

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50034582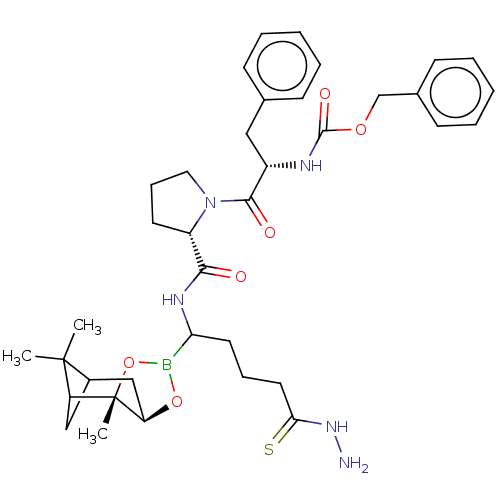 (CHEMBL2448441 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034581 (CHEMBL36744 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034581 (CHEMBL36744 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50034582 (CHEMBL2448441 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against kallikrein | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034574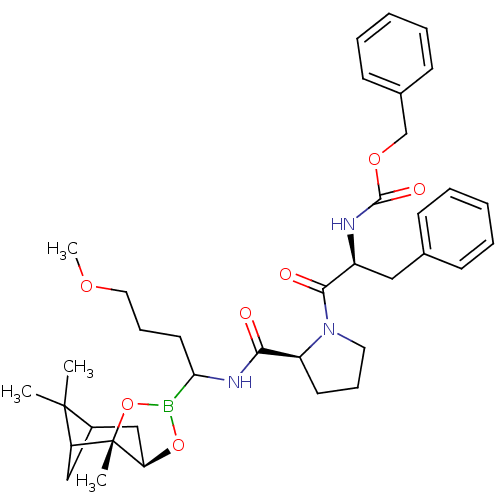 (CHEMBL288150 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034579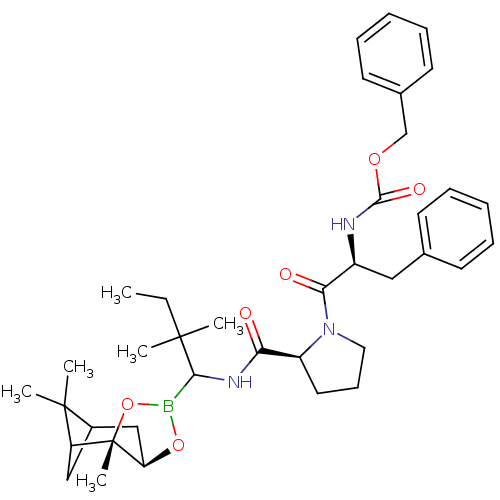 (CHEMBL290577 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50034585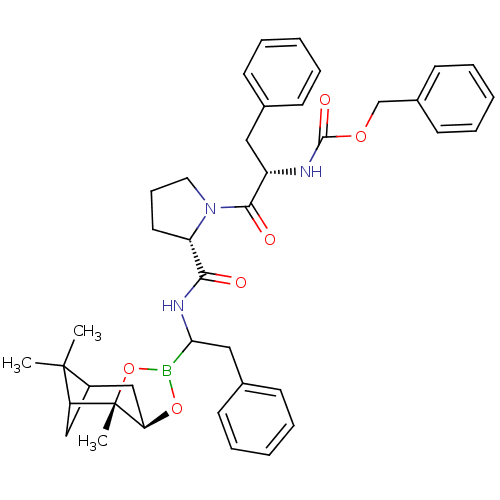 (CHEMBL285285 | Peptide boronate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Chymotrypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034585 (CHEMBL285285 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034585 (CHEMBL285285 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034577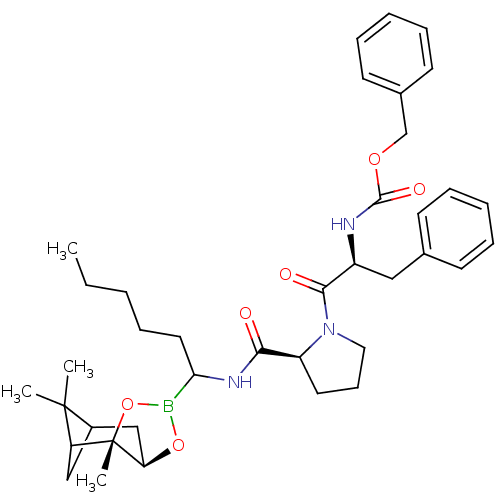 (CHEMBL291026 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034577 (CHEMBL291026 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50034580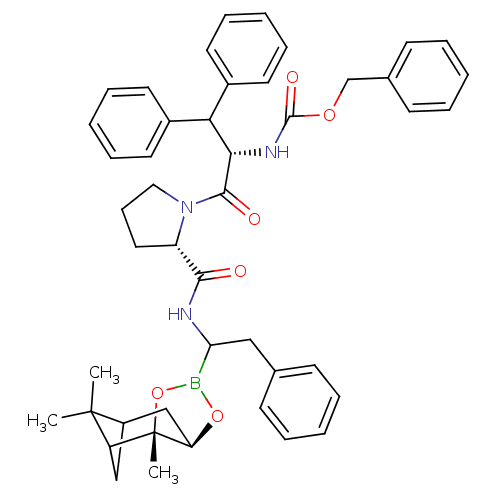 (CHEMBL418050 | Peptide boronate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Chymotrypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034579 (CHEMBL290577 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034583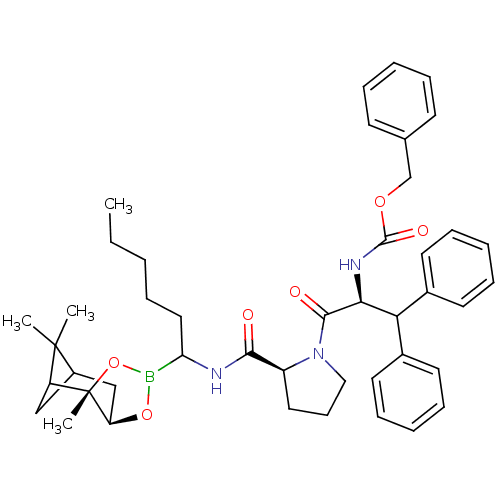 (CHEMBL287918 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034574 (CHEMBL288150 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034573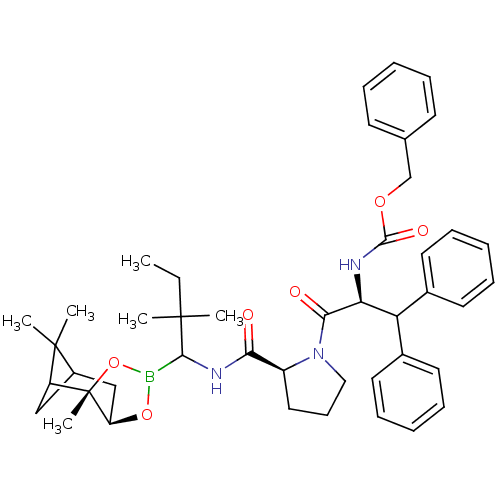 (CHEMBL291261 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034573 (CHEMBL291261 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034583 (CHEMBL287918 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034581 (CHEMBL36744 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034580 (CHEMBL418050 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031895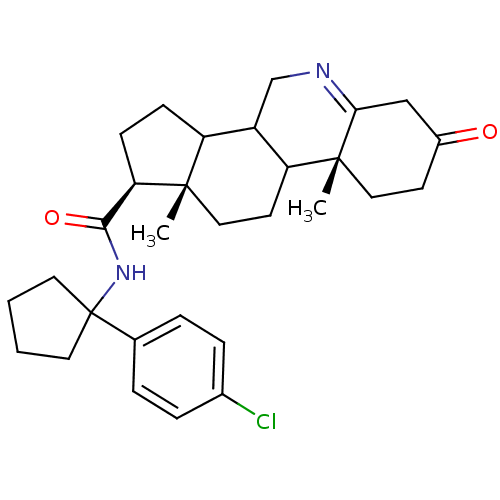 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034576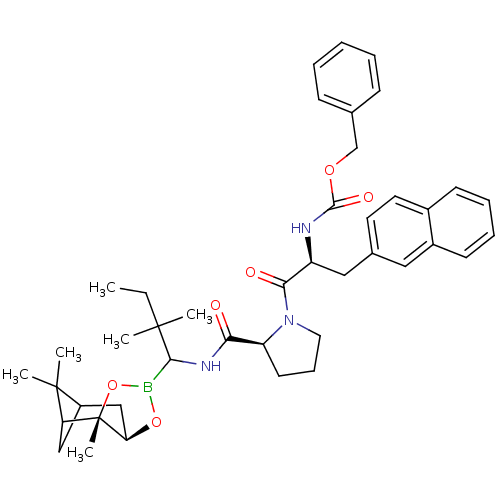 (CHEMBL288176 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031877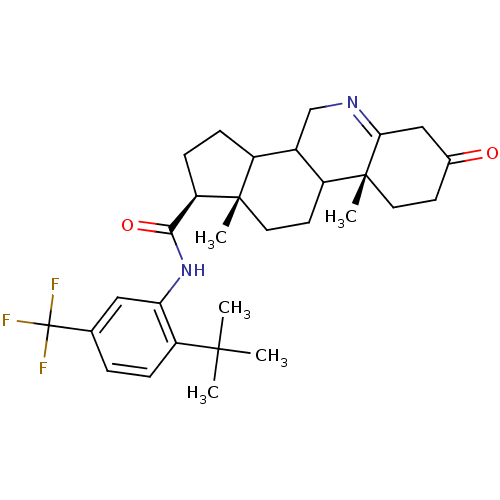 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994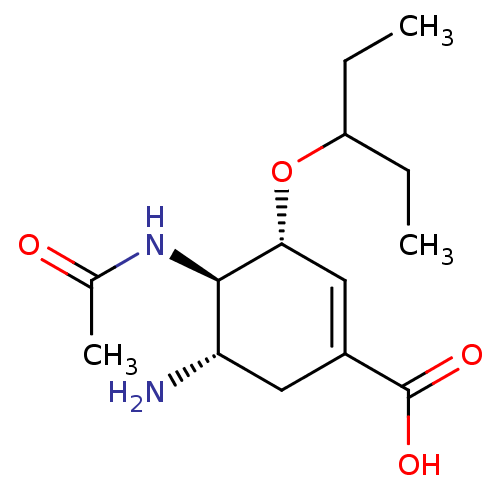 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50034577 (CHEMBL291026 | Peptide boronate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Chymotrypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031895 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031874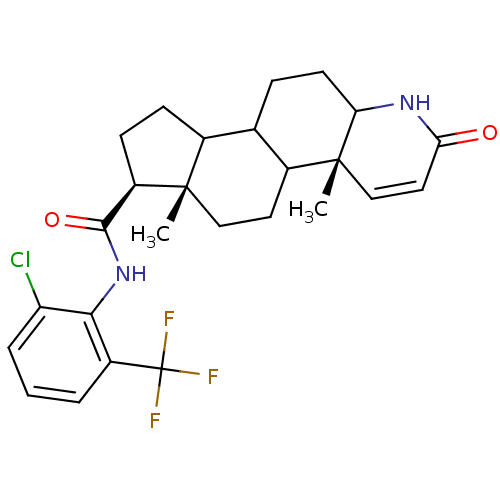 ((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Binding affinity to recombinant human Steroid 5-alpha-reductase type I was evaluated | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034580 (CHEMBL418050 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50034576 (CHEMBL288176 | Peptide boronate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against gamma thrombin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034585 (CHEMBL285285 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50034579 (CHEMBL290577 | Peptide boronate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Chymotrypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031877 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50034580 (CHEMBL418050 | Peptide boronate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against cathepsin G | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50034585 (CHEMBL285285 | Peptide boronate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against cathepsin G | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50407405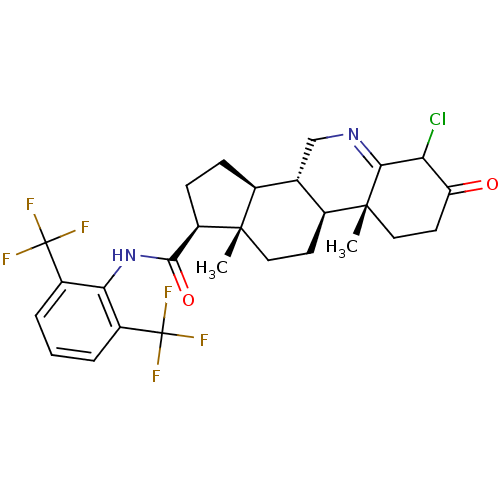 (CHEMBL2115222) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031883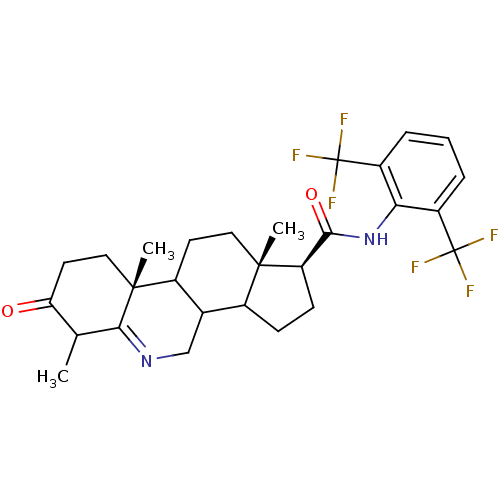 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50407405 (CHEMBL2115222) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173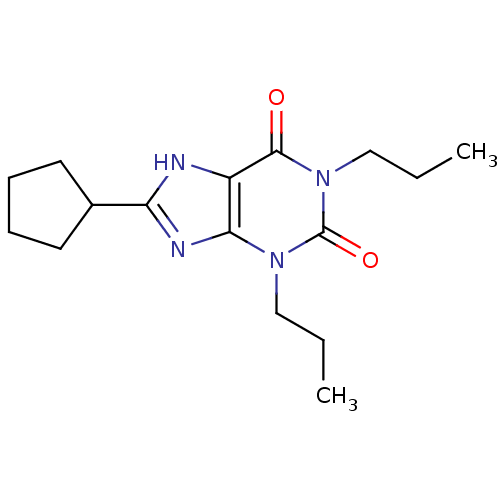 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031896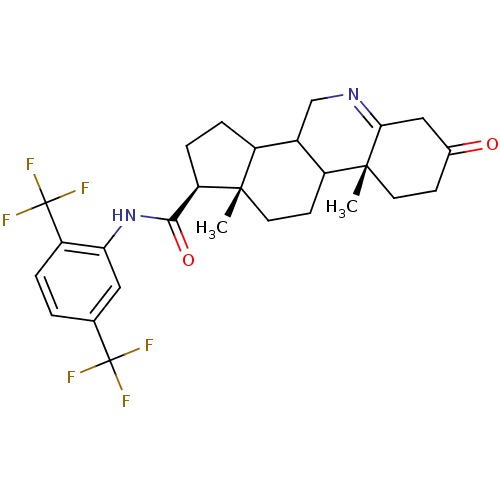 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034579 (CHEMBL290577 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031889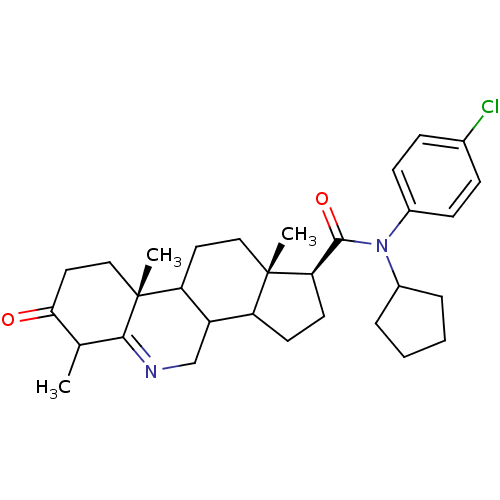 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50007838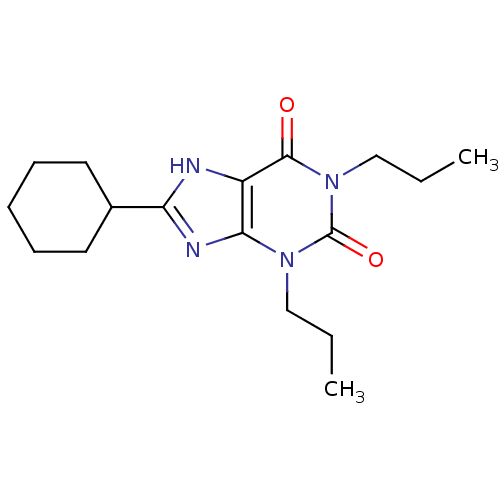 (8-Cyclohexyl-1,3-dipropyl-3,7-dihydro-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034577 (CHEMBL291026 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50034579 (CHEMBL290577 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards porcine pancreatic elastase | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368883 (CHEMBL1159458) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326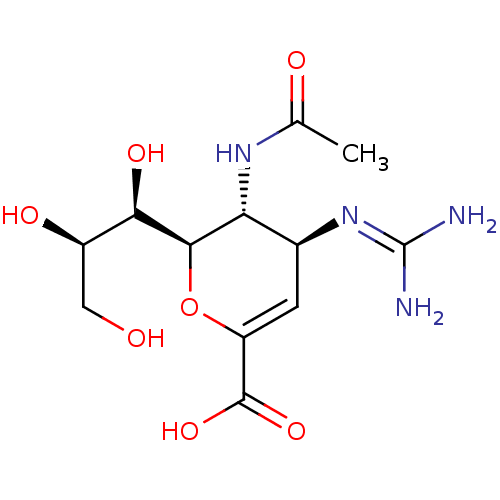 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034580 (CHEMBL418050 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50034583 (CHEMBL287918 | Peptide boronate) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Thrombosis Research Institute Curated by ChEMBL | Assay Description Binding affinity against Trypsin | J Med Chem 38: 1511-22 (1995) BindingDB Entry DOI: 10.7270/Q2QR4W57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 3927 total ) | Next | Last >> |