)/b26-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1B1 (unknown origin) |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50394657

(CHEMBL270067)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17+,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human intestinal N-terminal 6-His-tagged AKR1B10 expressed in Escherichia coli BL21 (DE3) pLysS cells using pyridine-3-alde... |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081803

(CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081806
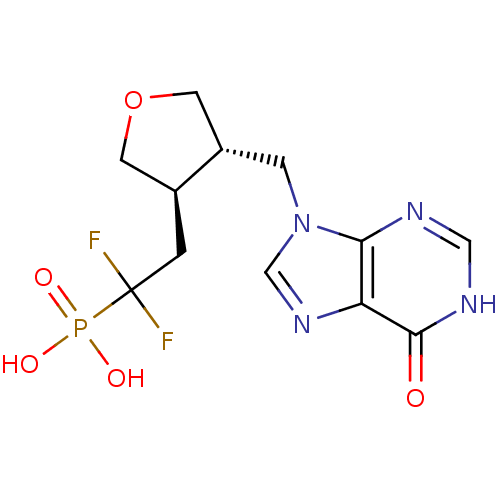
(CHEMBL94920 | {1,1-Difluoro-2-[(3R,4S)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM16314

(2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...)Show InChI InChI=1S/C16H14F3NO3S/c1-20(8-13(21)22)15(24)11-5-3-4-10-9(11)6-7-12(23-2)14(10)16(17,18)19/h3-7H,8H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged AKR1B10 expressed in Escherichia coli BL21 using retinaldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50059989
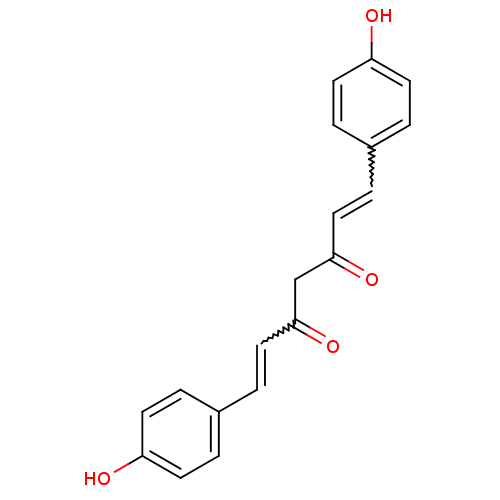
((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...)Show SMILES Oc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)cc2)cc1 |w:12.11,5.4| Show InChI InChI=1S/C19H16O4/c20-16-7-1-14(2-8-16)5-11-18(22)13-19(23)12-6-15-3-9-17(21)10-4-15/h1-12,20-21H,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of AKR1B10 (unknown origin) |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081804

((Difluoro-{(1R,2S)-2-[2-(6-oxo-1,6-dihydro-purin-9...)Show SMILES OP(O)(=O)C(F)(F)[C@@H]1C[C@H]1CCn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H13F2N4O4P/c12-11(13,22(19,20)21)7-3-6(7)1-2-17-5-16-8-9(17)14-4-15-10(8)18/h4-7H,1-3H2,(H,14,15,18)(H2,19,20,21)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM200221
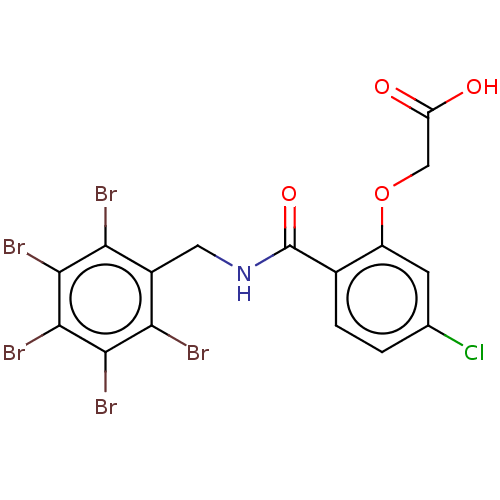
(2-(5-chloro-2-(((perbromophenyl)methyl)carbamoyl)p...)Show SMILES OC(=O)COc1cc(Cl)ccc1C(=O)NCc1c(Br)c(Br)c(Br)c(Br)c1Br Show InChI InChI=1S/C16H9Br5ClNO4/c17-11-8(12(18)14(20)15(21)13(11)19)4-23-16(26)7-2-1-6(22)3-9(7)27-5-10(24)25/h1-3H,4-5H2,(H,23,26)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1B10 expressed in Escherichia coli BL21(DE3) using pyridine-3-aldehyde as substrate measured for 3 mins by UV-vis spectrophoto... |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081803

(CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241830

(CHEMBL3318218)Show InChI InChI=1S/C18H15NO5/c20-13-4-1-11(2-5-13)7-8-19-17(22)15-9-12-3-6-14(21)10-16(12)24-18(15)23/h1-6,9-10,20-21H,7-8H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241827

(CHEMBL4071421)Show InChI InChI=1S/C19H17NO5/c21-14-5-1-3-12(9-14)4-2-8-20-18(23)16-10-13-6-7-15(22)11-17(13)25-19(16)24/h1,3,5-7,9-11,21-22H,2,4,8H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241822

(CHEMBL4062779)Show InChI InChI=1S/C20H19NO4/c1-13-4-6-14(7-5-13)3-2-10-21-19(23)17-11-15-8-9-16(22)12-18(15)25-20(17)24/h4-9,11-12,22H,2-3,10H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241817

(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241816

(CHEMBL4098452)Show InChI InChI=1S/C19H17NO5/c21-14-8-7-13-10-15(19(24)25-17(13)11-14)18(23)20-9-3-5-12-4-1-2-6-16(12)22/h1-2,4,6-8,10-11,21-22H,3,5,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241828

(CHEMBL4089817)Show InChI InChI=1S/C19H16FNO4/c20-14-6-3-12(4-7-14)2-1-9-21-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,22H,1-2,9H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241829

(CHEMBL4071420)Show InChI InChI=1S/C20H19NO5/c22-15-7-4-13(5-8-15)3-1-2-10-21-19(24)17-11-14-6-9-16(23)12-18(14)26-20(17)25/h4-9,11-12,22-23H,1-3,10H2,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081806

(CHEMBL94920 | {1,1-Difluoro-2-[(3R,4S)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241821

(CHEMBL4068704)Show SMILES Oc1ccc2cc(C(=O)NCCCc3cc(F)cc(F)c3)c(=O)oc2c1 Show InChI InChI=1S/C19H15F2NO4/c20-13-6-11(7-14(21)9-13)2-1-5-22-18(24)16-8-12-3-4-15(23)10-17(12)26-19(16)25/h3-4,6-10,23H,1-2,5H2,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081807

(({(1R,2S)-2-[2-(2-Amino-6-oxo-1,6-dihydro-purin-9-...)Show SMILES Nc1nc2n(CC[C@@H]3C[C@H]3C(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H14F2N5O4P/c12-11(13,23(20,21)22)6-3-5(6)1-2-18-4-15-7-8(18)16-10(14)17-9(7)19/h4-6H,1-3H2,(H2,20,21,22)(H3,14,16,17,19)/t5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241818

(CHEMBL4060049)Show InChI InChI=1S/C19H16FNO4/c20-14-5-1-3-12(9-14)4-2-8-21-18(23)16-10-13-6-7-15(22)11-17(13)25-19(16)24/h1,3,5-7,9-11,22H,2,4,8H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1B1 using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081804

((Difluoro-{(1R,2S)-2-[2-(6-oxo-1,6-dihydro-purin-9...)Show SMILES OP(O)(=O)C(F)(F)[C@@H]1C[C@H]1CCn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H13F2N4O4P/c12-11(13,22(19,20)21)7-3-6(7)1-2-17-5-16-8-9(17)14-4-15-10(8)18/h4-7H,1-3H2,(H,14,15,18)(H2,19,20,21)/t6-,7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM50241828

(CHEMBL4089817)Show InChI InChI=1S/C19H16FNO4/c20-14-6-3-12(4-7-14)2-1-9-21-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,22H,1-2,9H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1C4 using S-tetralol as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214705

(5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...)Show SMILES Nc1nc2n(CCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C10H14F2N5O4P/c11-10(12,22(19,20)21)3-1-2-4-17-5-14-6-7(17)15-9(13)16-8(6)18/h5H,1-4H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50241823

(CHEMBL4084456)Show InChI InChI=1S/C20H19NO5/c1-25-16-8-4-13(5-9-16)3-2-10-21-19(23)17-11-14-6-7-15(22)12-18(14)26-20(17)24/h4-9,11-12,22H,2-3,10H2,1H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptors from rat brain membranes using [3H]CCPA |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081807

(({(1R,2S)-2-[2-(2-Amino-6-oxo-1,6-dihydro-purin-9-...)Show SMILES Nc1nc2n(CC[C@@H]3C[C@H]3C(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C11H14F2N5O4P/c12-11(13,23(20,21)22)6-3-5(6)1-2-18-4-15-7-8(18)16-10(14)17-9(7)19/h4-6H,1-3H2,(H2,20,21,22)(H3,14,16,17,19)/t5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50241828

(CHEMBL4089817)Show InChI InChI=1S/C19H16FNO4/c20-14-6-3-12(4-7-14)2-1-9-21-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,22H,1-2,9H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1C3 using S-tetralol as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214705

(5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...)Show SMILES Nc1nc2n(CCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C10H14F2N5O4P/c11-10(12,22(19,20)21)3-1-2-4-17-5-14-6-7(17)15-9(13)16-8(6)18/h5H,1-4H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50241828

(CHEMBL4089817)Show InChI InChI=1S/C19H16FNO4/c20-14-6-3-12(4-7-14)2-1-9-21-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,22H,1-2,9H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1C2 using S-tetralol as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM50241817

(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1C4 using S-tetralol as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B10
(Homo sapiens (Human)) | BDBM50241815
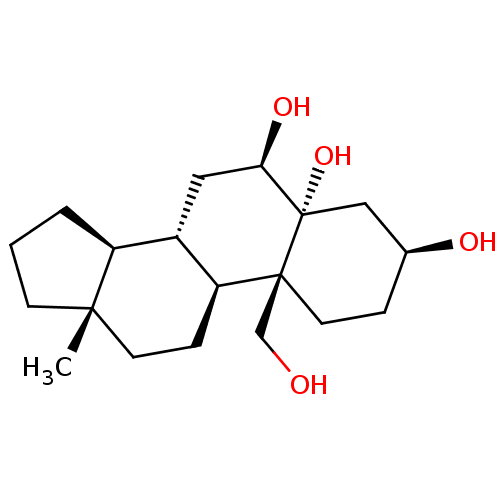
(CHEMBL4082424)Show SMILES [H][C@@]12CCC[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@@H](O)[C@@]2(O)C[C@@H](O)CC[C@]12CO |r| Show InChI InChI=1S/C19H32O4/c1-17-6-2-3-14(17)13-9-16(22)19(23)10-12(21)4-8-18(19,11-20)15(13)5-7-17/h12-16,20-23H,2-11H2,1H3/t12-,13-,14-,15-,16+,17-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1B10 (1 to 316 residues) expressed in Escherichia coli using pyridine-3-aldehyde as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50241817

(CHEMBL4081954)Show InChI InChI=1S/C19H17NO5/c21-14-6-3-12(4-7-14)2-1-9-20-18(23)16-10-13-5-8-15(22)11-17(13)25-19(16)24/h3-8,10-11,21-22H,1-2,9H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AKR1C1 using S-tetralol as substrate |
J Med Chem 60: 8441-8455 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00830
BindingDB Entry DOI: 10.7270/Q2NZ89S7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data