

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077661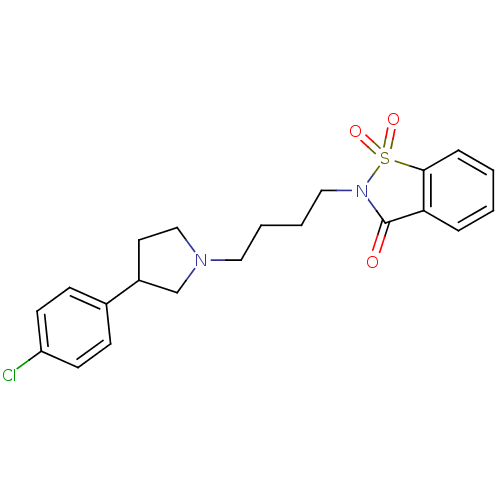 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077658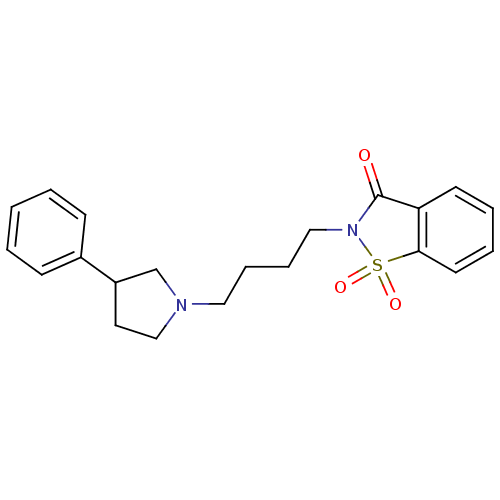 (1,1-Dioxo-2-[4-(3-phenyl-pyrrolidin-1-yl)-butyl]-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071729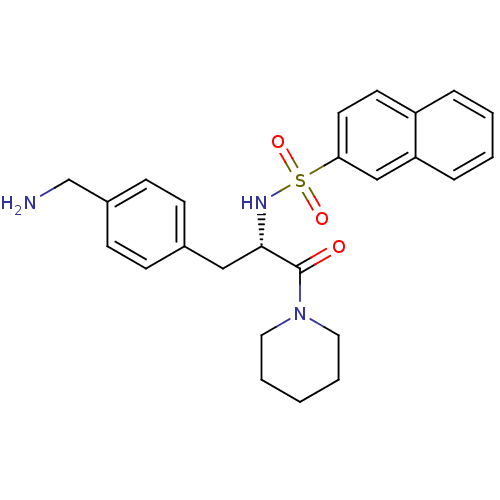 (CHEMBL313826 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071723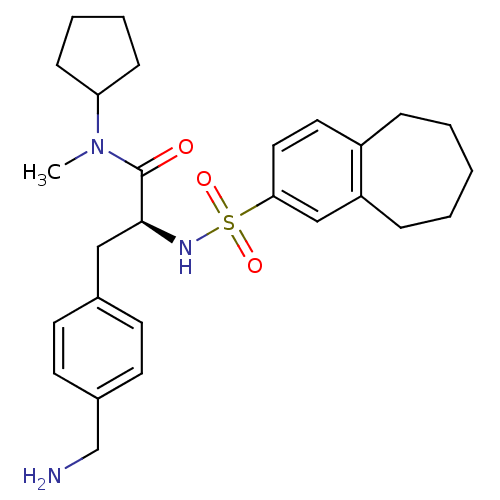 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077664 (2-{4-[3-(4-Fluoro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318133 ((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17,2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318123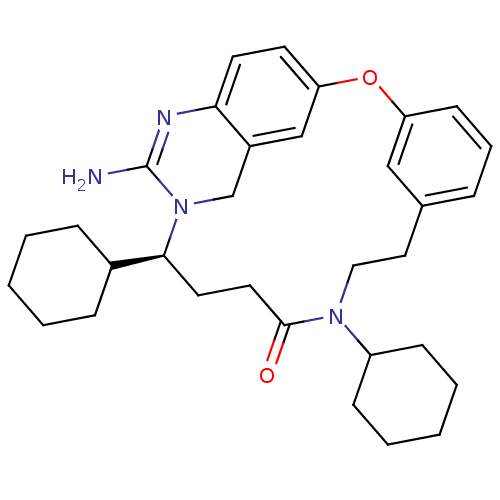 ((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071725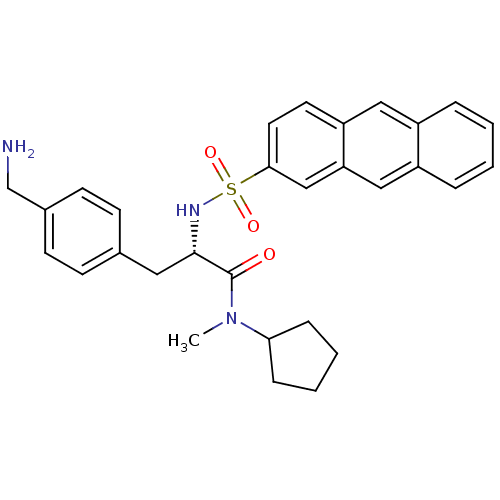 ((S)-3-(4-Aminomethyl-phenyl)-2-(anthracene-2-sulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071722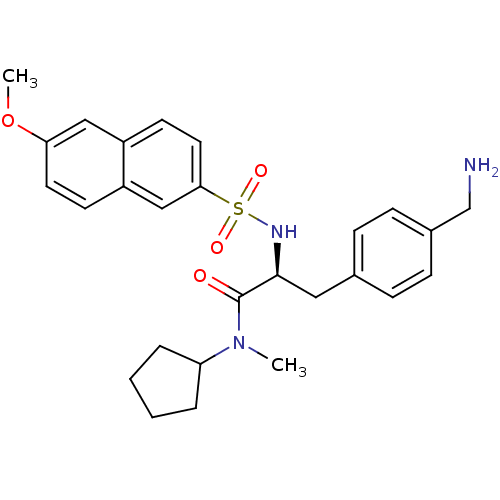 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070783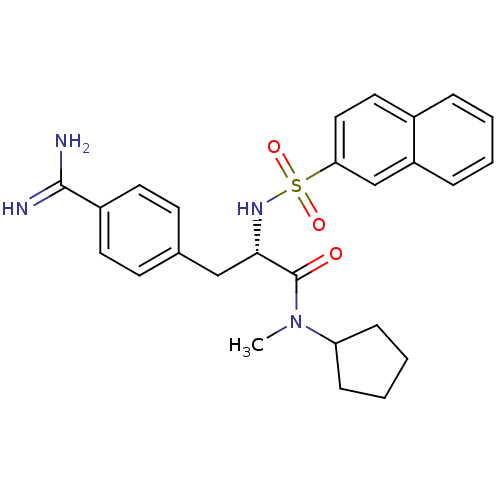 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318125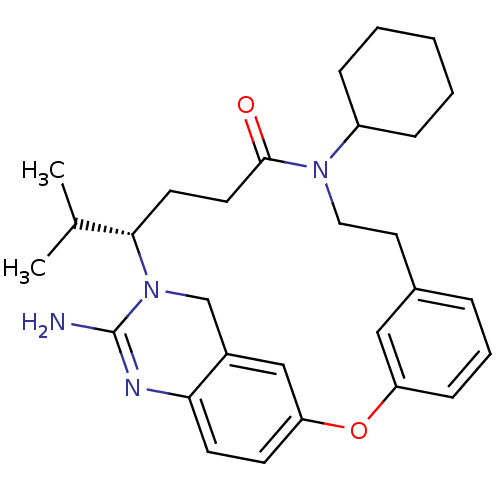 ((14S)-16-amino-10-cyclohexyl-14-(propan-2-yl)-2-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318131 ((14S)-16-amino-10,14-dicyclohexyl-20-fluoro-2-oxa-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071726 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077662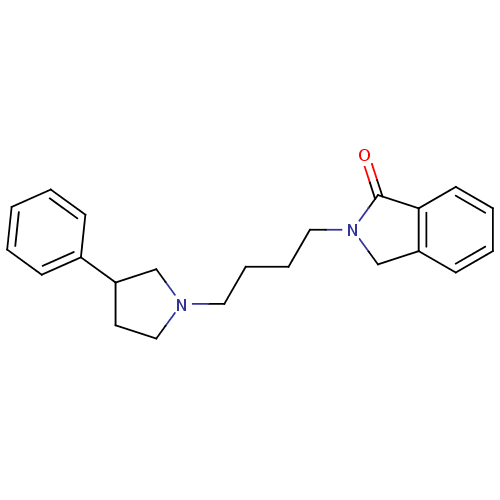 (2-[4-(3-Phenyl-pyrrolidin-1-yl)-butyl]-2,3-dihydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071724 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077660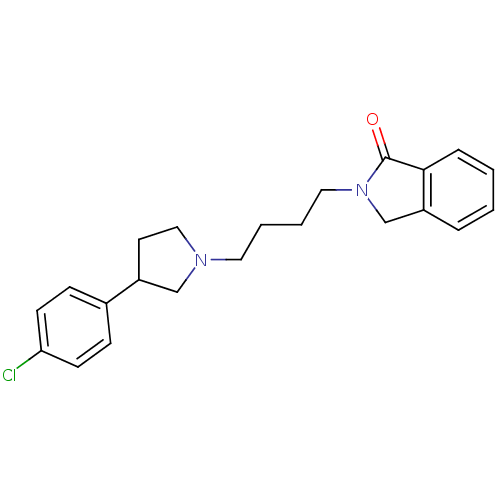 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077662 (2-[4-(3-Phenyl-pyrrolidin-1-yl)-butyl]-2,3-dihydro...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318132 ((14S)-16-amino-10,14-dicyclohexyl-20-methoxy-2-oxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077663 (CHEMBL280534 | {3-[3-(4-Chloro-phenyl)-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077658 (1,1-Dioxo-2-[4-(3-phenyl-pyrrolidin-1-yl)-butyl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318124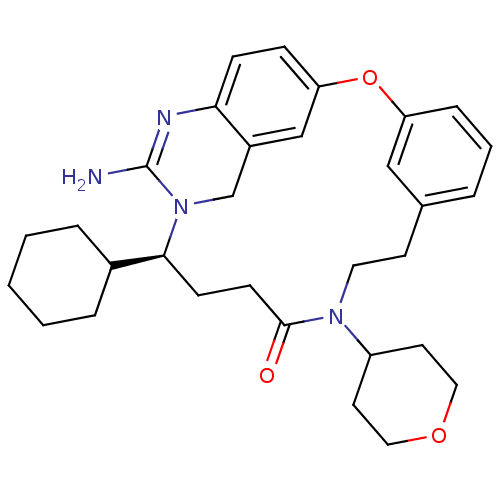 ((14S)-16-amino-14-cyclohexyl-10-(oxan-4-yl)-2-oxa-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069053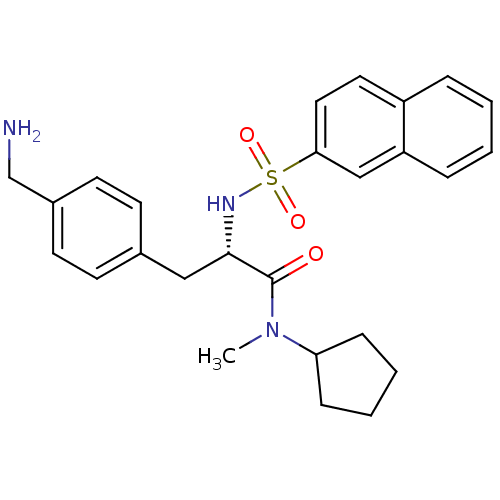 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318129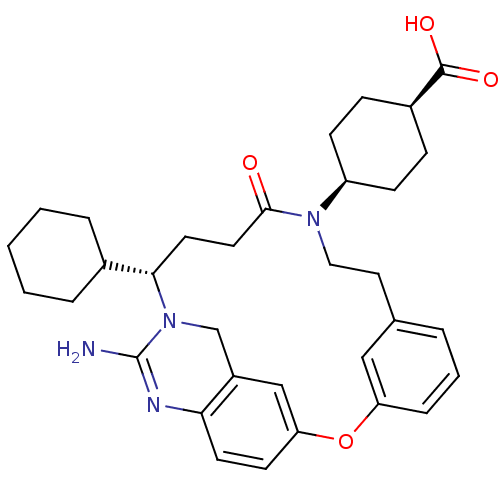 (4-[(14S)-16-amino-14-cyclohexyl-11-oxo-2-oxa-10,15...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071721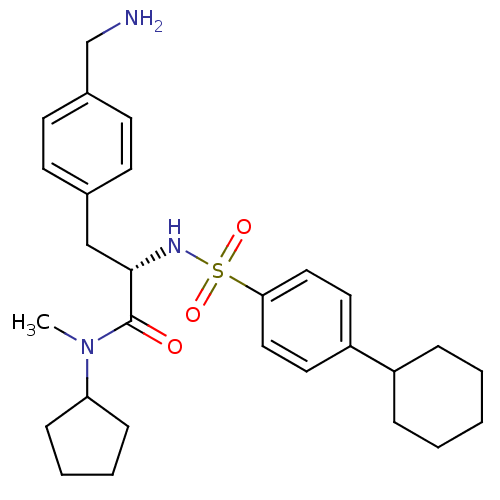 ((S)-3-(4-Aminomethyl-phenyl)-2-(4-cyclohexyl-benze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318128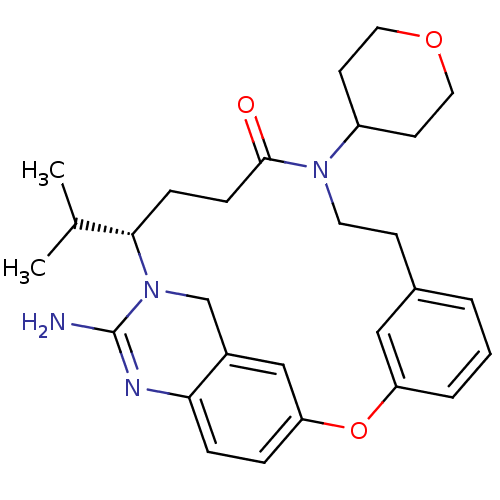 ((14S)-16-amino-10-(oxan-4-yl)-14-(propan-2-yl)-2-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077659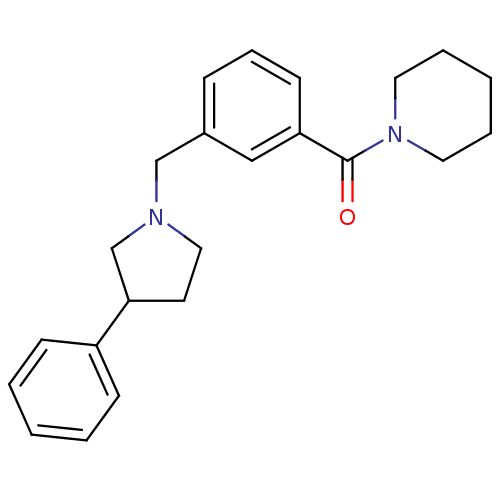 (CHEMBL281214 | [3-(3-Phenyl-pyrrolidin-1-ylmethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077660 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318127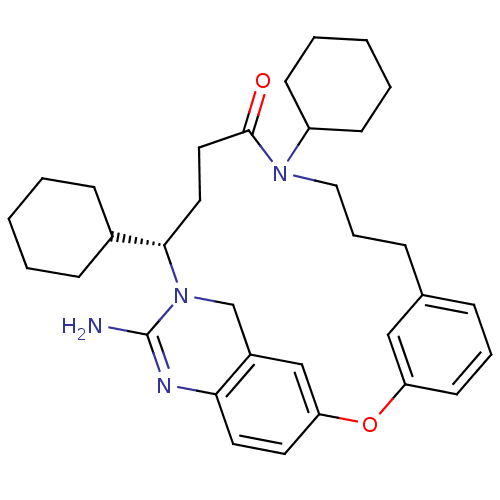 ((15S)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077659 (CHEMBL281214 | [3-(3-Phenyl-pyrrolidin-1-ylmethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT1A receptor of rat hippocampus using [3H]-8-OH-DPAT | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50077658 (1,1-Dioxo-2-[4-(3-phenyl-pyrrolidin-1-yl)-butyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT2A receptor of rat cerebrial cortex using [3H]-ketanserin | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077663 (CHEMBL280534 | {3-[3-(4-Chloro-phenyl)-pyrrolidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50077663 (CHEMBL280534 | {3-[3-(4-Chloro-phenyl)-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT2A receptor of rat cerebrial cortex using [3H]-ketanserin | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50077662 (2-[4-(3-Phenyl-pyrrolidin-1-yl)-butyl]-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT2A receptor of rat cerebrial cortex using [3H]-ketanserin | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069053 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50077661 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-2 adrenergic receptor of rat cerebral cortex using [3H]RX-821002 | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071728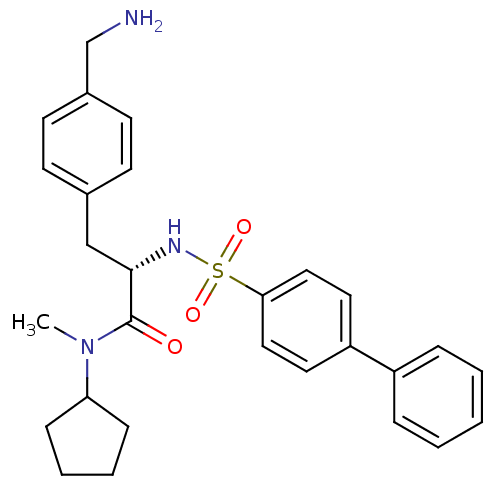 ((S)-3-(4-Aminomethyl-phenyl)-2-(biphenyl-4-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077662 (2-[4-(3-Phenyl-pyrrolidin-1-yl)-butyl]-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 of rat striatum using [3H]-raclopride | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071727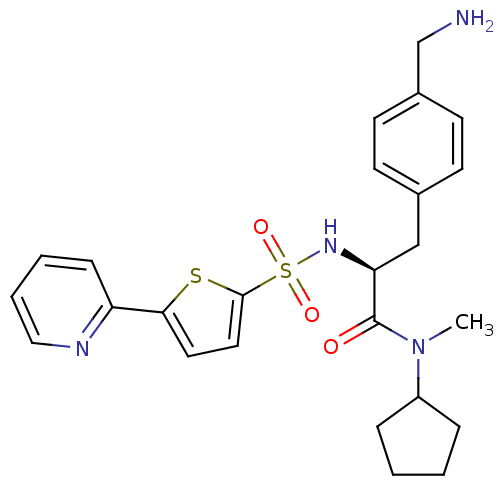 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50077660 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT2A receptor of rat cerebrial cortex using [3H]-ketanserin | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077660 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 of rat striatum using [3H]-raclopride | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50318126 ((15R)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 3158-60 (2010) Article DOI: 10.1016/j.bmcl.2010.03.097 BindingDB Entry DOI: 10.7270/Q2C53M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077658 (1,1-Dioxo-2-[4-(3-phenyl-pyrrolidin-1-yl)-butyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 of rat striatum using [3H]-raclopride | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069052 (CHEMBL164661 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50077661 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT2A receptor of rat cerebrial cortex using [3H]-ketanserin | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077664 (2-{4-[3-(4-Fluoro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 of rat striatum using [3H]-raclopride | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50077659 (CHEMBL281214 | [3-(3-Phenyl-pyrrolidin-1-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against 5-HT2A receptor of rat cerebrial cortex using [3H]-ketanserin | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077661 (2-{4-[3-(4-Chloro-phenyl)-pyrrolidin-1-yl]-butyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
POSTECH Curated by ChEMBL | Assay Description In vitro binding affinity against Dopamine receptor D2 of rat striatum using [3H]-raclopride | Bioorg Med Chem Lett 9: 1379-84 (1999) BindingDB Entry DOI: 10.7270/Q2862FMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 315 total ) | Next | Last >> |