 Found 21 hits with Last Name = 'ho' and Initial = 'mn'
Found 21 hits with Last Name = 'ho' and Initial = 'mn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cruzipain
(Trypanosoma cruzi) | BDBM50331787
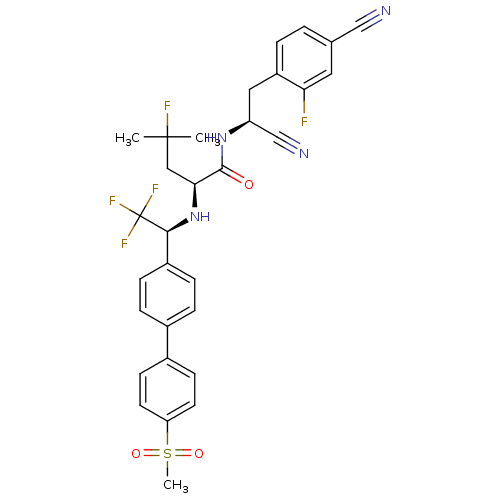
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H29F5N4O3S/c1-30(2,33)16-27(29(41)39-24(18-38)15-23-5-4-19(17-37)14-26(23)32)40-28(31(34,35)36)22-8-6-20(7-9-22)21-10-12-25(13-11-21)44(3,42)43/h4-14,24,27-28,40H,15-16H2,1-3H3,(H,39,41)/t24-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116365
BindingDB Entry DOI: 10.7270/Q2QZ2G27 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM19518

((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35)/t23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116365
BindingDB Entry DOI: 10.7270/Q2QZ2G27 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277781
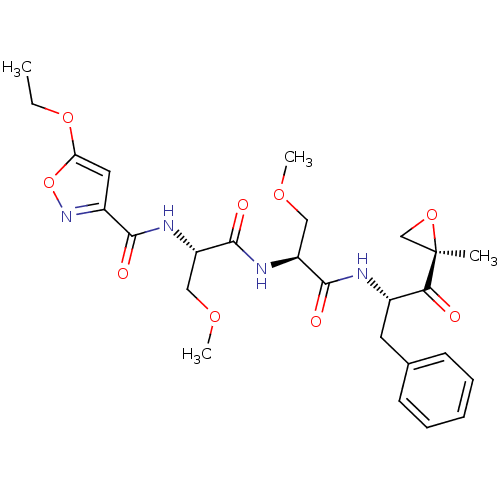
((2S)-2-[(2S)-2-[(5-ethoxy-1,2-oxazol-3-yl)formamid...)Show SMILES CCOc1cc(no1)C(=O)N[C@@H](COC)C(=O)N[C@@H](COC)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C26H34N4O9/c1-5-37-21-12-18(30-39-21)23(32)28-20(14-36-4)25(34)29-19(13-35-3)24(33)27-17(22(31)26(2)15-38-26)11-16-9-7-6-8-10-16/h6-10,12,17,19-20H,5,11,13-15H2,1-4H3,(H,27,33)(H,28,32)(H,29,34)/t17-,19-,20-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277889
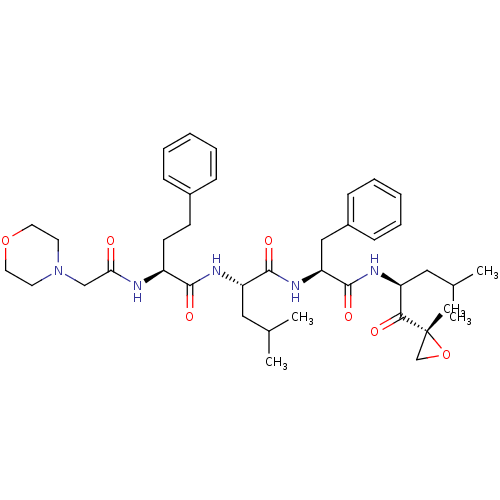
(CARFILZOMIB | CHEMBL451887)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277779
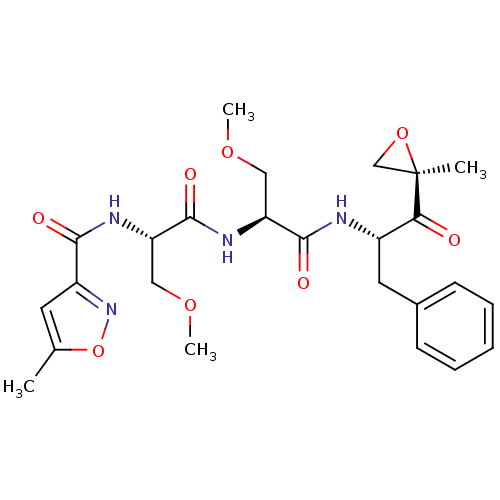
(CHEMBL484003 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(C)on1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O8/c1-15-10-18(29-37-15)22(31)27-20(13-35-4)24(33)28-19(12-34-3)23(32)26-17(21(30)25(2)14-36-25)11-16-8-6-5-7-9-16/h5-10,17,19-20H,11-14H2,1-4H3,(H,26,32)(H,27,31)(H,28,33)/t17-,19-,20-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277815
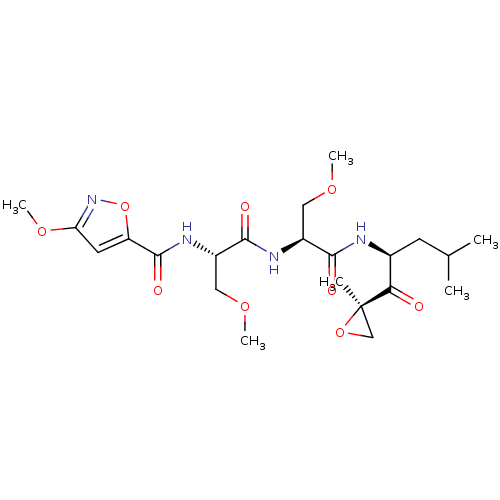
(3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(OC)no1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C22H34N4O9/c1-12(2)7-13(18(27)22(3)11-34-22)23-19(28)14(9-31-4)24-20(29)15(10-32-5)25-21(30)16-8-17(33-6)26-35-16/h8,12-15H,7,9-11H2,1-6H3,(H,23,28)(H,24,29)(H,25,30)/t13-,14-,15-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277816
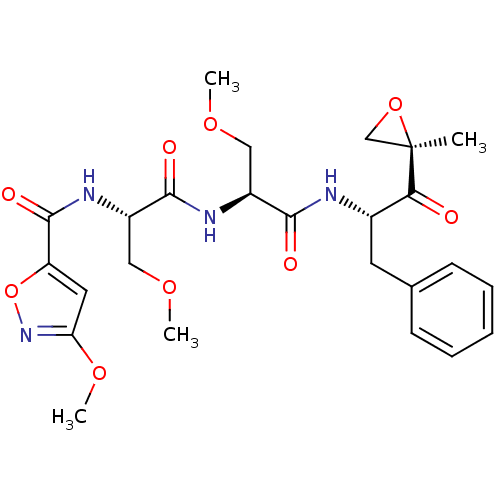
(3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(OC)no1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O9/c1-25(14-37-25)21(30)16(10-15-8-6-5-7-9-15)26-22(31)17(12-34-2)27-23(32)18(13-35-3)28-24(33)19-11-20(36-4)29-38-19/h5-9,11,16-18H,10,12-14H2,1-4H3,(H,26,31)(H,27,32)(H,28,33)/t16-,17-,18-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277780

(5-ethoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)-...)Show SMILES CCOc1cc(no1)C(=O)N[C@@H](COC)C(=O)N[C@@H](COC)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C23H36N4O9/c1-7-34-18-9-15(27-36-18)20(29)25-17(11-33-6)22(31)26-16(10-32-5)21(30)24-14(8-13(2)3)19(28)23(4)12-35-23/h9,13-14,16-17H,7-8,10-12H2,1-6H3,(H,24,30)(H,25,29)(H,26,31)/t14-,16-,17-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277818
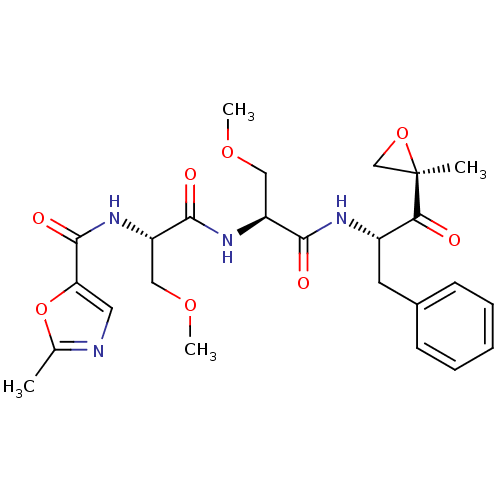
(2-Me-5-thiazole-Ser(OMe)-Ser(OMe)-Phe-ketoepoxide ...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cnc(C)o1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O8/c1-15-26-11-20(37-15)24(33)29-19(13-35-4)23(32)28-18(12-34-3)22(31)27-17(21(30)25(2)14-36-25)10-16-8-6-5-7-9-16/h5-9,11,17-19H,10,12-14H2,1-4H3,(H,27,31)(H,28,32)(H,29,33)/t17-,18-,19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277778
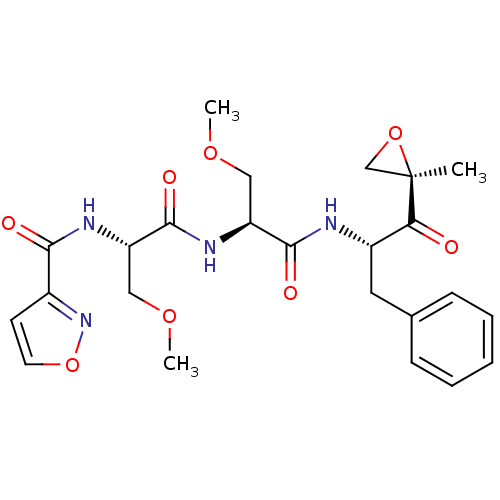
(CHEMBL484002 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1ccon1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C24H30N4O8/c1-24(14-35-24)20(29)17(11-15-7-5-4-6-8-15)25-22(31)18(12-33-2)27-23(32)19(13-34-3)26-21(30)16-9-10-36-28-16/h4-10,17-19H,11-14H2,1-3H3,(H,25,31)(H,26,30)(H,27,32)/t17-,18-,19-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277733

((2S)-3-methoxy-2-[(2S)-3-methoxy-2-[(5-methyl-1,2-...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(C)on1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C22H34N4O8/c1-12(2)7-14(18(27)22(4)11-33-22)23-20(29)16(9-31-5)25-21(30)17(10-32-6)24-19(28)15-8-13(3)34-26-15/h8,12,14,16-17H,7,9-11H2,1-6H3,(H,23,29)(H,24,28)(H,25,30)/t14-,16-,17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277734
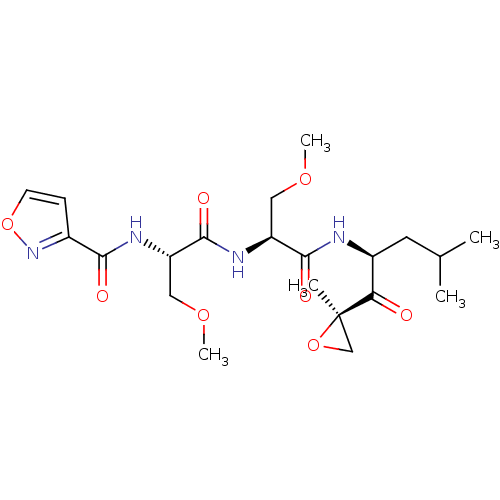
(CHEMBL484157 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1ccon1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C21H32N4O8/c1-12(2)8-14(17(26)21(3)11-32-21)22-19(28)15(9-30-4)24-20(29)16(10-31-5)23-18(27)13-6-7-33-25-13/h6-7,12,14-16H,8-11H2,1-5H3,(H,22,28)(H,23,27)(H,24,29)/t14-,15-,16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 607 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277817

(CHEMBL483740 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cnc(C)o1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C22H34N4O8/c1-12(2)7-14(18(27)22(4)11-33-22)24-19(28)15(9-31-5)25-20(29)16(10-32-6)26-21(30)17-8-23-13(3)34-17/h8,12,14-16H,7,9-11H2,1-6H3,(H,24,28)(H,25,29)(H,26,30)/t14-,15-,16-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 784 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50176699
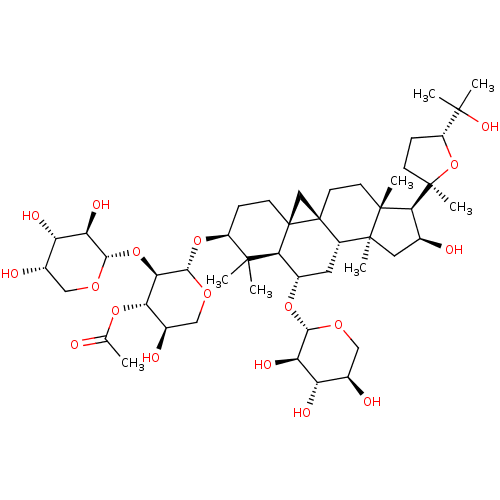
(CHEMBL411981 | askendoside B)Show SMILES CC(=O)O[C@H]1[C@H](O)CO[C@@H](O[C@H]2CC[C@]34C[C@]33CC[C@]5(C)[C@H]([C@@H](O)C[C@@]5(C)[C@@H]3C[C@H](O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)[C@H]4C2(C)C)[C@@]2(C)CC[C@@H](O2)C(C)(C)O)[C@@H]1O[C@@H]1OC[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C47H76O18/c1-21(48)61-34-25(52)19-60-40(35(34)64-39-33(56)31(54)24(51)18-59-39)63-28-10-12-47-20-46(47)14-13-43(6)36(45(8)11-9-29(65-45)42(4,5)57)22(49)16-44(43,7)27(46)15-26(37(47)41(28,2)3)62-38-32(55)30(53)23(50)17-58-38/h22-40,49-57H,9-20H2,1-8H3/t22-,23+,24-,25+,26-,27-,28-,29+,30-,31-,32+,33+,34-,35+,36-,37-,38-,39-,40-,43+,44-,45+,46-,47+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50176700
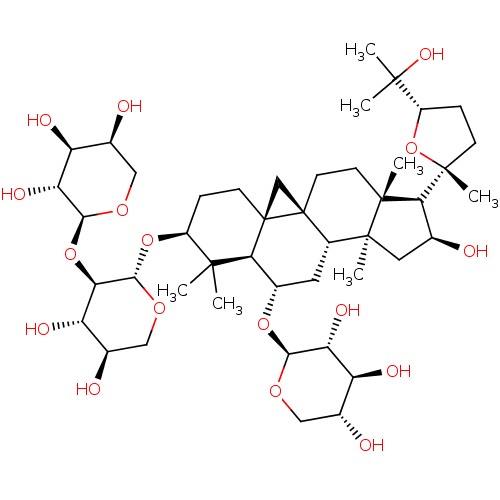
(CHEMBL371147 | askendoside D)Show SMILES CC(C)(O)[C@@H]1CC[C@@](C)(O1)[C@H]1[C@@H](O)C[C@@]2(C)[C@@H]3C[C@H](O[C@@H]4OC[C@@H](O)[C@H](O)[C@H]4O)[C@@H]4[C@]5(C[C@@]35CC[C@]12C)CC[C@H](O[C@@H]1OC[C@@H](O)[C@H](O)[C@H]1O[C@@H]1OC[C@H](O)[C@H](O)[C@H]1O)C4(C)C Show InChI InChI=1S/C45H74O17/c1-39(2)26(60-38-33(30(52)23(49)18-58-38)61-37-32(54)29(51)22(48)17-57-37)9-11-45-19-44(45)13-12-41(5)34(43(7)10-8-27(62-43)40(3,4)55)20(46)15-42(41,6)25(44)14-24(35(39)45)59-36-31(53)28(50)21(47)16-56-36/h20-38,46-55H,8-19H2,1-7H3/t20-,21+,22-,23+,24-,25-,26-,27-,28-,29-,30-,31+,32+,33+,34-,35-,36-,37-,38-,41+,42-,43+,44-,45+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50176696

(CHEMBL372097 | cycloorbicoside G)Show SMILES C[C@@H]1C[C@H]2O[C@]3(C[C@@]4(C)[C@@H]5[C@@H](O)C[C@@H]6[C@]7(C[C@@]57CC[C@]4(C)[C@@H]13)CC[C@H](O[C@@H]1OC[C@@H](O)[C@H](O)[C@H]1O)C6(C)C)O[C@@H]2C(C)(C)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |THB:40:39:21.1.2:4| Show InChI InChI=1S/C41H66O14/c1-18-12-21-32(36(4,5)55-34-29(49)27(47)26(46)22(14-42)51-34)54-41(53-21)16-38(7)31-19(43)13-23-35(2,3)24(52-33-28(48)25(45)20(44)15-50-33)8-9-39(23)17-40(31,39)11-10-37(38,6)30(18)41/h18-34,42-49H,8-17H2,1-7H3/t18-,19+,20-,21-,22-,23+,24+,25+,26-,27+,28-,29-,30-,31+,32+,33+,34+,37-,38+,39-,40+,41-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50370694
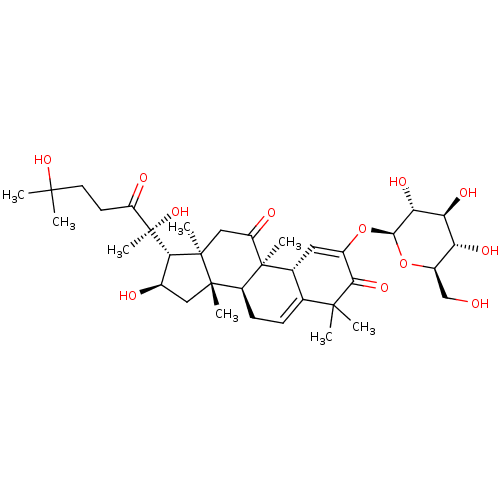
(BRYOAMARIDE)Show SMILES CC(C)(O)CCC(=O)[C@@](C)(O)[C@H]1[C@H](O)C[C@@]2(C)[C@@H]3CC=C4[C@@H](C=C(O[C@@H]5O[C@H](CO)[C@@H](O)[C@H](O)[C@H]5O)C(=O)C4(C)C)[C@]3(C)C(=O)C[C@]12C |r,c:19,t:22| Show InChI InChI=1S/C36H54O12/c1-31(2,45)12-11-23(39)36(8,46)28-19(38)14-33(5)22-10-9-17-18(35(22,7)24(40)15-34(28,33)6)13-20(29(44)32(17,3)4)47-30-27(43)26(42)25(41)21(16-37)48-30/h9,13,18-19,21-22,25-28,30,37-38,41-43,45-46H,10-12,14-16H2,1-8H3/t18-,19-,21-,22+,25-,26+,27-,28+,30-,33+,34-,35+,36-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50176701
(CHEMBL378140 | cyclosieversioside)Show SMILES CC(C)(O)[C@@H]1CC[C@@](C)(O1)[C@H]1[C@@H](O)C[C@@]2(C)[C@@H]3C[C@H](O[C@H]4O[C@@H](CO)[C@H](O)[C@H](O)[C@@H]4O)[C@@H]4[C@]5(C[C@@]35CC[C@]12C)CC[C@H](O[C@@H]1OC[C@@H](O)[C@H](O)[C@H]1O)C4(C)C Show InChI InChI=1S/C41H68O14/c1-35(2)24(54-33-29(48)26(45)20(44)17-51-33)9-11-41-18-40(41)13-12-37(5)31(39(7)10-8-25(55-39)36(3,4)50)19(43)15-38(37,6)23(40)14-21(32(35)41)52-34-30(49)28(47)27(46)22(16-42)53-34/h19-34,42-50H,8-18H2,1-7H3/t19-,20+,21-,22-,23-,24-,25-,26-,27-,28-,29+,30-,31-,32-,33-,34-,37+,38-,39+,40-,41+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50176698
(CHEMBL201643 | cyclocarposide)Show SMILES C[C@@H]1O[C@@H](O[C@H]2C[C@@H]3[C@@]4(C[C@@]44CC[C@H](O[C@@H]5OC[C@@H](O)[C@H](O)[C@H]5O)C(C)(C)[C@H]24)CC[C@]2(C)[C@H]([C@@H](O)C[C@@]32C)[C@@]2(C)CC[C@H](O2)C(C)(C)O)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C41H68O13/c1-19-26(44)28(46)30(48)34(51-19)52-22-15-23-38(7)16-20(42)31(39(8)11-9-25(54-39)36(4,5)49)37(38,6)13-14-40(23)18-41(40)12-10-24(35(2,3)32(22)41)53-33-29(47)27(45)21(43)17-50-33/h19-34,42-49H,9-18H2,1-8H3/t19-,20-,21+,22-,23-,24-,25-,26-,27-,28+,29+,30+,31-,32-,33-,34-,37+,38-,39+,40-,41+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas
Curated by ChEMBL
| Assay Description
Inhibitory activity against tyrosinase |
Bioorg Med Chem Lett 16: 324-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.085
BindingDB Entry DOI: 10.7270/Q2S75H42 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data