 Found 38 hits with Last Name = 'hirst' and Initial = 'm'
Found 38 hits with Last Name = 'hirst' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Lactobacillus casei) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498799
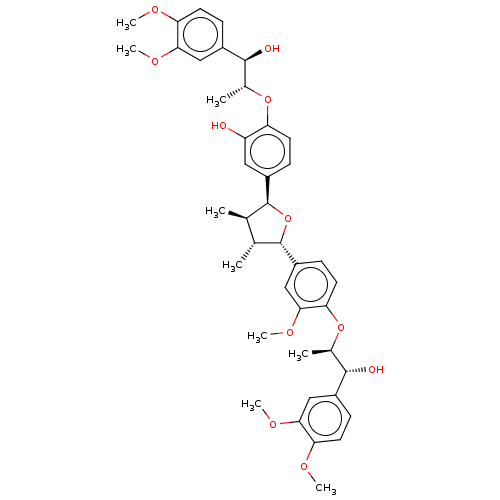
(Manassantin A | Manassantin B1)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1O)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C41H50O11/c1-22-23(2)41(29-13-17-34(37(21-29)49-9)51-25(4)39(44)27-11-16-33(46-6)36(20-27)48-8)52-40(22)28-12-14-31(30(42)18-28)50-24(3)38(43)26-10-15-32(45-5)35(19-26)47-7/h10-25,38-44H,1-9H3/t22-,23-,24-,25-,38+,39+,40+,41+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016789
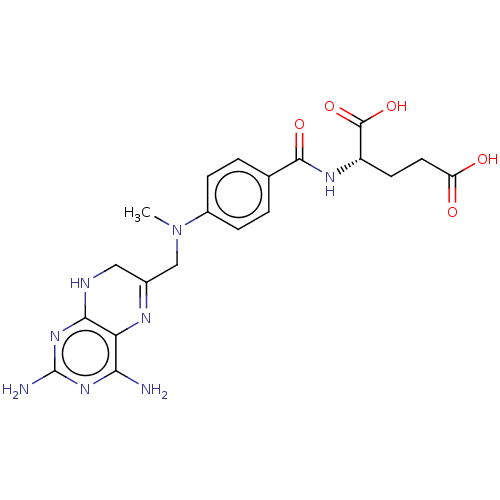
(CHEMBL3273846)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2NC1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C20H24N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,13H,6-9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H5,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Mus musculus) | BDBM50414393
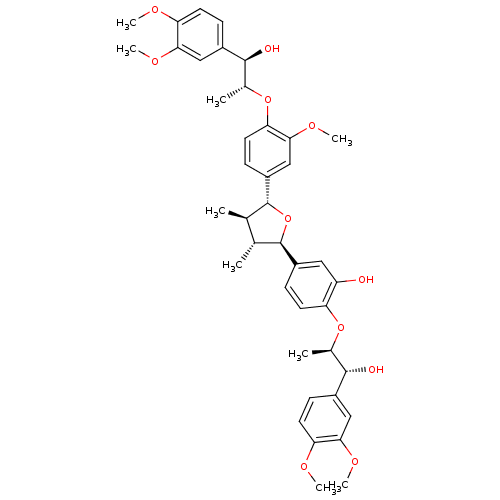
(CHEMBL2021443)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1O)[C@@H]1O[C@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C41H50O11/c1-22-23(2)41(29-13-17-34(37(21-29)49-9)51-25(4)39(44)27-11-16-33(46-6)36(20-27)48-8)52-40(22)28-12-14-31(30(42)18-28)50-24(3)38(43)26-10-15-32(45-5)35(19-26)47-7/h10-25,38-44H,1-9H3/t22-,23-,24-,25-,38+,39+,40-,41-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of CoCl2-induced HIF1alpha expression in mouse 4T1 cells transfected with oxygen-dependent-degradation domain of HIFalpha by luciferase re... |
Bioorg Med Chem Lett 19: 3783-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.071
BindingDB Entry DOI: 10.7270/Q2959HMT |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016790
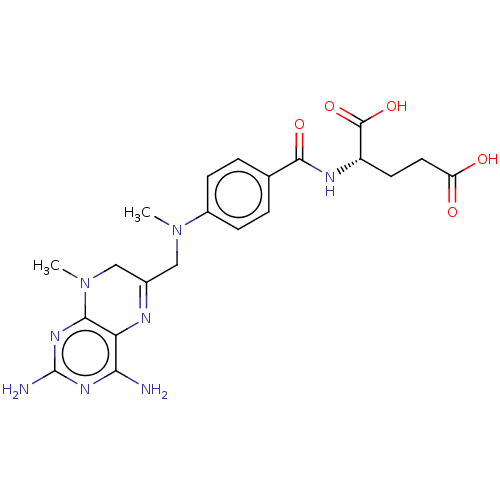
(CHEMBL3273847)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(C)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C21H26N8O5/c1-28(9-12-10-29(2)18-16(24-12)17(22)26-21(23)27-18)13-5-3-11(4-6-13)19(32)25-14(20(33)34)7-8-15(30)31/h3-6,14H,7-10H2,1-2H3,(H,25,32)(H,30,31)(H,33,34)(H4,22,23,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016791
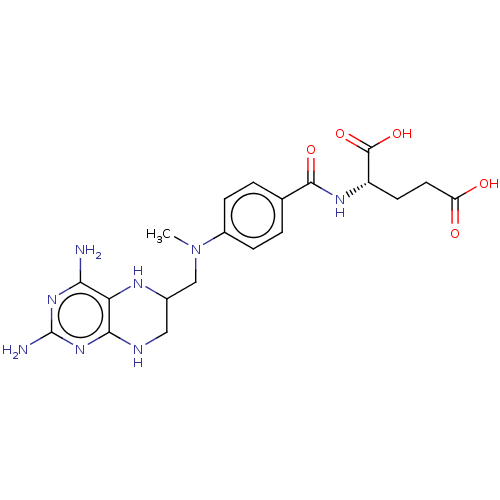
(CHEMBL3273855)Show SMILES CN(CC1CNc2nc(N)nc(N)c2N1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H26N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,11,13,24H,6-9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H5,21,22,23,26,27)/t11?,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498797

(CHEMBL3105545)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C32H40O8/c1-18-19(2)32(40-31(18)22-10-13-25(35-5)28(16-22)37-7)23-11-14-26(29(17-23)38-8)39-20(3)30(33)21-9-12-24(34-4)27(15-21)36-6/h9-20,30-33H,1-8H3/t18-,19-,20-,30+,31+,32+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498805
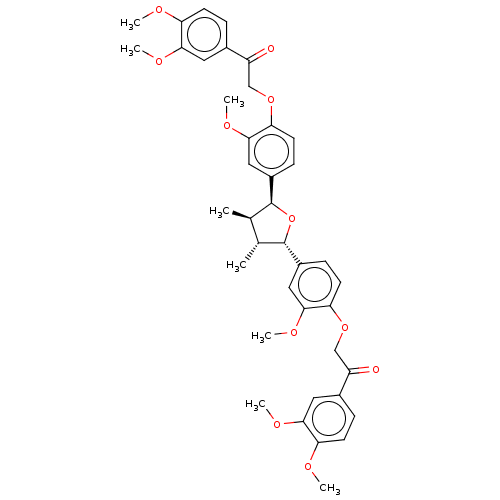
(CHEMBL3623807)Show SMILES COc1ccc(cc1OC)C(=O)COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OCC(=O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C40H44O11/c1-23-24(2)40(28-12-16-34(38(20-28)48-8)50-22-30(42)26-10-14-32(44-4)36(18-26)46-6)51-39(23)27-11-15-33(37(19-27)47-7)49-21-29(41)25-9-13-31(43-3)35(17-25)45-5/h9-20,23-24,39-40H,21-22H2,1-8H3/t23-,24-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498796

(CHEMBL3623808)Show SMILES COc1ccc(cc1OC)[C@@H](O)COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC[C@H](O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C40H48O11/c1-23-24(2)40(28-12-16-34(38(20-28)48-8)50-22-30(42)26-10-14-32(44-4)36(18-26)46-6)51-39(23)27-11-15-33(37(19-27)47-7)49-21-29(41)25-9-13-31(43-3)35(17-25)45-5/h9-20,23-24,29-30,39-42H,21-22H2,1-8H3/t23-,24-,29+,30+,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016801
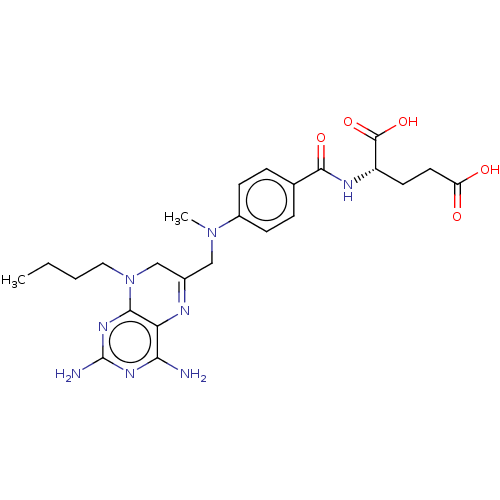
(CHEMBL3273849)Show SMILES CCCCN1CC(CN(C)c2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=Nc2c(N)nc(N)nc12 |r,c:28| Show InChI InChI=1S/C24H32N8O5/c1-3-4-11-32-13-15(27-19-20(25)29-24(26)30-21(19)32)12-31(2)16-7-5-14(6-8-16)22(35)28-17(23(36)37)9-10-18(33)34/h5-8,17H,3-4,9-13H2,1-2H3,(H,28,35)(H,33,34)(H,36,37)(H4,25,26,29,30)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498801
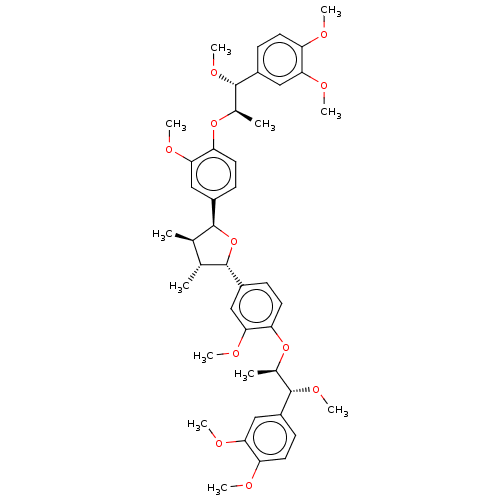
(CHEMBL3623806)Show SMILES CO[C@@H]([C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](OC)c2ccc(OC)c(OC)c2)c(OC)c1)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C44H56O11/c1-25-26(2)42(30-16-20-36(40(22-30)50-10)54-28(4)44(52-12)32-14-18-34(46-6)38(24-32)48-8)55-41(25)29-15-19-35(39(21-29)49-9)53-27(3)43(51-11)31-13-17-33(45-5)37(23-31)47-7/h13-28,41-44H,1-12H3/t25-,26-,27-,28-,41+,42+,43+,44+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498794
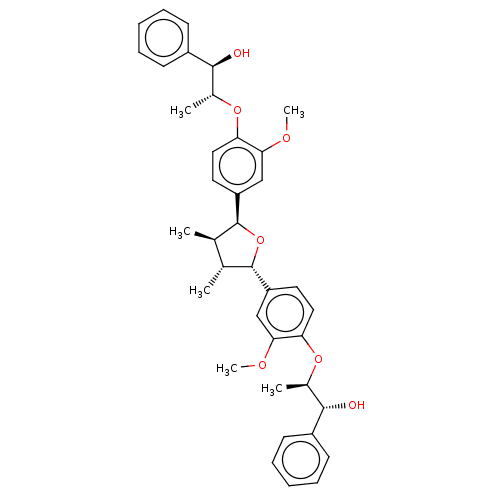
(CHEMBL3623812)Show SMILES COc1cc(ccc1O[C@H](C)[C@H](O)c1ccccc1)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)[C@H](O)c2ccccc2)c(OC)c1 |r| Show InChI InChI=1S/C38H44O7/c1-23-24(2)38(30-18-20-32(34(22-30)42-6)44-26(4)36(40)28-15-11-8-12-16-28)45-37(23)29-17-19-31(33(21-29)41-5)43-25(3)35(39)27-13-9-7-10-14-27/h7-26,35-40H,1-6H3/t23-,24-,25-,26-,35+,36+,37+,38+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498800
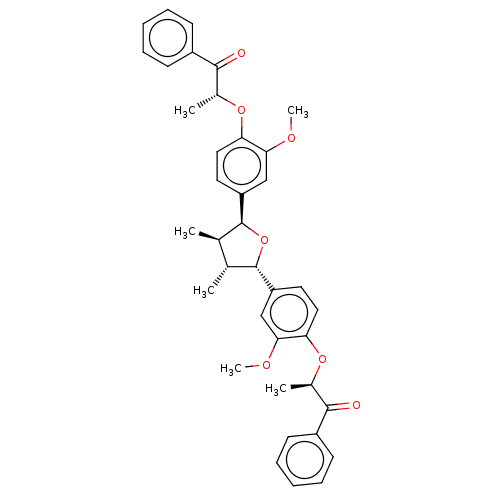
(CHEMBL3623811)Show SMILES COc1cc(ccc1O[C@H](C)C(=O)c1ccccc1)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccccc2)c(OC)c1 |r| Show InChI InChI=1S/C38H40O7/c1-23-24(2)38(30-18-20-32(34(22-30)42-6)44-26(4)36(40)28-15-11-8-12-16-28)45-37(23)29-17-19-31(33(21-29)41-5)43-25(3)35(39)27-13-9-7-10-14-27/h7-26,37-38H,1-6H3/t23-,24-,25-,26-,37+,38+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016796
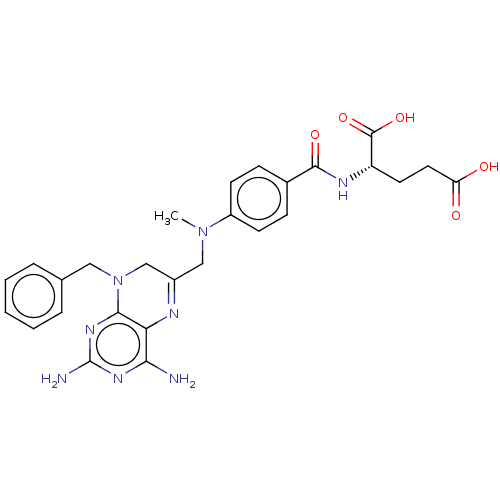
(CHEMBL3273852)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(Cc2ccccc2)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C27H30N8O5/c1-34(19-9-7-17(8-10-19)25(38)31-20(26(39)40)11-12-21(36)37)14-18-15-35(13-16-5-3-2-4-6-16)24-22(30-18)23(28)32-27(29)33-24/h2-10,20H,11-15H2,1H3,(H,31,38)(H,36,37)(H,39,40)(H4,28,29,32,33)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016800
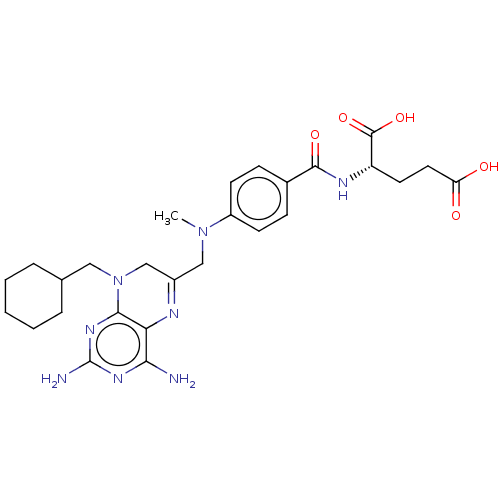
(CHEMBL3273851)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(CC2CCCCC2)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C27H36N8O5/c1-34(19-9-7-17(8-10-19)25(38)31-20(26(39)40)11-12-21(36)37)14-18-15-35(13-16-5-3-2-4-6-16)24-22(30-18)23(28)32-27(29)33-24/h7-10,16,20H,2-6,11-15H2,1H3,(H,31,38)(H,36,37)(H,39,40)(H4,28,29,32,33)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 869 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016802
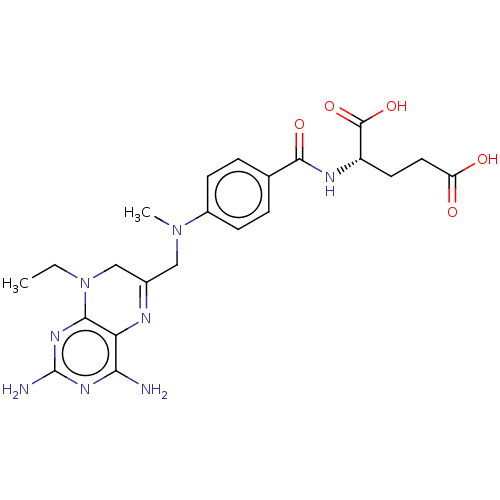
(CHEMBL3273848)Show SMILES CCN1CC(CN(C)c2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=Nc2c(N)nc(N)nc12 |r,c:26| Show InChI InChI=1S/C22H28N8O5/c1-3-30-11-13(25-17-18(23)27-22(24)28-19(17)30)10-29(2)14-6-4-12(5-7-14)20(33)26-15(21(34)35)8-9-16(31)32/h4-7,15H,3,8-11H2,1-2H3,(H,26,33)(H,31,32)(H,34,35)(H4,23,24,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498804
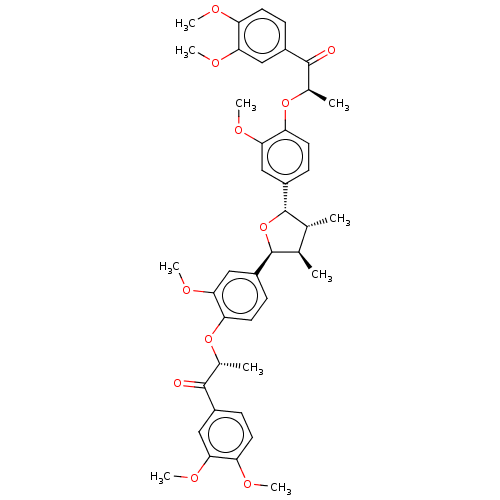
(CHEMBL3623805)Show SMILES COc1ccc(cc1OC)C(=O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C42H48O11/c1-23-24(2)42(30-14-18-34(38(22-30)50-10)52-26(4)40(44)28-12-16-32(46-6)36(20-28)48-8)53-41(23)29-13-17-33(37(21-29)49-9)51-25(3)39(43)27-11-15-31(45-5)35(19-27)47-7/h11-26,41-42H,1-10H3/t23-,24-,25-,26-,41+,42+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016789

(CHEMBL3273846)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2NC1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C20H24N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,13H,6-9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H5,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498803

(CHEMBL3623810)Show SMILES COc1ccc(CCOc2ccc(cc2OC)[C@H]2O[C@@H]([C@H](C)[C@H]2C)c2ccc(OCCc3ccc(OC)c(OC)c3)c(OC)c2)cc1OC |r| Show InChI InChI=1S/C40H48O9/c1-25-26(2)40(30-12-16-34(38(24-30)46-8)48-20-18-28-10-14-32(42-4)36(22-28)44-6)49-39(25)29-11-15-33(37(23-29)45-7)47-19-17-27-9-13-31(41-3)35(21-27)43-5/h9-16,21-26,39-40H,17-20H2,1-8H3/t25-,26-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016804
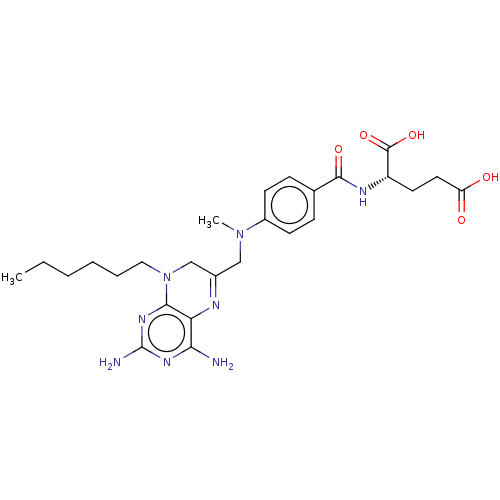
(CHEMBL3273850)Show SMILES CCCCCCN1CC(CN(C)c2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=Nc2c(N)nc(N)nc12 |r,c:30| Show InChI InChI=1S/C26H36N8O5/c1-3-4-5-6-13-34-15-17(29-21-22(27)31-26(28)32-23(21)34)14-33(2)18-9-7-16(8-10-18)24(37)30-19(25(38)39)11-12-20(35)36/h7-10,19H,3-6,11-15H2,1-2H3,(H,30,37)(H,35,36)(H,38,39)(H4,27,28,31,32)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498795
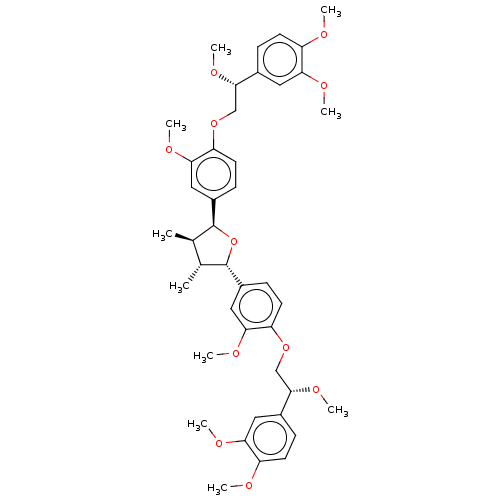
(CHEMBL3623809)Show SMILES CO[C@@H](COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC[C@H](OC)c2ccc(OC)c(OC)c2)c(OC)c1)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C42H52O11/c1-25-26(2)42(30-14-18-34(38(22-30)48-8)52-24-40(50-10)28-12-16-32(44-4)36(20-28)46-6)53-41(25)29-13-17-33(37(21-29)47-7)51-23-39(49-9)27-11-15-31(43-3)35(19-27)45-5/h11-22,25-26,39-42H,23-24H2,1-10H3/t25-,26-,39+,40+,41+,42+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498793
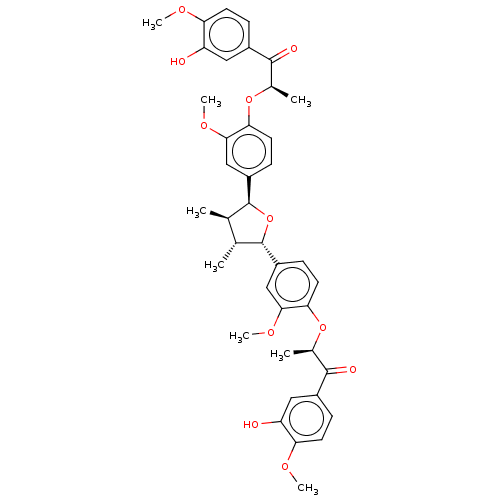
(CHEMBL3623803)Show SMILES COc1ccc(cc1O)C(=O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(O)c2)c(OC)c1 |r| Show InChI InChI=1S/C40H44O11/c1-21-22(2)40(28-12-16-34(36(20-28)48-8)50-24(4)38(44)26-10-14-32(46-6)30(42)18-26)51-39(21)27-11-15-33(35(19-27)47-7)49-23(3)37(43)25-9-13-31(45-5)29(41)17-25/h9-24,39-42H,1-8H3/t21-,22-,23-,24-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016803
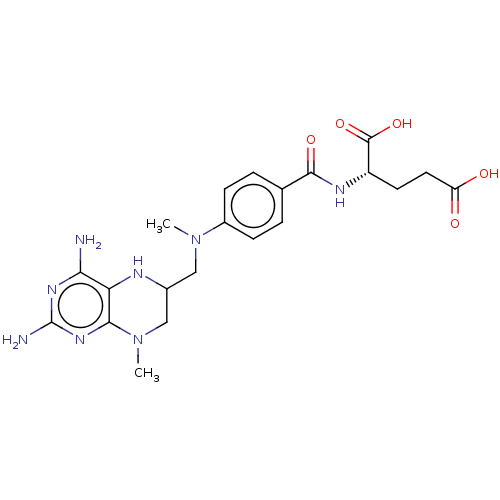
(CHEMBL3274179)Show SMILES CN(CC1CN(C)c2nc(N)nc(N)c2N1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H28N8O5/c1-28(9-12-10-29(2)18-16(24-12)17(22)26-21(23)27-18)13-5-3-11(4-6-13)19(32)25-14(20(33)34)7-8-15(30)31/h3-6,12,14,24H,7-10H2,1-2H3,(H,25,32)(H,30,31)(H,33,34)(H4,22,23,26,27)/t12?,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016797
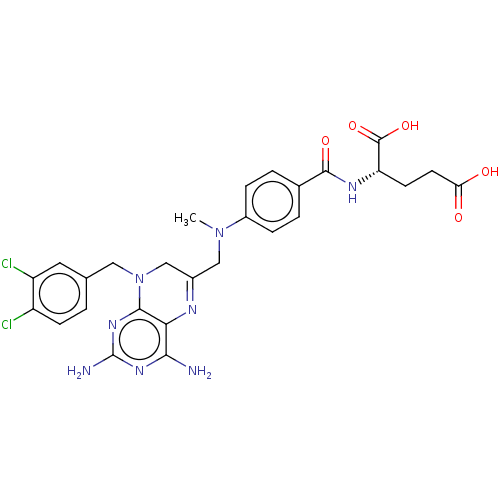
(CHEMBL3273853)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(Cc2ccc(Cl)c(Cl)c2)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C27H28Cl2N8O5/c1-36(17-5-3-15(4-6-17)25(40)33-20(26(41)42)8-9-21(38)39)12-16-13-37(11-14-2-7-18(28)19(29)10-14)24-22(32-16)23(30)34-27(31)35-24/h2-7,10,20H,8-9,11-13H2,1H3,(H,33,40)(H,38,39)(H,41,42)(H4,30,31,34,35)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498802

(CHEMBL3621232)Show SMILES COc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C32H38O8/c1-18-19(2)32(40-31(18)22-10-13-25(35-5)28(16-22)37-7)23-11-14-26(29(17-23)38-8)39-20(3)30(33)21-9-12-24(34-4)27(15-21)36-6/h9-20,31-32H,1-8H3/t18-,19-,20-,31+,32+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016790

(CHEMBL3273847)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(C)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C21H26N8O5/c1-28(9-12-10-29(2)18-16(24-12)17(22)26-21(23)27-18)13-5-3-11(4-6-13)19(32)25-14(20(33)34)7-8-15(30)31/h3-6,14H,7-10H2,1-2H3,(H,25,32)(H,30,31)(H,33,34)(H4,22,23,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Lactobacillus casei) | BDBM50016798

(CHEMBL3273854)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(Cc2cccc3ccccc23)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C31H32N8O5/c1-38(22-11-9-19(10-12-22)29(42)35-24(30(43)44)13-14-25(40)41)16-21-17-39(28-26(34-21)27(32)36-31(33)37-28)15-20-7-4-6-18-5-2-3-8-23(18)20/h2-12,24H,13-17H2,1H3,(H,35,42)(H,40,41)(H,43,44)(H4,32,33,36,37)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei dihydrofolate reductase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016791

(CHEMBL3273855)Show SMILES CN(CC1CNc2nc(N)nc(N)c2N1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H26N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,11,13,24H,6-9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H5,21,22,23,26,27)/t11?,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016796

(CHEMBL3273852)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(Cc2ccccc2)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C27H30N8O5/c1-34(19-9-7-17(8-10-19)25(38)31-20(26(39)40)11-12-21(36)37)14-18-15-35(13-16-5-3-2-4-6-16)24-22(30-18)23(28)32-27(29)33-24/h2-10,20H,11-15H2,1H3,(H,31,38)(H,36,37)(H,39,40)(H4,28,29,32,33)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016797

(CHEMBL3273853)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(Cc2ccc(Cl)c(Cl)c2)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C27H28Cl2N8O5/c1-36(17-5-3-15(4-6-17)25(40)33-20(26(41)42)8-9-21(38)39)12-16-13-37(11-14-2-7-18(28)19(29)10-14)24-22(32-16)23(30)34-27(31)35-24/h2-7,10,20H,8-9,11-13H2,1H3,(H,33,40)(H,38,39)(H,41,42)(H4,30,31,34,35)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016798

(CHEMBL3273854)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(Cc2cccc3ccccc23)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C31H32N8O5/c1-38(22-11-9-19(10-12-22)29(42)35-24(30(43)44)13-14-25(40)41)16-21-17-39(28-26(34-21)27(32)36-31(33)37-28)15-20-7-4-6-18-5-2-3-8-23(18)20/h2-12,24H,13-17H2,1H3,(H,35,42)(H,40,41)(H,43,44)(H4,32,33,36,37)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016800

(CHEMBL3273851)Show SMILES CN(CC1=Nc2c(N)nc(N)nc2N(CC2CCCCC2)C1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,t:3| Show InChI InChI=1S/C27H36N8O5/c1-34(19-9-7-17(8-10-19)25(38)31-20(26(39)40)11-12-21(36)37)14-18-15-35(13-16-5-3-2-4-6-16)24-22(30-18)23(28)32-27(29)33-24/h7-10,16,20H,2-6,11-15H2,1H3,(H,31,38)(H,36,37)(H,39,40)(H4,28,29,32,33)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016801

(CHEMBL3273849)Show SMILES CCCCN1CC(CN(C)c2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=Nc2c(N)nc(N)nc12 |r,c:28| Show InChI InChI=1S/C24H32N8O5/c1-3-4-11-32-13-15(27-19-20(25)29-24(26)30-21(19)32)12-31(2)16-7-5-14(6-8-16)22(35)28-17(23(36)37)9-10-18(33)34/h5-8,17H,3-4,9-13H2,1-2H3,(H,28,35)(H,33,34)(H,36,37)(H4,25,26,29,30)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016802

(CHEMBL3273848)Show SMILES CCN1CC(CN(C)c2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=Nc2c(N)nc(N)nc12 |r,c:26| Show InChI InChI=1S/C22H28N8O5/c1-3-30-11-13(25-17-18(23)27-22(24)28-19(17)30)10-29(2)14-6-4-12(5-7-14)20(33)26-15(21(34)35)8-9-16(31)32/h4-7,15H,3,8-11H2,1-2H3,(H,26,33)(H,31,32)(H,34,35)(H4,23,24,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016803

(CHEMBL3274179)Show SMILES CN(CC1CN(C)c2nc(N)nc(N)c2N1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H28N8O5/c1-28(9-12-10-29(2)18-16(24-12)17(22)26-21(23)27-18)13-5-3-11(4-6-13)19(32)25-14(20(33)34)7-8-15(30)31/h3-6,12,14,24H,7-10H2,1-2H3,(H,25,32)(H,30,31)(H,33,34)(H4,22,23,26,27)/t12?,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50016804

(CHEMBL3273850)Show SMILES CCCCCCN1CC(CN(C)c2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=Nc2c(N)nc(N)nc12 |r,c:30| Show InChI InChI=1S/C26H36N8O5/c1-3-4-5-6-13-34-15-17(29-21-22(27)31-26(28)32-23(21)34)14-33(2)18-9-7-16(8-10-18)24(37)30-19(25(38)39)11-12-20(35)36/h7-10,19H,3-6,11-15H2,1-2H3,(H,30,37)(H,35,36)(H,38,39)(H4,27,28,31,32)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MTX-resistant Lactobacillus casei thymidylate synthetase |
J Med Chem 20: 1323-7 (1977)
BindingDB Entry DOI: 10.7270/Q2SX6FRK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498798
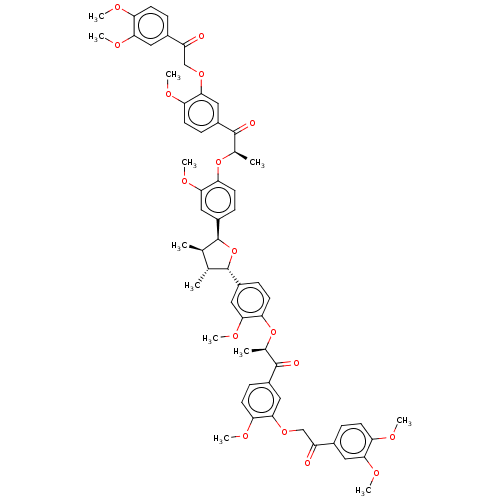
(CHEMBL3623804)Show SMILES COc1ccc(cc1OC)C(=O)COc1cc(ccc1OC)C(=O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(O[C@H](C)C(=O)c2ccc(OC)c(OCC(=O)c3ccc(OC)c(OC)c3)c2)c(OC)c1 |r| Show InChI InChI=1S/C60H64O17/c1-33-34(2)60(42-18-24-50(54(30-42)72-12)76-36(4)58(64)40-16-22-48(68-8)56(28-40)74-32-44(62)38-14-20-46(66-6)52(26-38)70-10)77-59(33)41-17-23-49(53(29-41)71-11)75-35(3)57(63)39-15-21-47(67-7)55(27-39)73-31-43(61)37-13-19-45(65-5)51(25-37)69-9/h13-30,33-36,59-60H,31-32H2,1-12H3/t33-,34-,35-,36-,59+,60+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HIF1 in human HEK293T cells transfected with pGL3-5HRE-VEGF and pRL-SV40 after 24 hrs by dual luciferase reporter assay |
J Med Chem 58: 7659-71 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01220
BindingDB Entry DOI: 10.7270/Q2T156N9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data