 Found 803 hits with Last Name = 'hird' and Initial = 'aw'
Found 803 hits with Last Name = 'hird' and Initial = 'aw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508937

(CHEMBL4448046)Show SMILES Cn1nc2CSCc3nn(C)c(Cl)c3-c3c(Cl)ccc4c(CCCOc5cc(SCc1c2)cc1ccccc51)c(C(O)=O)n(C)c34 Show InChI InChI=1S/C34H31Cl2N5O3S2/c1-39-31-25-10-11-26(35)29(31)30-27(38-41(3)33(30)36)18-45-16-20-14-21(40(2)37-20)17-46-22-13-19-7-4-5-8-23(19)28(15-22)44-12-6-9-24(25)32(39)34(42)43/h4-5,7-8,10-11,13-15H,6,9,12,16-18H2,1-3H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203869
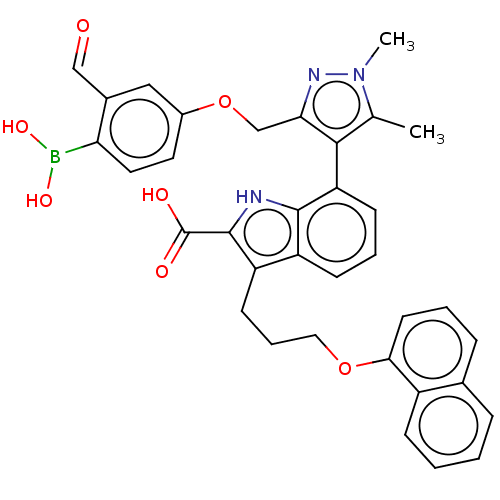
(7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...)Show SMILES Cc1c(c(COc2ccc(B(O)O)c(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C35H32BN3O7/c1-21-32(30(38-39(21)2)20-46-24-15-16-29(36(43)44)23(18-24)19-40)28-12-6-11-26-27(34(35(41)42)37-33(26)28)13-7-17-45-31-14-5-9-22-8-3-4-10-25(22)31/h3-6,8-12,14-16,18-19,37,43-44H,7,13,17,20H2,1-2H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | -51.5 | 3.40 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203875
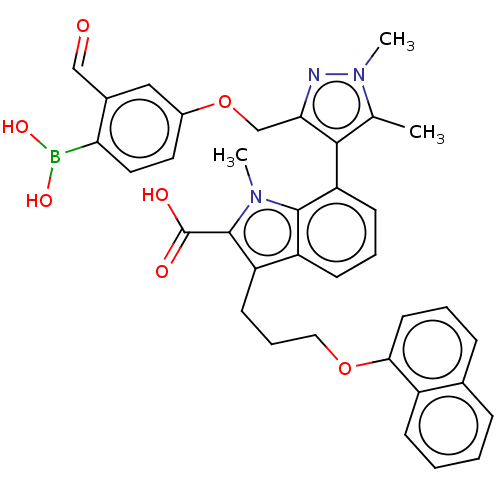
(7-(3-((4-Borono-3-formylphenoxy)methyl)-1,5-dimeth...)Show SMILES Cc1c(c(COc2ccc(B(O)O)c(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c(C(O)=O)n(C)c12 |(.34,-2.6,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;2.77,.94,;3.86,-.15,;3.86,2.02,;.32,-.31,;1.22,-1.52,;-1.14,.17,)| Show InChI InChI=1S/C36H34BN3O7/c1-22-33(31(38-40(22)3)21-47-25-16-17-30(37(44)45)24(19-25)20-41)29-13-7-12-27-28(35(36(42)43)39(2)34(27)29)14-8-18-46-32-15-6-10-23-9-4-5-11-26(23)32/h4-7,9-13,15-17,19-20,44-45H,8,14,18,21H2,1-3H3,(H,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | -50.7 | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203870
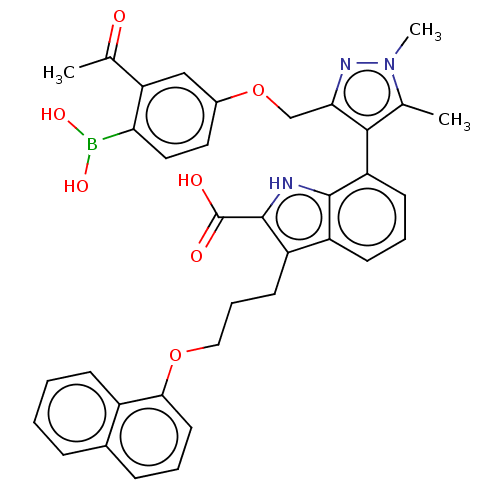
(7-(3-((3-Acetyl-4-boronophenoxy)methyl)-1,5-dimeth...)Show SMILES CC(=O)c1cc(OCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)ccc1B(O)O |(-13.02,-2.49,;-11.53,-2.88,;-12.16,-4.44,;-10.2,-2.11,;-8.87,-2.88,;-7.53,-2.11,;-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;.05,-1.8,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-11.29,.51,;-10.89,2,;-12.78,.91,)| Show InChI InChI=1S/C36H34BN3O7/c1-21-33(31(39-40(21)3)20-47-24-16-17-30(37(44)45)29(19-24)22(2)41)28-13-7-12-26-27(35(36(42)43)38-34(26)28)14-8-18-46-32-15-6-10-23-9-4-5-11-25(23)32/h4-7,9-13,15-17,19,38,44-45H,8,14,18,20H2,1-3H3,(H,42,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | -50.7 | 4.70 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203876
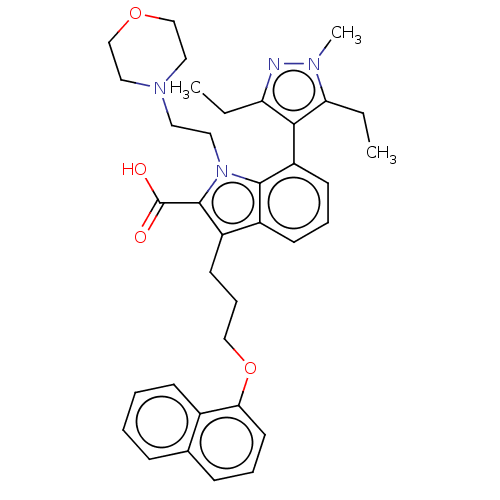
(Mcl-1 inhibitor 12)Show SMILES CCc1nn(C)c(CC)c1-c1cccc2c(CCCOc3cccc4ccccc34)c(C(O)=O)n(CCN3CCOCC3)c12 |(-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;-.02,-2.6,;.3,-4.24,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;2.77,.94,;3.86,-.15,;3.86,2.02,;.32,-.31,;1.22,-1.52,;2.76,-1.52,;3.16,-3.01,;1.82,-3.78,;1.82,-5.32,;3.16,-6.09,;4.49,-5.32,;4.49,-3.78,;-1.14,.17,)| Show InChI InChI=1S/C36H42N4O4/c1-4-30-33(31(5-2)38(3)37-30)29-15-9-14-27-28(16-10-22-44-32-17-8-12-25-11-6-7-13-26(25)32)35(36(41)42)40(34(27)29)19-18-39-20-23-43-24-21-39/h6-9,11-15,17H,4-5,10,16,18-24H2,1-3H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | -50.0 | 5.96 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203871
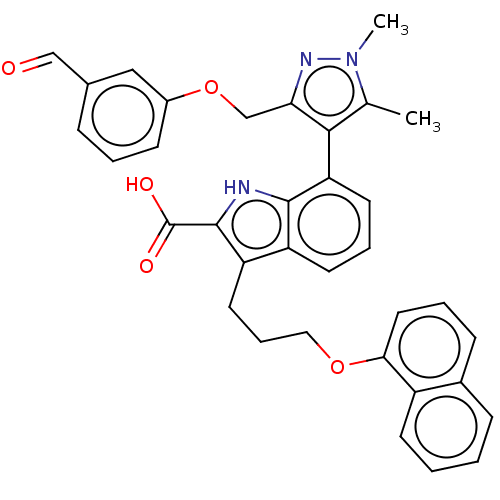
(7-(3-((3-formylphenoxy)methyl)-1,5-dimethyl-1H-pyr...)Show SMILES Cc1c(c(COc2cccc(C=O)c2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-10.2,-2.11,;-11.53,-2.88,;-10.76,-4.22,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C35H31N3O5/c1-22-32(30(37-38(22)2)21-43-25-12-5-9-23(19-25)20-39)29-15-7-14-27-28(34(35(40)41)36-33(27)29)16-8-18-42-31-17-6-11-24-10-3-4-13-26(24)31/h3-7,9-15,17,19-20,36H,8,16,18,21H2,1-2H3,(H,40,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | -44.3 | 59.5 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508939

(CHEMBL4443085)Show SMILES Cc1cc(CSCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)nn1C |(21.19,-26.33,;22.68,-25.93,;23.37,-24.56,;24.9,-24.79,;25.99,-23.71,;27.47,-24.11,;28.56,-23.02,;30.05,-23.42,;30.52,-24.88,;32.06,-24.88,;32.84,-26.21,;32.54,-23.42,;34.03,-23.02,;31.29,-22.51,;31.29,-20.97,;29.96,-20.21,;29.96,-18.67,;31.29,-17.89,;32.62,-18.66,;34.09,-18.19,;34.49,-16.7,;35.97,-16.3,;36.37,-14.82,;37.86,-14.42,;38.26,-12.93,;37.17,-11.83,;37.57,-10.35,;39.06,-9.95,;40.14,-11.03,;41.62,-10.63,;42.72,-11.71,;42.34,-13.2,;40.85,-13.61,;39.75,-12.52,;34.99,-19.43,;34.09,-20.68,;32.62,-20.2,;36.53,-19.43,;37.3,-20.76,;37.3,-18.1,;25.13,-26.32,;23.76,-27.02,;23.36,-28.5,)| Show InChI InChI=1S/C34H35N5O3S/c1-21-18-24(36-38(21)3)19-43-20-29-31(22(2)39(4)37-29)28-14-8-13-26-27(33(34(40)41)35-32(26)28)15-9-17-42-30-16-7-11-23-10-5-6-12-25(23)30/h5-8,10-14,16,18,35H,9,15,17,19-20H2,1-4H3,(H,40,41) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203873
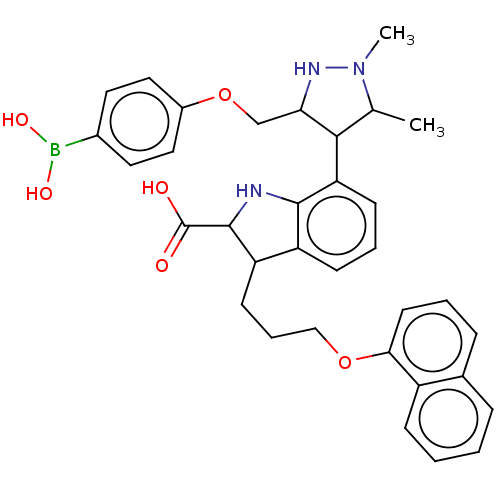
(7-(3-((4-Boronophenoxy)methyl)-1,5-dimethyl-1H-pyr...)Show SMILES CC1C(C(COc2ccc(cc2)B(O)O)NN1C)c1cccc2C(CCCOc3cccc4ccccc34)C(Nc12)C(O)=O Show InChI InChI=1S/C34H38BN3O6/c1-21-31(29(37-38(21)2)20-44-24-17-15-23(16-18-24)35(41)42)28-12-6-11-26-27(33(34(39)40)36-32(26)28)13-7-19-43-30-14-5-9-22-8-3-4-10-25(22)30/h3-6,8-12,14-18,21,27,29,31,33,36-37,41-42H,7,13,19-20H2,1-2H3,(H,39,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | -41.8 | 162 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508950

(CHEMBL4472439)Show SMILES C[C@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203872
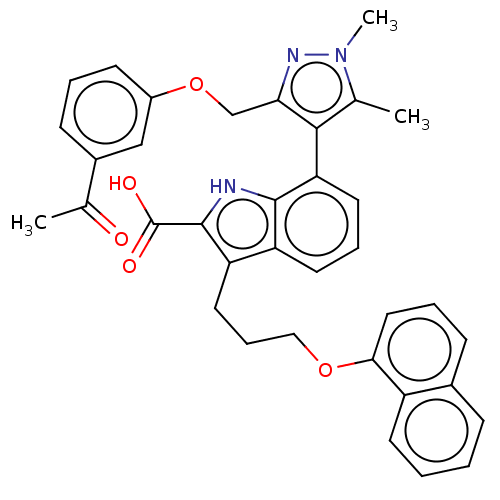
(7-(3-((3-Acetylphenoxy)methyl)-1,5-dimethyl-1H-pyr...)Show SMILES CC(=O)c1cccc(OCc2nn(C)c(C)c2-c2cccc3c(CCCOc4cccc5ccccc45)c([nH]c23)C(O)=O)c1 |(-12.87,-2.11,;-11.53,-2.88,;-12.01,-4.36,;-10.2,-2.11,;-10.2,-.57,;-8.87,.2,;-7.53,-.57,;-7.53,-2.11,;-6.2,-2.88,;-5.11,-1.8,;-3.78,-2.57,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-1.28,-2.57,;.05,-1.8,;-2.53,-1.66,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,;-8.87,-2.88,)| Show InChI InChI=1S/C36H33N3O5/c1-22-33(31(38-39(22)3)21-44-26-13-6-12-25(20-26)23(2)40)30-16-8-15-28-29(35(36(41)42)37-34(28)30)17-9-19-43-32-18-7-11-24-10-4-5-14-27(24)32/h4-8,10-16,18,20,37H,9,17,19,21H2,1-3H3,(H,41,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | -40.9 | 237 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327]
(Homo sapiens (Human)) | BDBM203874
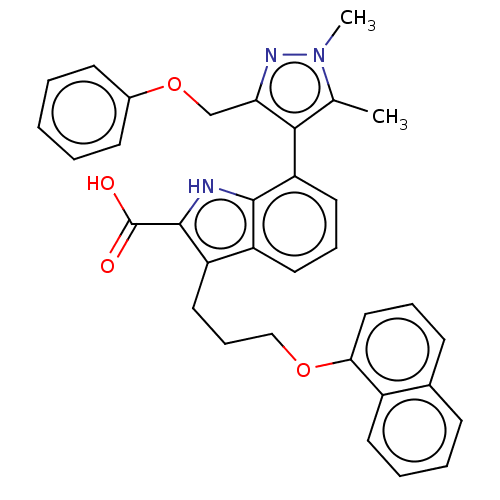
(Mcl-1 inhibitor 10)Show SMILES Cc1c(c(COc2ccccc2)nn1C)-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(.05,-1.8,;-1.28,-2.57,;-2.53,-1.66,;-3.78,-2.57,;-5.11,-1.8,;-6.2,-2.88,;-7.53,-2.11,;-7.53,-.57,;-8.87,.2,;-10.2,-.57,;-10.2,-2.11,;-8.87,-2.88,;-3.3,-4.03,;-1.76,-4.03,;-.67,-5.12,;-2.48,-.6,;-3.81,.17,;-3.81,1.71,;-2.48,2.48,;-1.14,1.71,;.32,2.18,;-.08,3.67,;1.01,4.76,;.62,6.24,;1.7,7.33,;1.31,8.82,;-.03,9.59,;-.03,11.13,;1.31,11.9,;2.64,11.13,;3.97,11.9,;5.31,11.13,;5.31,9.59,;3.97,8.82,;2.64,9.59,;1.23,.94,;.32,-.31,;-1.14,.17,;2.77,.94,;3.86,-.15,;3.86,2.02,)| Show InChI InChI=1S/C34H31N3O4/c1-22-31(29(36-37(22)2)21-41-24-13-4-3-5-14-24)28-17-9-16-26-27(33(34(38)39)35-32(26)28)18-10-20-40-30-19-8-12-23-11-6-7-15-25(23)30/h3-9,11-17,19,35H,10,18,20-21H2,1-2H3,(H,38,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 104 | -39.7 | 383 | n/a | n/a | n/a | n/a | 7.4 | 24 |
Oncology Innovative Medicines Unit
| Assay Description
TR-FRET assay was used to assess the ability of compounds to disrupt the interaction between recombinant human Mcl-1 and a labeled BIM peptide probe.... |
Nat Chem Biol 12: 931-936 (2016)
Article DOI: 10.1038/nchembio.2174
BindingDB Entry DOI: 10.7270/Q2FN1513 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508947
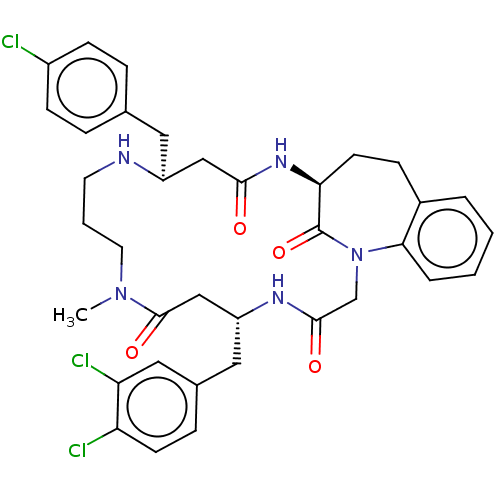
(CHEMBL4460550)Show SMILES CN1CCCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC1=O)C2=O |r| Show InChI InChI=1S/C36H40Cl3N5O4/c1-43-16-4-15-40-27(17-23-7-11-26(37)12-8-23)20-33(45)42-31-14-10-25-5-2-3-6-32(25)44(36(31)48)22-34(46)41-28(21-35(43)47)18-24-9-13-29(38)30(39)19-24/h2-3,5-9,11-13,19,27-28,31,40H,4,10,14-18,20-22H2,1H3,(H,41,46)(H,42,45)/t27-,28+,31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508938

(CHEMBL1984039)Show SMILES Cc1nn(C)c(C)c1-c1cccc2c(CCCOc3cccc4ccccc34)c([nH]c12)C(O)=O |(-6.44,-15.46,;-4.9,-15.46,;-3.99,-16.71,;-2.53,-16.23,;-1.28,-17.14,;-2.53,-14.69,;-1.28,-13.79,;-3.99,-14.22,;-4.47,-12.75,;-5.97,-12.43,;-6.45,-10.97,;-5.42,-9.83,;-3.91,-10.15,;-2.67,-9.24,;-2.67,-7.7,;-1.33,-6.93,;-1.33,-5.39,;,-4.62,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;2.67,-3.08,;1.33,-2.31,;-1.42,-10.15,;-1.9,-11.61,;-3.44,-11.61,;.04,-9.67,;1.19,-10.7,;.36,-8.16,)| Show InChI InChI=1S/C28H27N3O3/c1-17-25(18(2)31(3)30-17)23-13-7-12-21-22(27(28(32)33)29-26(21)23)14-8-16-34-24-15-6-10-19-9-4-5-11-20(19)24/h4-7,9-13,15,29H,8,14,16H2,1-3H3,(H,32,33) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508940
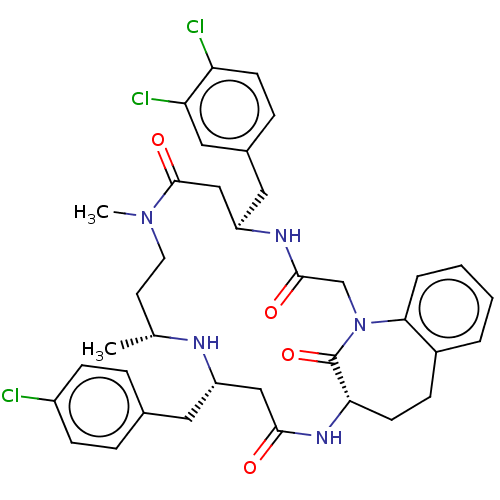
(CHEMBL4582512)Show SMILES C[C@@H]1CCN(C)C(=O)C[C@@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CN2c3ccccc3CC[C@H](NC(=O)C[C@H](Cc3ccc(Cl)cc3)N1)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-44(2)36(48)21-29(18-25-9-13-30(39)31(40)19-25)42-35(47)22-45-33-6-4-3-5-26(33)10-14-32(37(45)49)43-34(46)20-28(41-23)17-24-7-11-27(38)12-8-24/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23-,28+,29-,32+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 739 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508942
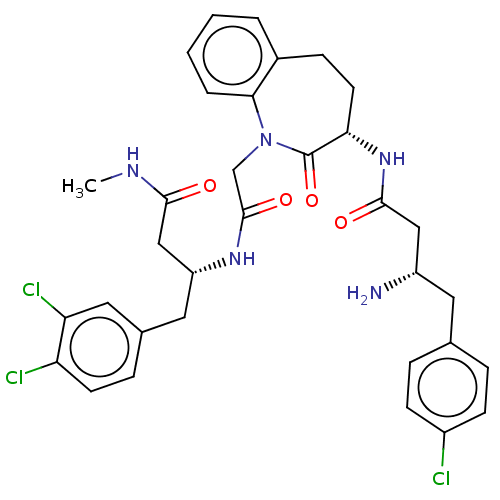
(CHEMBL4449849)Show SMILES CNC(=O)C[C@@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)CN1c2ccccc2CC[C@H](NC(=O)C[C@@H](N)Cc2ccc(Cl)cc2)C1=O |r| Show InChI InChI=1S/C33H36Cl3N5O4/c1-38-30(42)18-25(15-21-8-12-26(35)27(36)16-21)39-32(44)19-41-29-5-3-2-4-22(29)9-13-28(33(41)45)40-31(43)17-24(37)14-20-6-10-23(34)11-7-20/h2-8,10-12,16,24-25,28H,9,13-15,17-19,37H2,1H3,(H,38,42)(H,39,44)(H,40,43)/t24-,25+,28-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508941

(CHEMBL4442625)Show SMILES CC1CCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC(=O)N1C)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-41-28(17-24-7-11-27(38)12-8-24)20-34(46)43-32-14-10-26-5-3-4-6-33(26)45(37(32)49)22-35(47)42-29(21-36(48)44(23)2)18-25-9-13-30(39)31(40)19-25/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23?,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508941

(CHEMBL4442625)Show SMILES CC1CCN[C@@H](Cc2ccc(Cl)cc2)CC(=O)N[C@H]2CCc3ccccc3N(CC(=O)N[C@H](Cc3ccc(Cl)c(Cl)c3)CC(=O)N1C)C2=O |r| Show InChI InChI=1S/C37H42Cl3N5O4/c1-23-15-16-41-28(17-24-7-11-27(38)12-8-24)20-34(46)43-32-14-10-26-5-3-4-6-33(26)45(37(32)49)22-35(47)42-29(21-36(48)44(23)2)18-25-9-13-30(39)31(40)19-25/h3-9,11-13,19,23,28-29,32,41H,10,14-18,20-22H2,1-2H3,(H,42,47)(H,43,46)/t23?,28-,29+,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508936
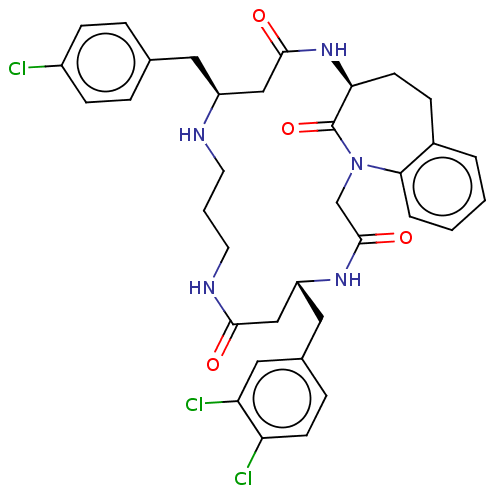
(CHEMBL4590345)Show SMILES Clc1ccc(C[C@H]2CC(=O)N[C@H]3CCc4ccccc4N(CC(=O)N[C@H](Cc4ccc(Cl)c(Cl)c4)CC(=O)NCCCN2)C3=O)cc1 |r| Show InChI InChI=1S/C35H38Cl3N5O4/c36-25-10-6-22(7-11-25)16-26-19-33(45)42-30-13-9-24-4-1-2-5-31(24)43(35(30)47)21-34(46)41-27(20-32(44)40-15-3-14-39-26)17-23-8-12-28(37)29(38)18-23/h1-2,4-8,10-12,18,26-27,30,39H,3,9,13-17,19-21H2,(H,40,44)(H,41,46)(H,42,45)/t26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50539187

(CHEMBL4632769)Show SMILES Cc1cc(C)c(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1ncc[nH]1 Show InChI InChI=1S/C24H22N4O2/c1-16-13-17(2)22(14-21(16)23-26-11-12-27-23)28-24(29)18-6-8-20(9-7-18)30-15-19-5-3-4-10-25-19/h3-14H,15H2,1-2H3,(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50539188
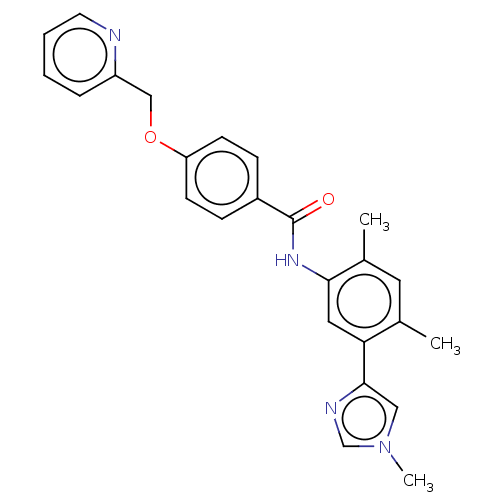
(CHEMBL4637222)Show SMILES Cc1cc(C)c(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1cn(C)cn1 Show InChI InChI=1S/C25H24N4O2/c1-17-12-18(2)23(13-22(17)24-14-29(3)16-27-24)28-25(30)19-7-9-21(10-8-19)31-15-20-6-4-5-11-26-20/h4-14,16H,15H2,1-3H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50249522

(2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...)Show SMILES CS(=O)(=O)c1ccc(C(=O)Nc2ccc(Cl)c(c2)-c2ccccn2)c(Cl)c1 Show InChI InChI=1S/C19H14Cl2N2O3S/c1-27(25,26)13-6-7-14(17(21)11-13)19(24)23-12-5-8-16(20)15(10-12)18-4-2-3-9-22-18/h2-11H,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388722
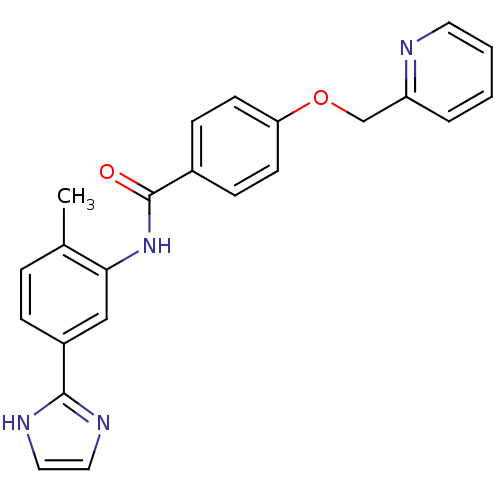
(CHEMBL2059865)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1ncc[nH]1 Show InChI InChI=1S/C23H20N4O2/c1-16-5-6-18(22-25-12-13-26-22)14-21(16)27-23(28)17-7-9-20(10-8-17)29-15-19-4-2-3-11-24-19/h2-14H,15H2,1H3,(H,25,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115227
BindingDB Entry DOI: 10.7270/Q2CF9TNC |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388706
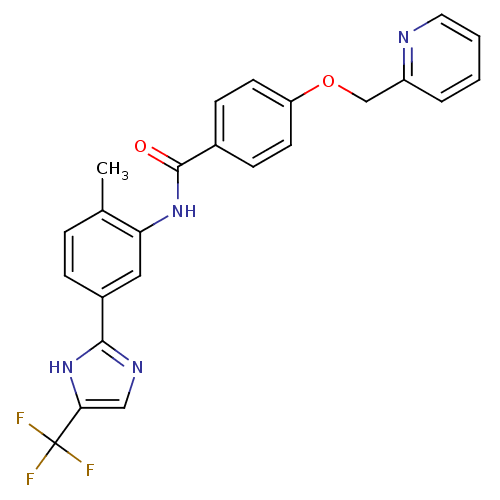
(CHEMBL2059863)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1ncc([nH]1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O2/c1-15-5-6-17(22-29-13-21(31-22)24(25,26)27)12-20(15)30-23(32)16-7-9-19(10-8-16)33-14-18-4-2-3-11-28-18/h2-13H,14H2,1H3,(H,29,31)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388707

(CHEMBL2059864)Show SMILES Cc1c[nH]c(n1)-c1ccc(C)c(NC(=O)c2ccc(OCc3ccccn3)cc2)c1 Show InChI InChI=1S/C24H22N4O2/c1-16-6-7-19(23-26-14-17(2)27-23)13-22(16)28-24(29)18-8-10-21(11-9-18)30-15-20-5-3-4-12-25-20/h3-14H,15H2,1-2H3,(H,26,27)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239363

(CHEMBL4094351)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)Nc4c(OC\C=C\CO2)c3)n2ncc(C#N)c2n1 |r,t:26| Show InChI InChI=1S/C25H25N7O4/c26-11-16-12-27-32-22-10-21(29-25(16)32)31-13-19(9-18(31)14-33)35-5-1-2-6-36-20-8-17(28-22)7-15-3-4-23(34)30-24(15)20/h1-2,7-8,10,12,18-19,28,33H,3-6,9,13-14H2,(H,30,34)/b2-1+/t18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Bcl6 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454567

(US10717746, Example 48)Show SMILES CC(=O)N[C@@H]1CC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(Cl)cn1 |r| Show InChI InChI=1S/C21H26ClN5O2/c1-12(28)25-14-5-4-13(6-14)20(29)26-19-7-15(17(22)10-23-19)16-9-24-27-11-21(2,3)8-18(16)27/h7,9-10,13-14H,4-6,8,11H2,1-3H3,(H,25,28)(H,23,26,29)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588261

(CHEMBL5170524)Show SMILES COc1cc(ccc1-c1ccnc2[nH]c(cc12)C1CCNCC1)C(=O)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588290
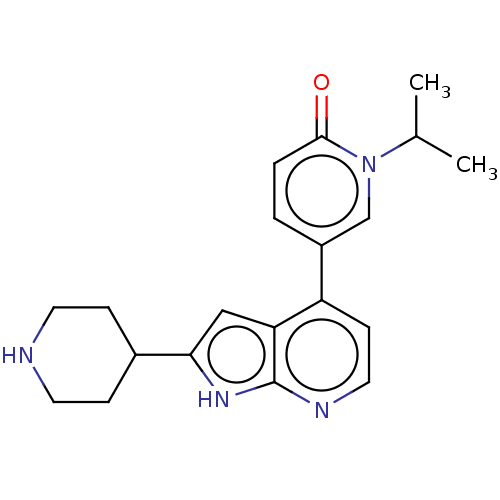
(CHEMBL5191725)Show SMILES CC(C)n1cc(ccc1=O)-c1ccnc2[nH]c(cc12)C1CCNCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454570
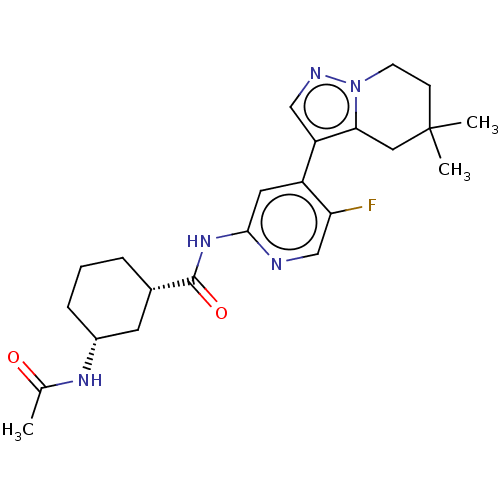
(US10717746, Example 51)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CCC(C)(C)Cc23)c(F)cn1 |r| Show InChI InChI=1S/C23H30FN5O2/c1-14(30)27-16-6-4-5-15(9-16)22(31)28-21-10-17(19(24)13-25-21)18-12-26-29-8-7-23(2,3)11-20(18)29/h10,12-13,15-16H,4-9,11H2,1-3H3,(H,27,30)(H,25,28,31)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM167534
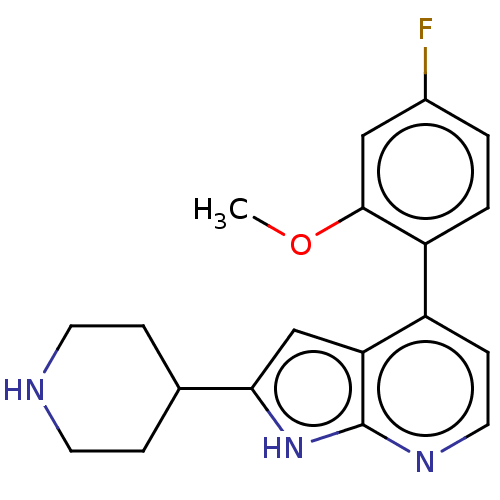
(US9073922, 18 | US9796708, Example 18)Show InChI InChI=1S/C19H20FN3O/c1-24-18-10-13(20)2-3-15(18)14-6-9-22-19-16(14)11-17(23-19)12-4-7-21-8-5-12/h2-3,6,9-12,21H,4-5,7-8H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588255
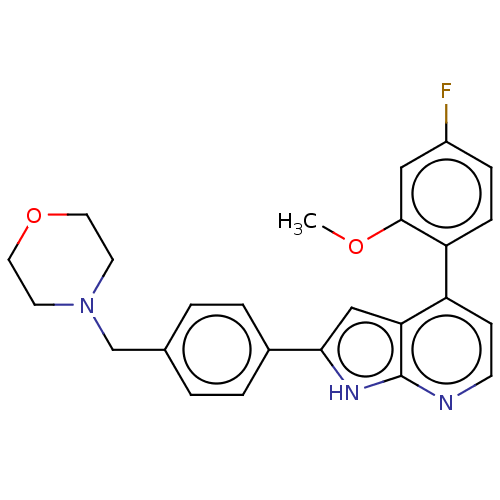
(CHEMBL5170195)Show SMILES COc1cc(F)ccc1-c1ccnc2[nH]c(cc12)-c1ccc(CN2CCOCC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588262

(CHEMBL5197170)Show SMILES CC(C)n1cc(ccc1=O)-c1ccnc2[nH]c(cc12)C1CCN(CC(=O)N2CCCCC2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588263
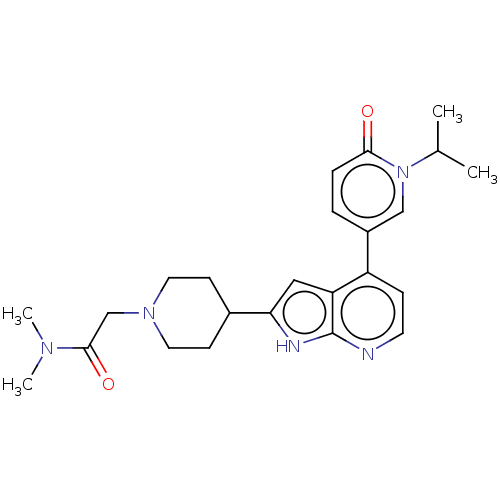
(CHEMBL5198038)Show SMILES CC(C)n1cc(ccc1=O)-c1ccnc2[nH]c(cc12)C1CCN(CC(=O)N(C)C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588264

(CHEMBL5207871)Show SMILES CC(C)n1cc(ccc1=O)-c1ccnc2[nH]c(cc12)C1CCN(CC(=O)N2CCCC2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50001129

(CHEMBL3236390)Show SMILES [H][C@]12C[C@@]3([H])CCN(C[C@@]3([H])N1C(=O)[C@H](CCC2)NC(=O)[C@H](C)NC)C(=O)C(OCC#CC#CCOC(C(=O)N1CC[C@]2([H])C[C@]3([H])CCC[C@H](NC(=O)[C@H](C)NC)C(=O)N3[C@]2([H])C1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C54H70N8O8/c1-35(55-3)49(63)57-43-23-15-21-41-31-39-25-27-59(33-45(39)61(41)51(43)65)53(67)47(37-17-9-7-10-18-37)69-29-13-5-6-14-30-70-48(38-19-11-8-12-20-38)54(68)60-28-26-40-32-42-22-16-24-44(58-50(64)36(2)56-4)52(66)62(42)46(40)34-60/h7-12,17-20,35-36,39-48,55-56H,15-16,21-34H2,1-4H3,(H,57,63)(H,58,64)/t35-,36-,39+,40+,41-,42-,43-,44-,45+,46+,47?,48?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 domain (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 1820-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.016
BindingDB Entry DOI: 10.7270/Q2416ZJ7 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50001129

(CHEMBL3236390)Show SMILES [H][C@]12C[C@@]3([H])CCN(C[C@@]3([H])N1C(=O)[C@H](CCC2)NC(=O)[C@H](C)NC)C(=O)C(OCC#CC#CCOC(C(=O)N1CC[C@]2([H])C[C@]3([H])CCC[C@H](NC(=O)[C@H](C)NC)C(=O)N3[C@]2([H])C1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C54H70N8O8/c1-35(55-3)49(63)57-43-23-15-21-41-31-39-25-27-59(33-45(39)61(41)51(43)65)53(67)47(37-17-9-7-10-18-37)69-29-13-5-6-14-30-70-48(38-19-11-8-12-20-38)54(68)60-28-26-40-32-42-22-16-24-44(58-50(64)36(2)56-4)52(66)62(42)46(40)34-60/h7-12,17-20,35-36,39-48,55-56H,15-16,21-34H2,1-4H3,(H,57,63)(H,58,64)/t35-,36-,39+,40+,41-,42-,43-,44-,45+,46+,47?,48?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cIAP1 BIR3 domain (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 1820-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.016
BindingDB Entry DOI: 10.7270/Q2416ZJ7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588263

(CHEMBL5198038)Show SMILES CC(C)n1cc(ccc1=O)-c1ccnc2[nH]c(cc12)C1CCN(CC(=O)N(C)C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588272
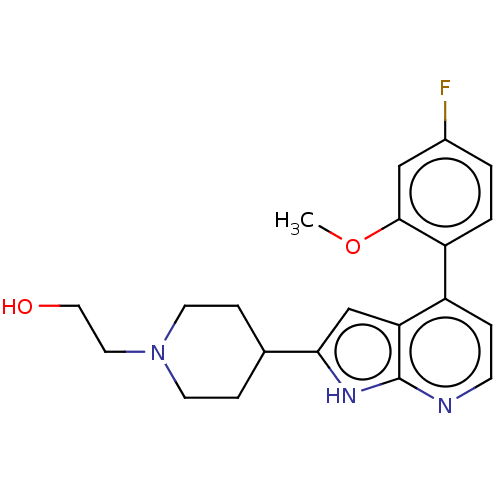
(CHEMBL5180640)Show SMILES COc1cc(F)ccc1-c1ccnc2[nH]c(cc12)C1CCN(CCO)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588266

(CHEMBL5181709)Show SMILES CC(C)n1c(C)nc2ccc(cc12)-c1ccnc2[nH]c(cc12)C1CCN(CC(=O)N(C)C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM454542

(US10717746, Example 25 | US10717746, Example 84)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(F)cn1 |r| Show InChI InChI=1S/C22H28FN5O2/c1-13(29)26-15-6-4-5-14(7-15)21(30)27-20-8-16(18(23)11-24-20)17-10-25-28-12-22(2,3)9-19(17)28/h8,10-11,14-15H,4-7,9,12H2,1-3H3,(H,26,29)(H,24,27,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50001129

(CHEMBL3236390)Show SMILES [H][C@]12C[C@@]3([H])CCN(C[C@@]3([H])N1C(=O)[C@H](CCC2)NC(=O)[C@H](C)NC)C(=O)C(OCC#CC#CCOC(C(=O)N1CC[C@]2([H])C[C@]3([H])CCC[C@H](NC(=O)[C@H](C)NC)C(=O)N3[C@]2([H])C1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C54H70N8O8/c1-35(55-3)49(63)57-43-23-15-21-41-31-39-25-27-59(33-45(39)61(41)51(43)65)53(67)47(37-17-9-7-10-18-37)69-29-13-5-6-14-30-70-48(38-19-11-8-12-20-38)54(68)60-28-26-40-32-42-22-16-24-44(58-50(64)36(2)56-4)52(66)62(42)46(40)34-60/h7-12,17-20,35-36,39-48,55-56H,15-16,21-34H2,1-4H3,(H,57,63)(H,58,64)/t35-,36-,39+,40+,41-,42-,43-,44-,45+,46+,47?,48?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of XIAP BIR3 domain (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 1820-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.016
BindingDB Entry DOI: 10.7270/Q2416ZJ7 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50001129

(CHEMBL3236390)Show SMILES [H][C@]12C[C@@]3([H])CCN(C[C@@]3([H])N1C(=O)[C@H](CCC2)NC(=O)[C@H](C)NC)C(=O)C(OCC#CC#CCOC(C(=O)N1CC[C@]2([H])C[C@]3([H])CCC[C@H](NC(=O)[C@H](C)NC)C(=O)N3[C@]2([H])C1)c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C54H70N8O8/c1-35(55-3)49(63)57-43-23-15-21-41-31-39-25-27-59(33-45(39)61(41)51(43)65)53(67)47(37-17-9-7-10-18-37)69-29-13-5-6-14-30-70-48(38-19-11-8-12-20-38)54(68)60-28-26-40-32-42-22-16-24-44(58-50(64)36(2)56-4)52(66)62(42)46(40)34-60/h7-12,17-20,35-36,39-48,55-56H,15-16,21-34H2,1-4H3,(H,57,63)(H,58,64)/t35-,36-,39+,40+,41-,42-,43-,44-,45+,46+,47?,48?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of XIAP BIR3 domain (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 1820-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.016
BindingDB Entry DOI: 10.7270/Q2416ZJ7 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588273
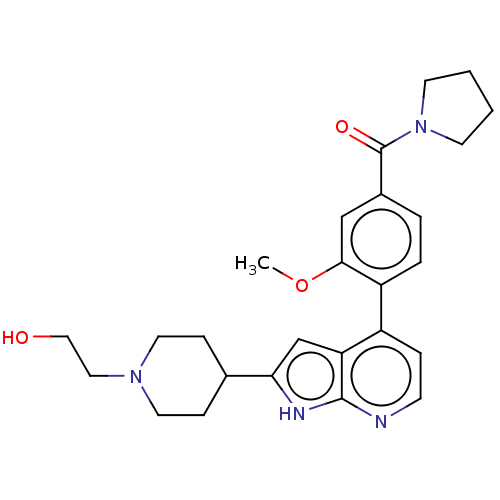
(CHEMBL5192311)Show SMILES COc1cc(ccc1-c1ccnc2[nH]c(cc12)C1CCN(CCO)CC1)C(=O)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588285
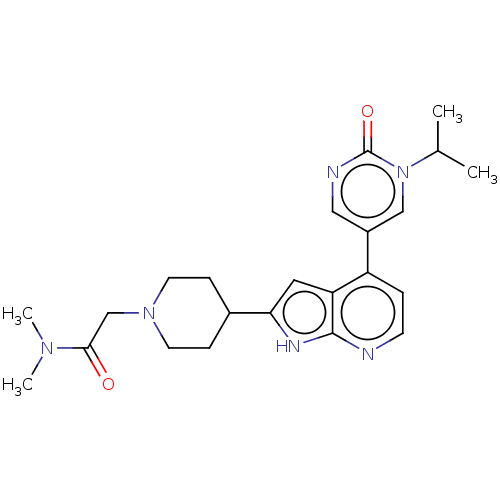
(CHEMBL5179773)Show SMILES CC(C)n1cc(cnc1=O)-c1ccnc2[nH]c(cc12)C1CCN(CC(=O)N(C)C)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50588261

(CHEMBL5170524)Show SMILES COc1cc(ccc1-c1ccnc2[nH]c(cc12)C1CCNCC1)C(=O)N1CCCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01249
BindingDB Entry DOI: 10.7270/Q2F193PM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50387299
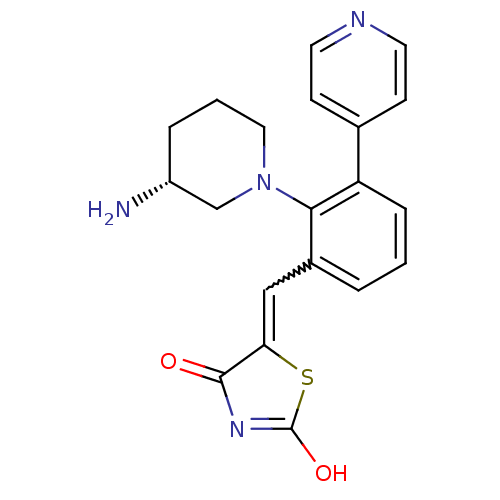
(CHEMBL2048873)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccncc1 |r,w:9.9,c:14| Show InChI InChI=1S/C20H20N4O2S/c21-15-4-2-10-24(12-15)18-14(11-17-19(25)23-20(26)27-17)3-1-5-16(18)13-6-8-22-9-7-13/h1,3,5-9,11,15H,2,4,10,12,21H2,(H,23,25,26)/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 50 uM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528817

(CHEMBL4462530 | US10717746, Example 14 | US2023041...)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(Cl)cn1 |r| Show InChI InChI=1S/C22H28ClN5O2/c1-13(29)26-15-6-4-5-14(7-15)21(30)27-20-8-16(18(23)11-24-20)17-10-25-28-12-22(2,3)9-19(17)28/h8,10-11,14-15H,4-7,9,12H2,1-3H3,(H,26,29)(H,24,27,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50387299

(CHEMBL2048873)Show SMILES N[C@@H]1CCCN(C1)c1c(C=C2SC(O)=NC2=O)cccc1-c1ccncc1 |r,w:9.9,c:14| Show InChI InChI=1S/C20H20N4O2S/c21-15-4-2-10-24(12-15)18-14(11-17-19(25)23-20(26)27-17)3-1-5-16(18)13-6-8-22-9-7-13/h1,3,5-9,11,15H,2,4,10,12,21H2,(H,23,25,26)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using FITC-(AHX)RSRHSSYPAGT-COOH as substrate after 90 mins by mobility shift assay in presence of 5 mM ATP |
Bioorg Med Chem Lett 22: 4599-604 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.098
BindingDB Entry DOI: 10.7270/Q2348MDS |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50388705
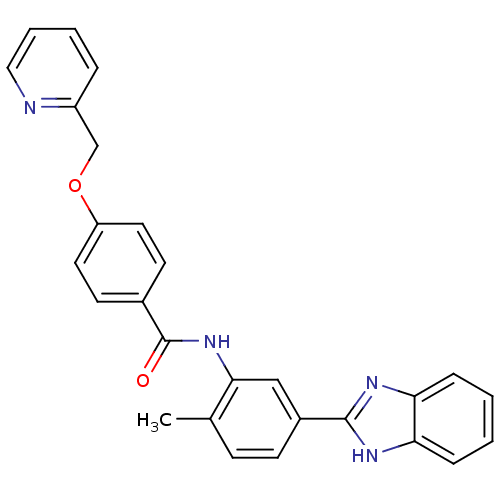
(CHEMBL2059859)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(OCc2ccccn2)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C27H22N4O2/c1-18-9-10-20(26-29-23-7-2-3-8-24(23)30-26)16-25(18)31-27(32)19-11-13-22(14-12-19)33-17-21-6-4-5-15-28-21/h2-16H,17H2,1H3,(H,29,30)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Smo expressed in mouse NIH/3T3 cells after 20 hrs by Gli reporter gene assay |
Bioorg Med Chem Lett 22: 4907-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.104
BindingDB Entry DOI: 10.7270/Q2W37XCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50389803
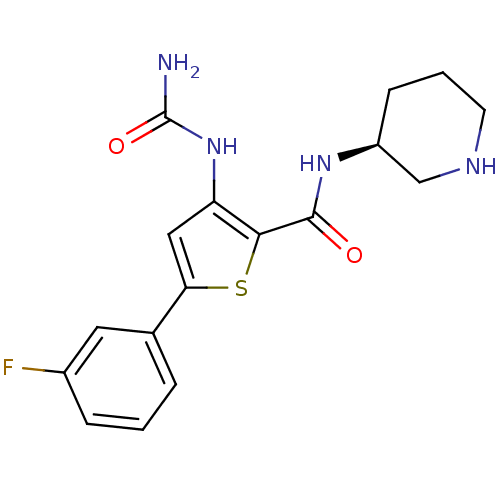
(AZD7762 | CHEMBL2041933)Show SMILES NC(=O)Nc1cc(sc1C(=O)N[C@H]1CCCNC1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C17H19FN4O2S/c18-11-4-1-3-10(7-11)14-8-13(22-17(19)24)15(25-14)16(23)21-12-5-2-6-20-9-12/h1,3-4,7-8,12,20H,2,5-6,9H2,(H,21,23)(H3,19,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Oncology Chemistry, IMED Biotech Unit, AstraZeneca , 35 Gatehouse Drive, Waltham, Massachusetts 02451, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) |
J Med Chem 61: 1061-1073 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01490
BindingDB Entry DOI: 10.7270/Q2PC34T5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data