 Found 48 hits with Last Name = 'heimrich' and Initial = 'em'
Found 48 hits with Last Name = 'heimrich' and Initial = 'em' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165
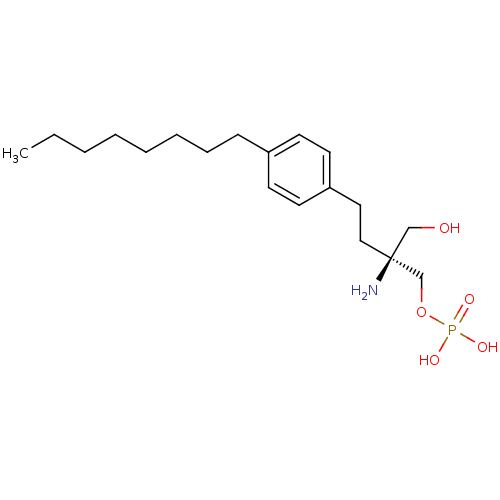
(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470
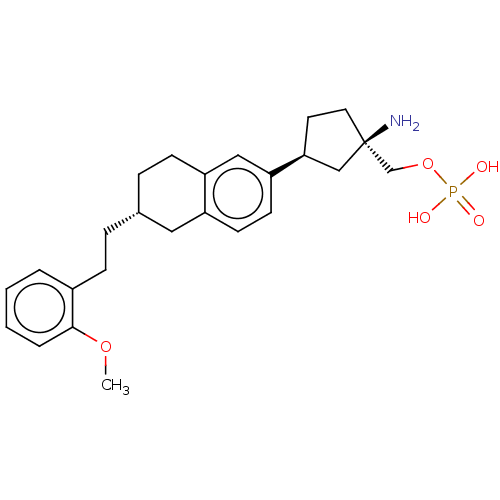
(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163
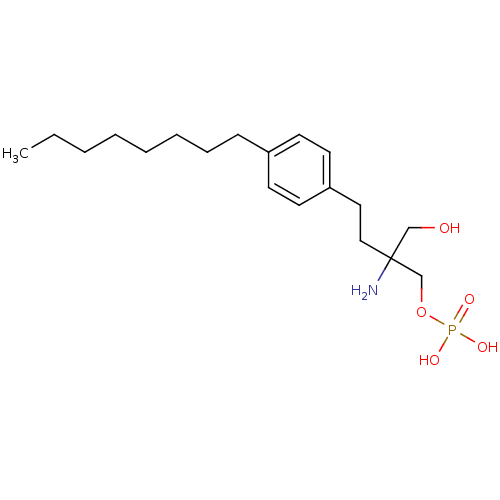
(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human S1P3 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50563973

(Bms-986166)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(CO)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cell membranes assessed as stimulation of cAMP accumulation incubated for 10 mins by HTRF... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cell membranes assessed as stimulation of cAMP accumulation incubated for 10 mins by HTRF... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cell membranes assessed as stimulation of cAMP accumulation incubated for 10 mins by HTRF... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant C-terminal GFP-fused S1P1 expressed in CHO cells assessed as induction of receptor internalization incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant C-terminal GFP-fused S1P1 expressed in CHO cells assessed as induction of receptor internalization incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant C-terminal GFP-fused S1P1 expressed in CHO cells assessed as induction of receptor internalization incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cells assessed as stimulation of ERK phosphorylation incubated for 7 mins by microplate r... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cells assessed as stimulation of ERK phosphorylation incubated for 7 mins by microplate r... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P1 expressed in CHO cells assessed as stimulation of ERK phosphorylation incubated for 7 mins by microplate r... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P3 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P3 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P3 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P4 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P4 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P4 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P5 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM258470

(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P5 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P5 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.377 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.901 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in CHO cells co-expressing GFP assessed as cellular internalization after 45 mins by Hoechst assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.114 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in CHO cells co-expressing GFP assessed as cellular internalization after 45 mins by Hoechst assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.00560 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in CHO cells assessed as ERK phosphorylation after 7 mins by alphaLISA |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in CHO cells assessed as ERK phosphorylation after 7 mins by alphaLISA |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in CHO cells assessed as cAMP accumulation after 30 mins by HTRF analysis |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor expressed in CHO cells assessed as cAMP accumulation after 30 mins by HTRF analysis |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P4 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P3 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P4 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data