

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030754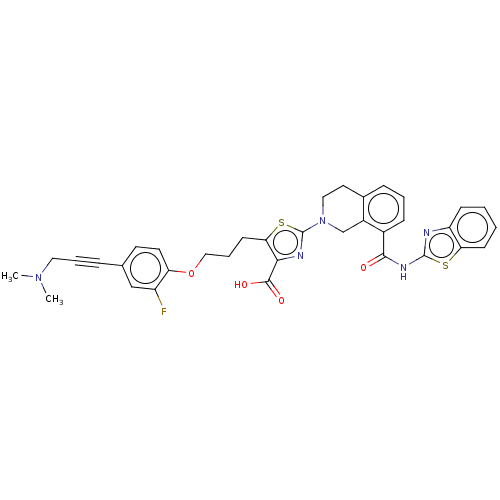 (CHEMBL3342332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162797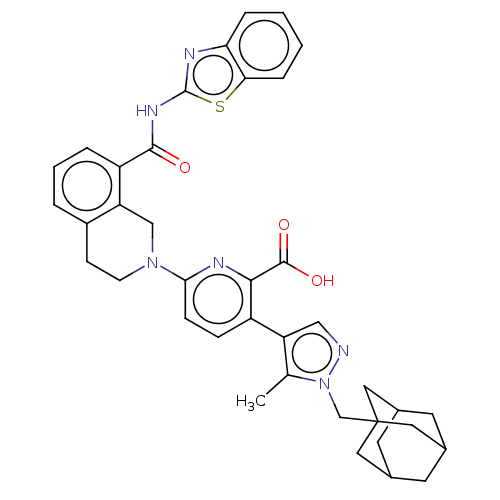 (CHEMBL3793424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM178570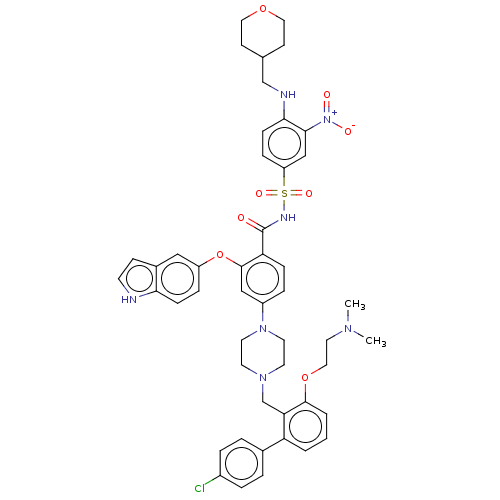 (US9125913, 129) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50270877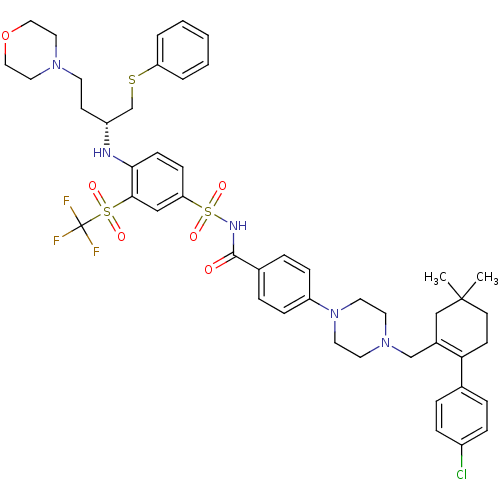 ((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50270877 ((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162797 (CHEMBL3793424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162727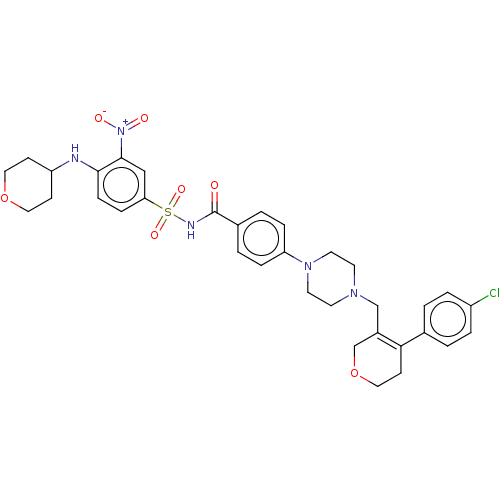 (CHEMBL3794524) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50030754 (CHEMBL3342332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM178570 (US9125913, 129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL (unknown origin) by FRET assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162727 (CHEMBL3794524) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to Bcl-XL (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 26: 2105-14 (2016) Article DOI: 10.1016/j.bmcl.2016.03.032 BindingDB Entry DOI: 10.7270/Q23F4RK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551542 (US11312719, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551543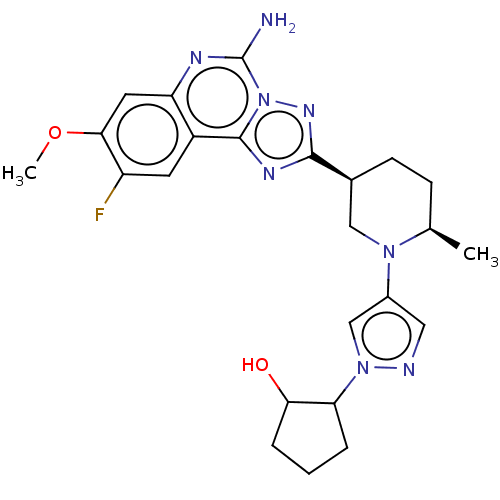 (US11312719, Example 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551571 (US11312719, Example 117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551564 (1-(4-((2S,5R)-5-(5-amino-8-chloro-9-fluoro-[1,2,4]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551576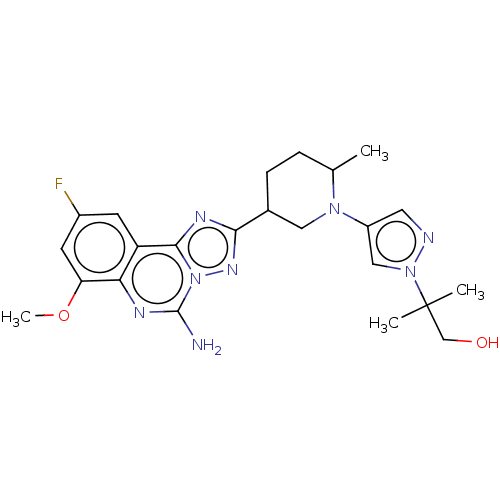 (2-(4-((2R or 2S,5R or 5S)-5-(5-amino-9-fluoro-7-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551576 (2-(4-((2R or 2S,5R or 5S)-5-(5-amino-9-fluoro-7-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551540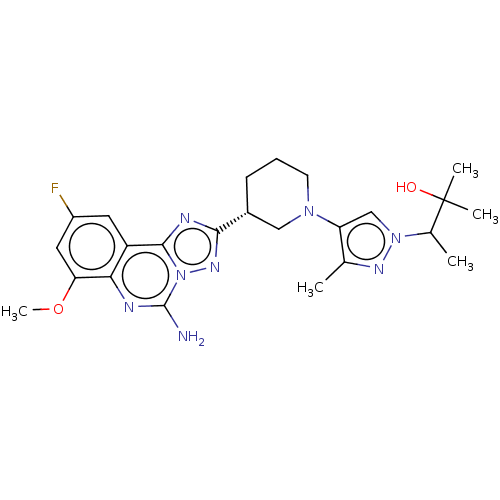 ((S or R)-3-(4-((R)-3-(5-amino-9-fluoro-7-methoxy-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551476 ((R)-1-(4-(3-(5-amino-9-fluoro-8-methoxy-[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551504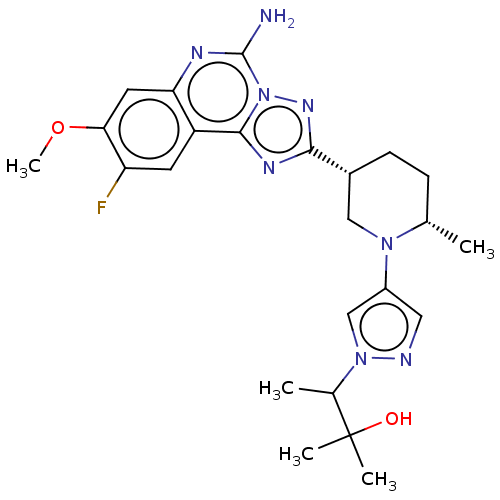 (US11312719, Example 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551580 (US11312719, Example 126 | US11312719, Example 127 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50201641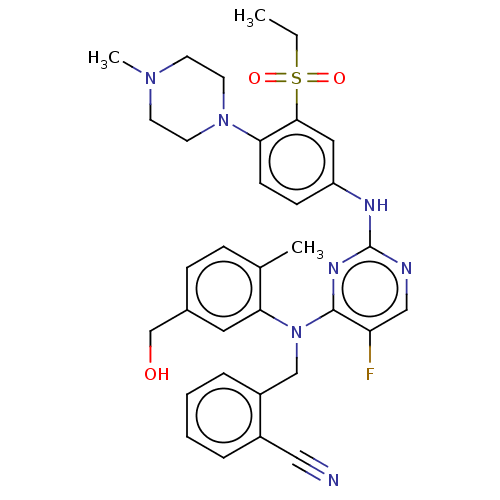 (CHEMBL3923175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) by ATP-kinaseGlo assay | ACS Med Chem Lett 7: 1118-1123 (2016) Article DOI: 10.1021/acsmedchemlett.6b00322 BindingDB Entry DOI: 10.7270/Q2PV6NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50201648 (CHEMBL3950903) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) by ATP-kinaseGlo assay | ACS Med Chem Lett 7: 1118-1123 (2016) Article DOI: 10.1021/acsmedchemlett.6b00322 BindingDB Entry DOI: 10.7270/Q2PV6NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551540 ((S or R)-3-(4-((R)-3-(5-amino-9-fluoro-7-methoxy-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551485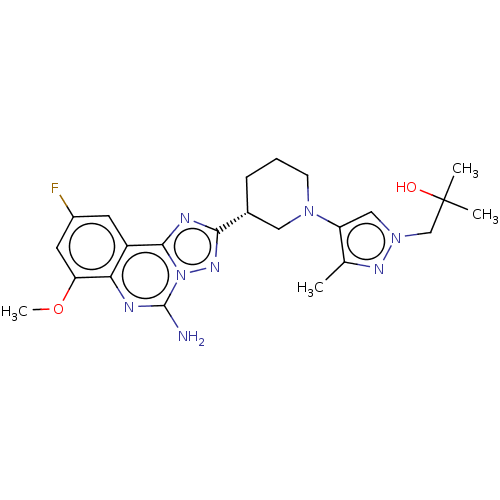 ((R)-1-(4-(3-(5-amino-9-fluoro-7-methoxy-[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551678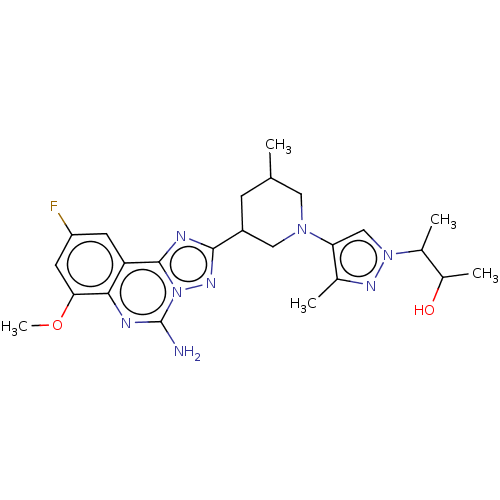 ((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551678 ((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551666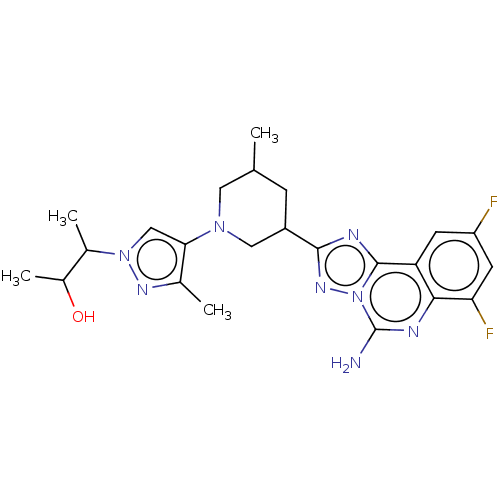 ((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551566 (1-(4-((2S,5R)-5-(5-amino-9-fluoro-8-methyl-[1,2,4]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551524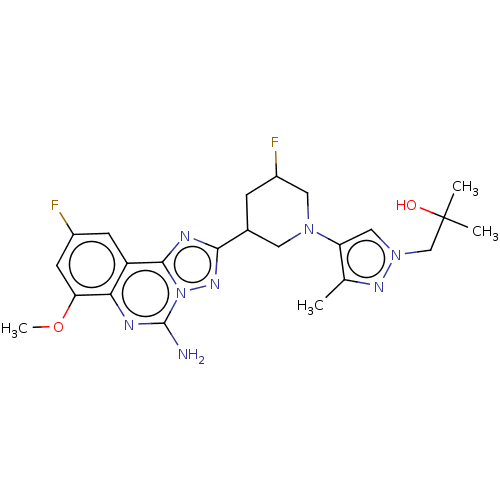 (1-(4-((3R,5S or 3S,5R)-3-(5-amino-9-fluoro-7-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50201651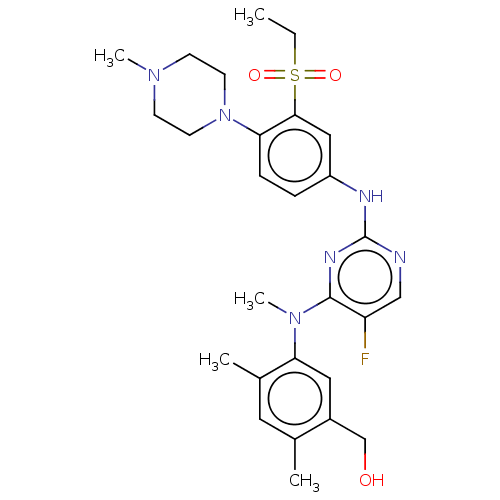 (CHEMBL3896232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) by ATP-kinaseGlo assay | ACS Med Chem Lett 7: 1118-1123 (2016) Article DOI: 10.1021/acsmedchemlett.6b00322 BindingDB Entry DOI: 10.7270/Q2PV6NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551557 ((R or S)-7,9-difluoro-2-(1-(1-methyl-1H-pyrazol-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551643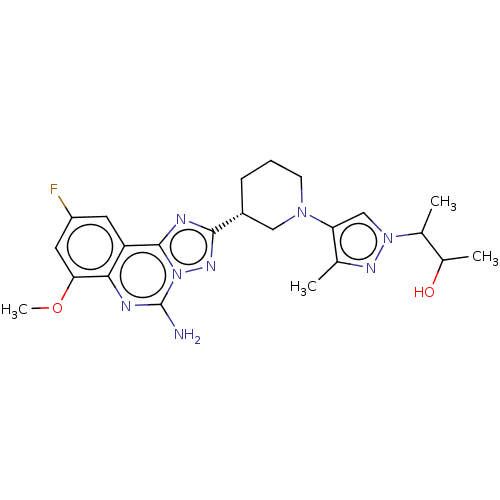 ((2S,3S or 2R,3R)-3-(4-((R)-3-(5-amino-9-fluoro-7- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551674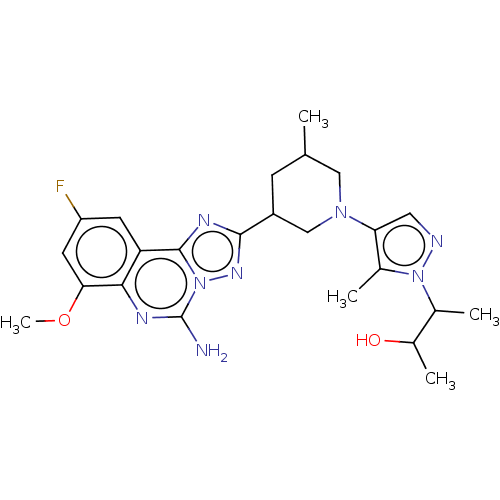 ((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551666 ((2S,3S or 2R,3R)-3-(4-((3R,5S or 3S,5R)-3-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551466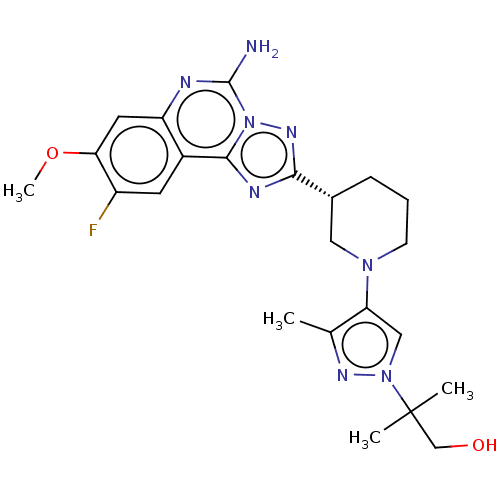 ((R)-2-(4-(3-(5-amino-9-fluoro-8-methoxy-[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551487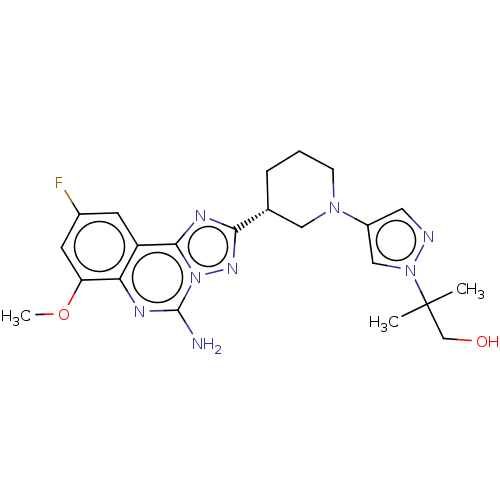 ((R)-2-(4-(3-(5-amino-9-fluoro-7-methoxy-[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551464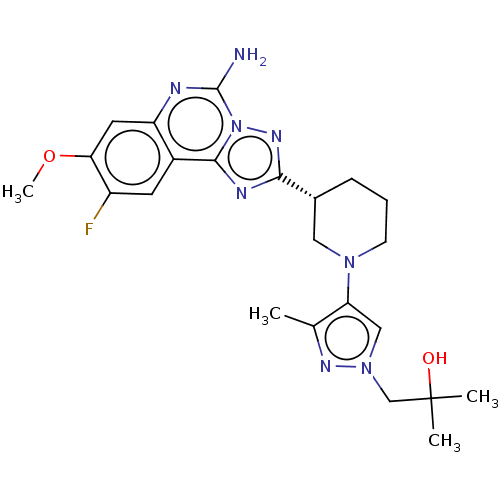 ((R)-1-(4-(3-(5-amino-9-fluoro-8-methoxy-[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [2-169,G12C,C51S,C80L,C118S] (Human sapiens (Human)) | BDBM50572114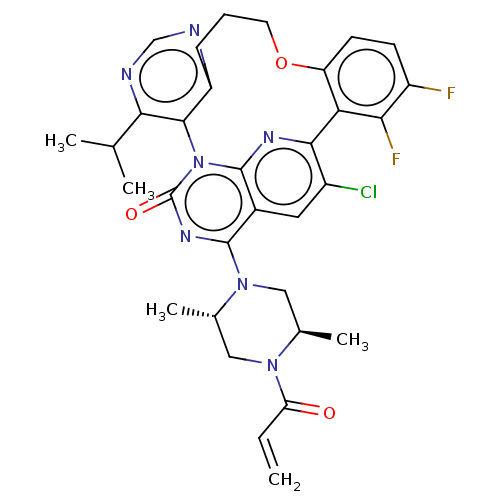 (CHEMBL4852458 | US20240043448, Example 74) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [2-169,G12C,C51S,C80L,C118S] (Human sapiens (Human)) | BDBM650996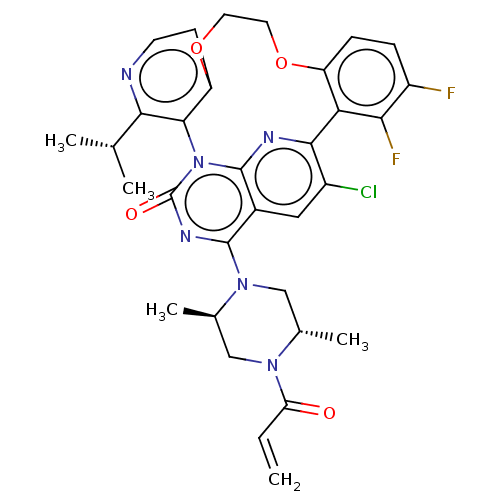 ((5aSa,17aRa)-20-chloro-2- [(2S,5R)-2,5-dimethyl-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [2-169,G12C,C51S,C80L,C118S] (Human sapiens (Human)) | BDBM651033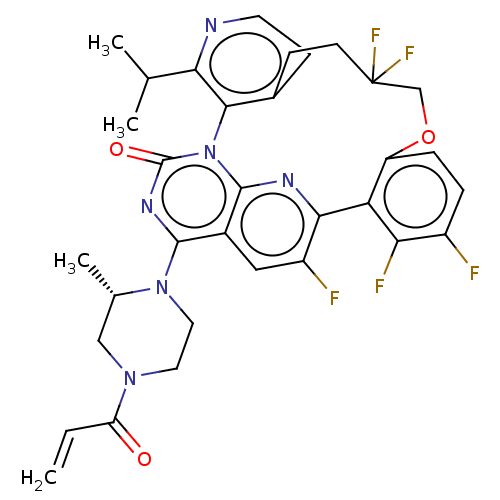 (US20240043448, Example 97) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50201654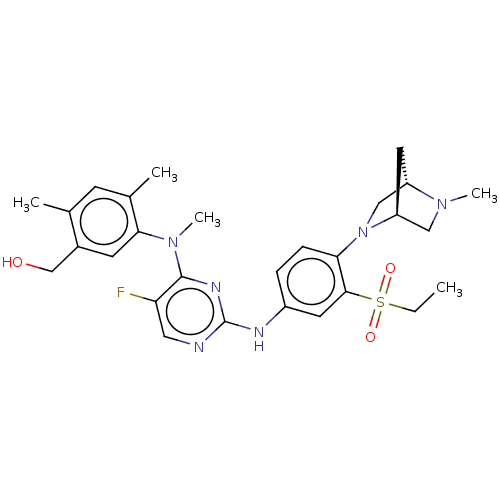 (CHEMBL3964858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) by ATP-kinaseGlo assay | ACS Med Chem Lett 7: 1118-1123 (2016) Article DOI: 10.1021/acsmedchemlett.6b00322 BindingDB Entry DOI: 10.7270/Q2PV6NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551627 (1-(4-((3R,5S or 3S,5R)-3-(5-amino-9-fluoro-7-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551631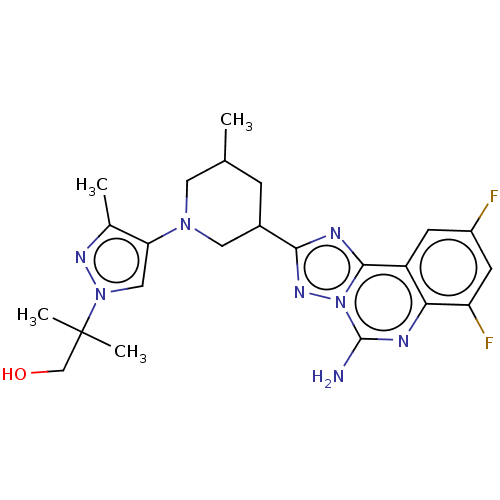 (2-(4-((3R,5S or 3S,5R)-3-(5-amino-7,9-difluoro- [1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551572 (1-(4-((2R or 2S,5R or 5S)-5-(5-amino-9-fluoro-7-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551621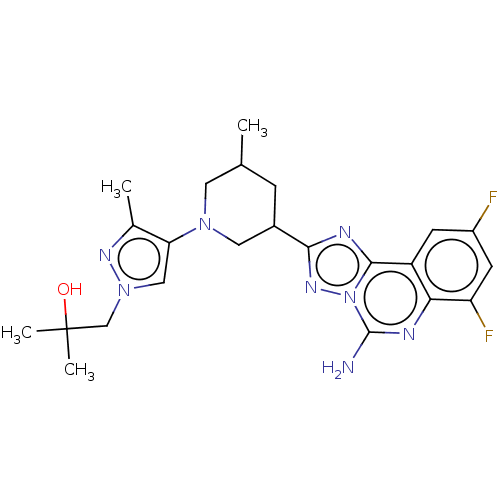 (1-(4-((3R,5S or 3S,5R)-3-(5-amino-7,9-difluoro- [1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to antagonize human A2A and A2B adenosine receptors was determined using a kit to measure changes in intracellular cyclic AM... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551521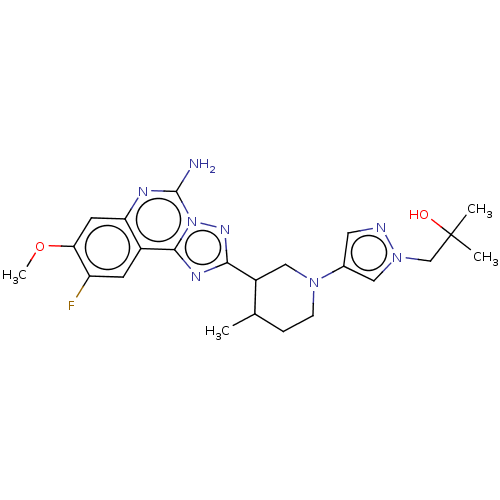 (1-(4-((3S or 3R,4S or 4R)-3-(5-amino-9-fluoro-8- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551524 (1-(4-((3R,5S or 3S,5R)-3-(5-amino-9-fluoro-7-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM551551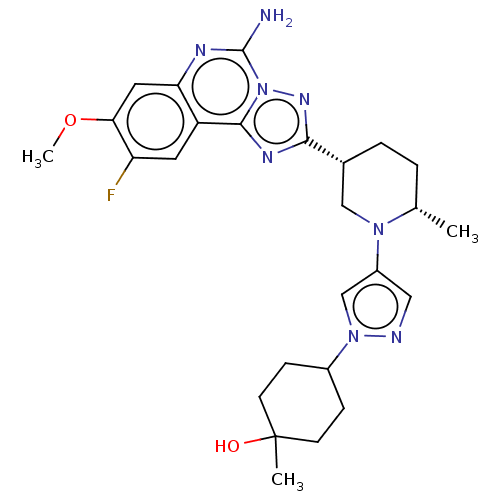 (US11312719, Example 97) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 148 μL (5 μg/mL) membranes (Perkin Elmer, Cat. No. RBHA2aM400UA) and 2 μL compounds of the invention to be tested (test compound) were... | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1433 total ) | Next | Last >> |