

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procathepsin L (Homo sapiens (Human)) | BDBM50505559 (CHEMBL4441303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505555 (CHEMBL4447348) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50033762 (Gallinamide A) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505557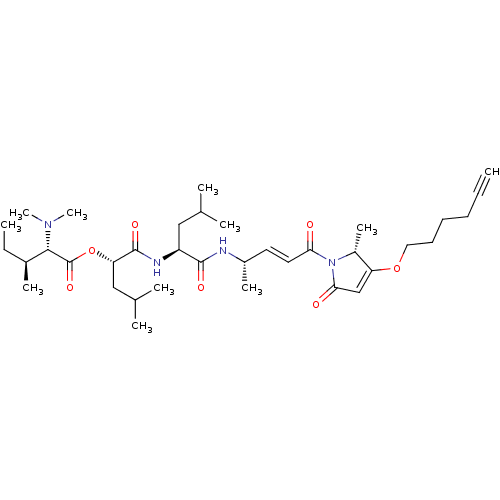 (CHEMBL4527930) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505568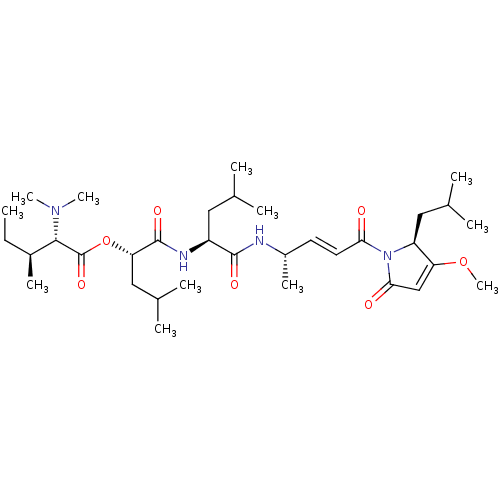 (CHEMBL4482954) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505555 (CHEMBL4447348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.632 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505562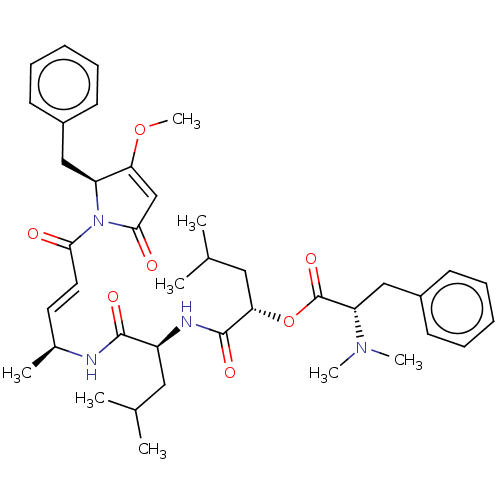 (CHEMBL4445744) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505554 (CHEMBL4564747) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505557 (CHEMBL4527930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505563 (CHEMBL4527329) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505566 (CHEMBL4582558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505559 (CHEMBL4441303) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505565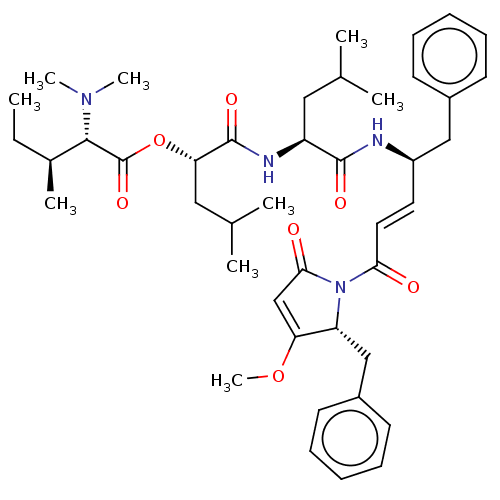 (CHEMBL4474668) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505564 (CHEMBL4527867) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505561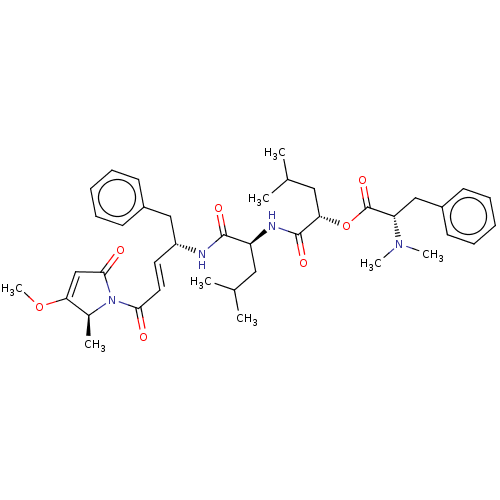 (CHEMBL4475810) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505560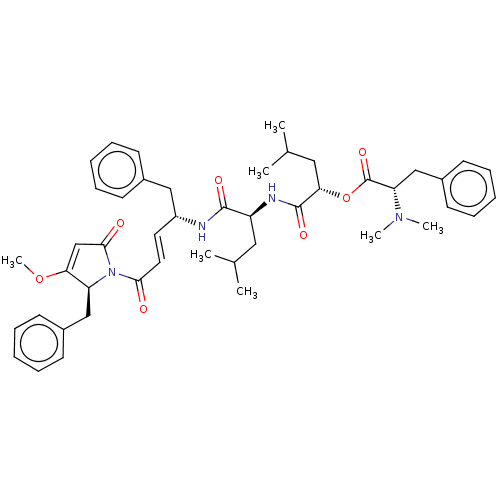 (CHEMBL4471760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505560 (CHEMBL4471760) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505562 (CHEMBL4445744) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505568 (CHEMBL4482954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505565 (CHEMBL4474668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505567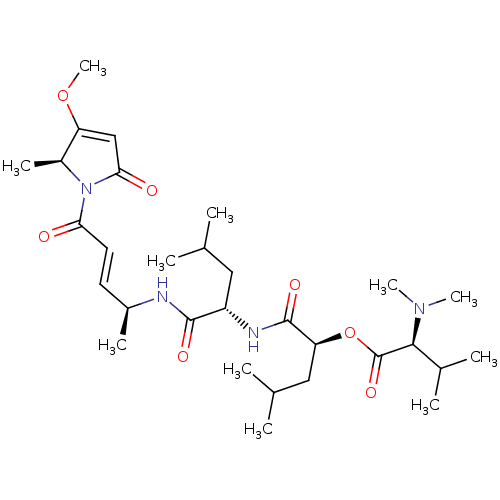 (CHEMBL4457500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505564 (CHEMBL4527867) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505563 (CHEMBL4527329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505561 (CHEMBL4475810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505566 (CHEMBL4582558) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337114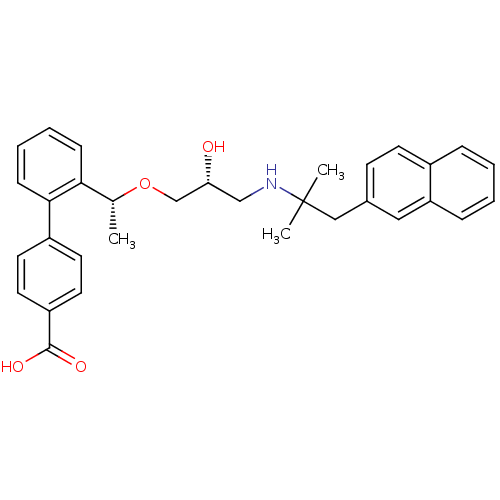 (2'-((1R)-1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505554 (CHEMBL4564747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50033762 (Gallinamide A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50033762 (Gallinamide A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337116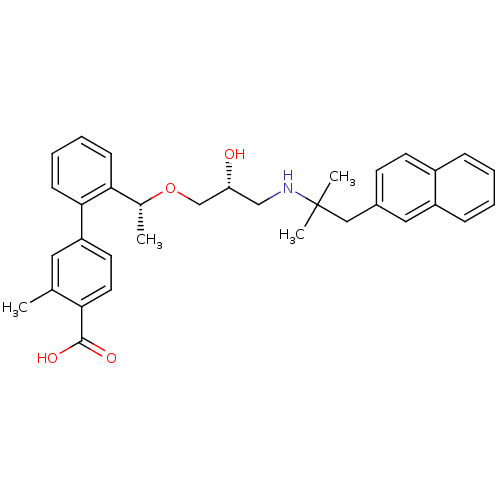 (2'-((1R)-1-{(2R)-3-[2-methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505567 (CHEMBL4457500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337113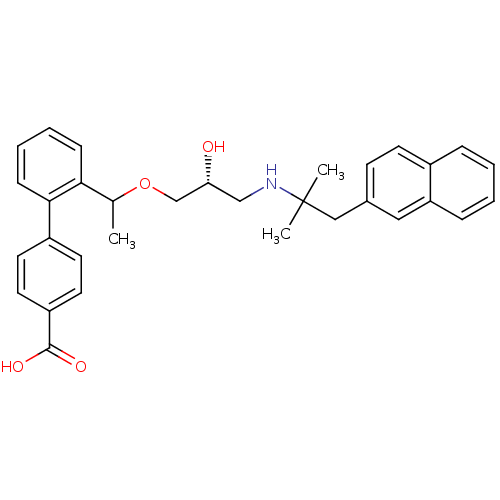 ((RS)-2'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337103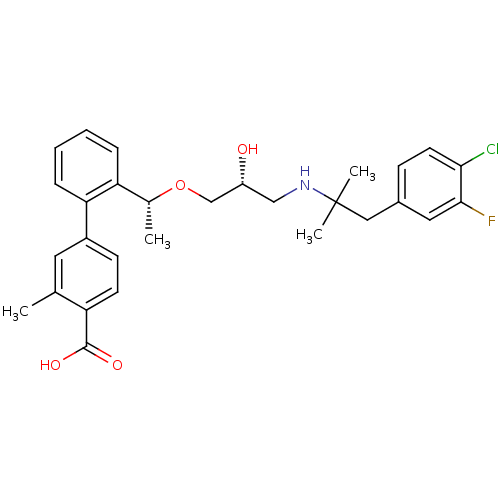 (2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337112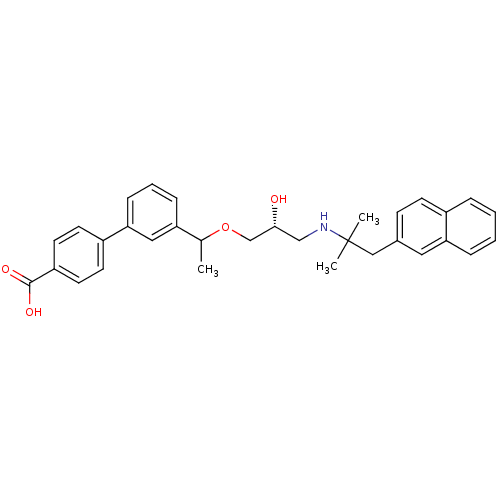 ((RS)-3'-(1-{(2R)-3-[2-Methyl-1-(naphthalen-2-yl)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337105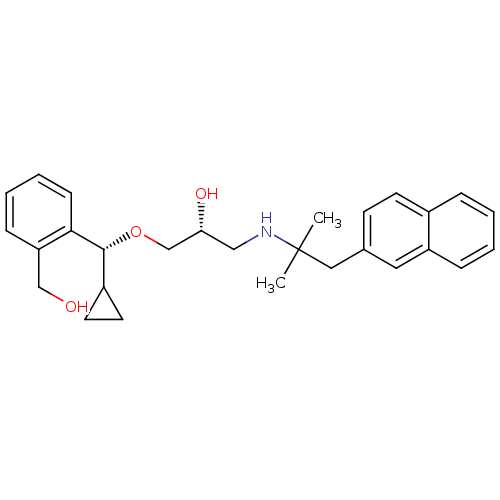 ((R)-1-((R)-cyclopropyl(2-(hydroxymethyl)phenyl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337115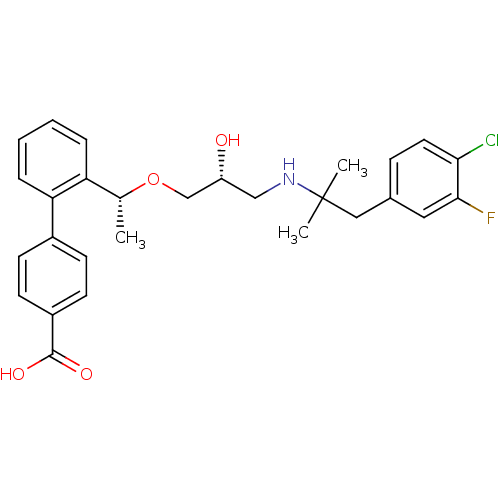 (2'-((1R)-1-{(2R)-3-[1-(4-Chloro-3-fluorophenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320009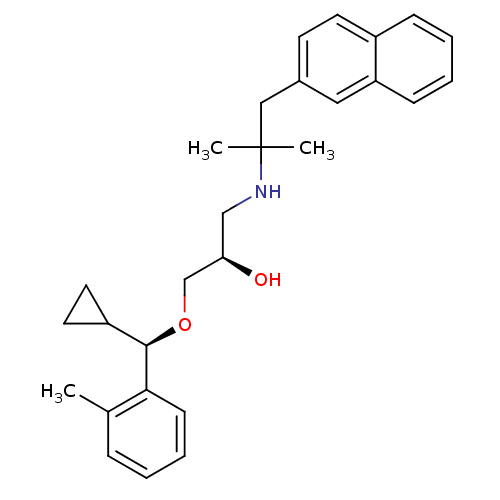 ((R)-1-((R)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320009 ((R)-1-((R)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50337104 (3-[2-((1R)-1-{(2R)-2-Hydroxy-3-[2-methyl-1-(naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12h cells by reporter gene assay | ACS Med Chem Lett 2: 238-242 (2011) Article DOI: 10.1021/ml100268k BindingDB Entry DOI: 10.7270/Q21R6QT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50161093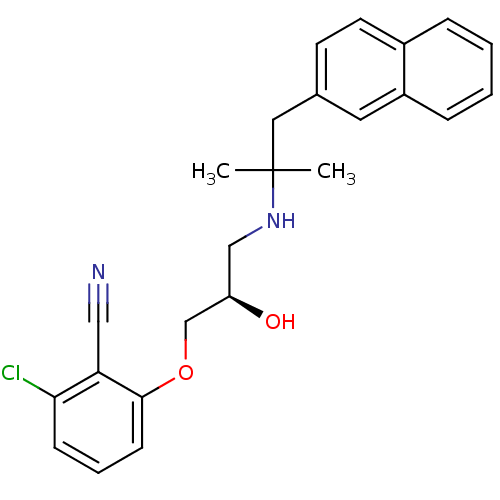 ((R)-2-chloro-6-(2-hydroxy-3-(2-methyl-1-(naphthale...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505558 (CHEMBL4514842) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320009 ((R)-1-((R)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320018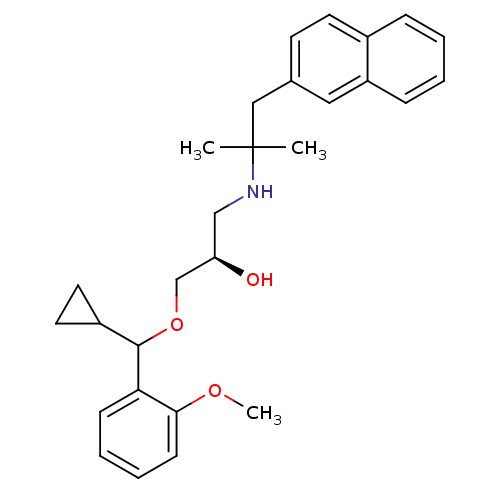 ((2R)-1-(cyclopropyl(2-methoxyphenyl)methoxy)-3-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320011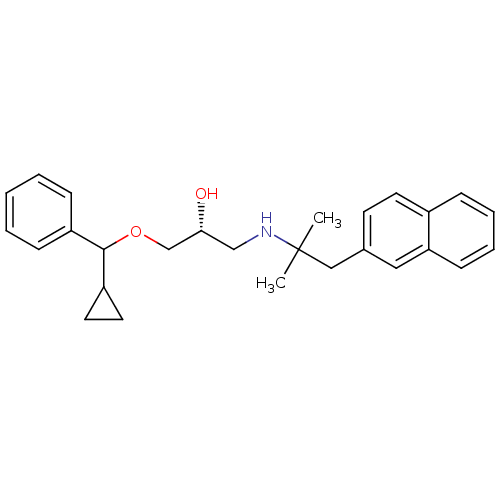 ((2R)-1-(cyclopropyl(phenyl)methoxy)-3-(2-methyl-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320012 ((2R)-1-(cyclobutyl(phenyl)methoxy)-3-(2-methyl-1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50505558 (CHEMBL4514842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using fluorogenic substrate cbz-FR-AMC monitored for 90 to 120 mins by spectrophotometry | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50505556 (CHEMBL4468530) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Jordan Curated by ChEMBL | Assay Description Inhibition of recombinant Trypanosoma cruzi Cruzain expressed in Pichia pastoris using Z-Phe-Arg-AMC substrate incubated for 30 mins by fluorescence ... | J Med Chem 62: 9026-9044 (2019) Article DOI: 10.1021/acs.jmedchem.9b00294 BindingDB Entry DOI: 10.7270/Q2H998GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320019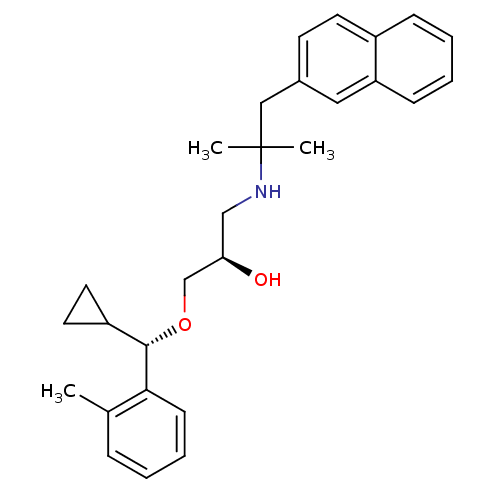 ((R)-1-((S)-cyclopropyl(o-tolyl)methoxy)-3-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50319998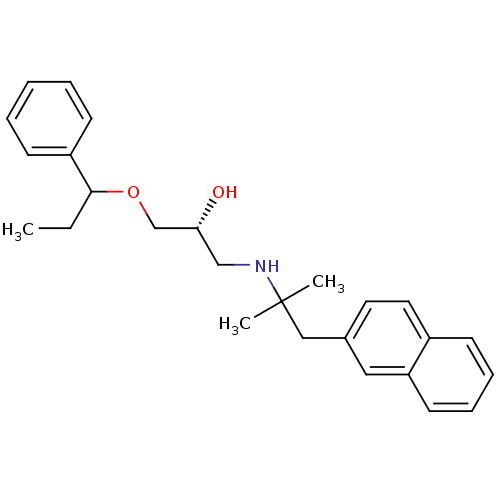 ((2R)-1-(2-methyl-1-(naphthalen-2-yl)propan-2-ylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50320008 ((2R)-1-(1-(2-methoxyphenyl)ethoxy)-3-(2-methyl-1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in rat PC12 cells transfected with zif promoter/luciferase by reporter gene assay | Bioorg Med Chem Lett 20: 3809-13 (2010) Article DOI: 10.1016/j.bmcl.2010.04.035 BindingDB Entry DOI: 10.7270/Q2765FGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 214 total ) | Next | Last >> |