 Found 48 hits with Last Name = 'hoff' and Initial = 'o'
Found 48 hits with Last Name = 'hoff' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224371

(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-(e...)Show SMILES CCSc1nc(Nc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19Cl2N5S/c1-2-29-21-26-19(25-16-10-6-9-15(22)11-16)17-12-24-28(20(17)27-21)13-18(23)14-7-4-3-5-8-14/h3-12,18H,2,13H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887
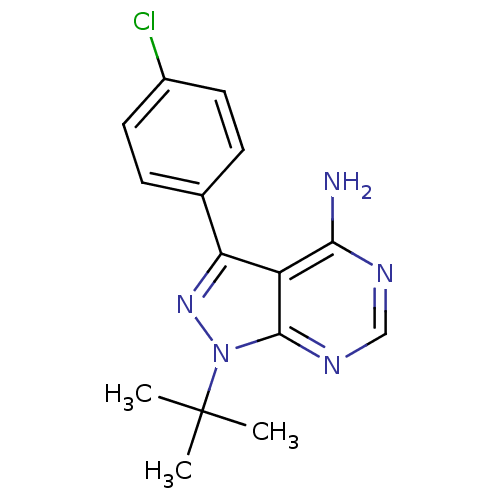
(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224368
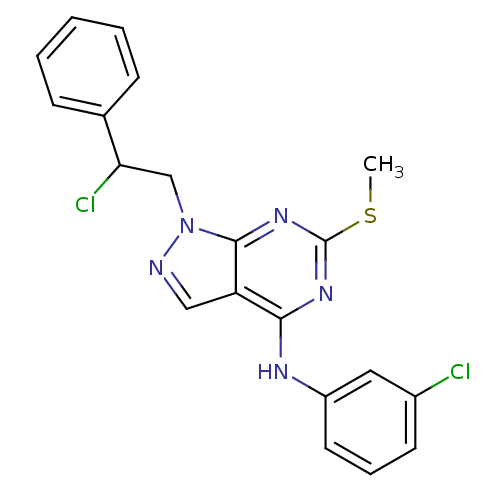
(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-(m...)Show SMILES CSc1nc(Nc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C20H17Cl2N5S/c1-28-20-25-18(24-15-9-5-8-14(21)10-15)16-11-23-27(19(16)26-20)12-17(22)13-6-3-2-4-7-13/h2-11,17H,12H2,1H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142880
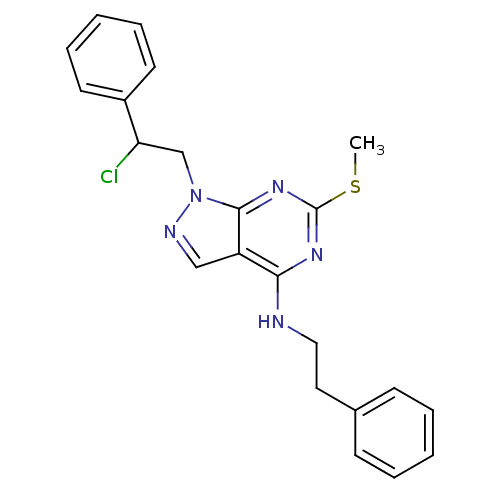
(1-(2-chloro-2-phenylethyl)-6-(methylthio)-N-phenet...)Show SMILES CSc1nc(NCCc2ccccc2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C22H22ClN5S/c1-29-22-26-20(24-13-12-16-8-4-2-5-9-16)18-14-25-28(21(18)27-22)15-19(23)17-10-6-3-7-11-17/h2-11,14,19H,12-13,15H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224385
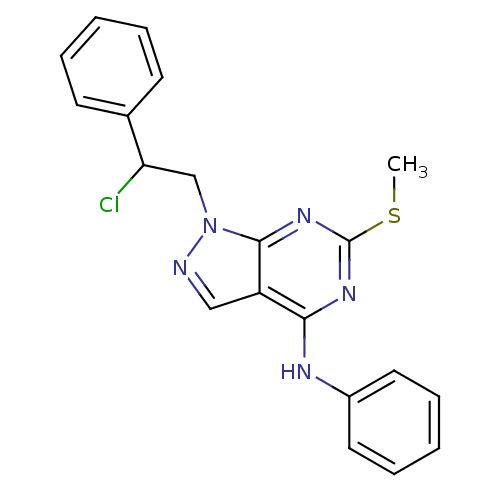
(1-(2-chloro-2-phenylethyl)-6-(methylthio)-N-phenyl...)Show InChI InChI=1S/C20H18ClN5S/c1-27-20-24-18(23-15-10-6-3-7-11-15)16-12-22-26(19(16)25-20)13-17(21)14-8-4-2-5-9-14/h2-12,17H,13H2,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224376
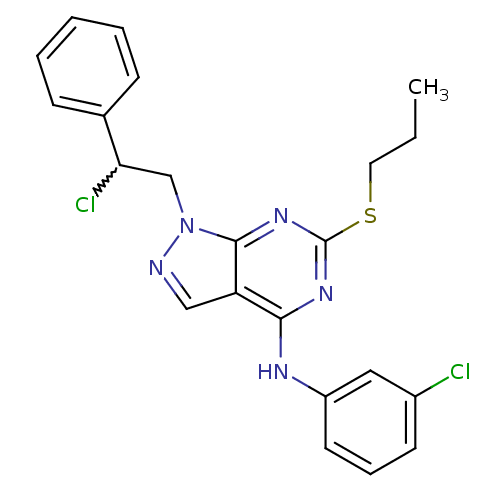
(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenyl)-6-(p...)Show SMILES CCCSc1nc(Nc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21Cl2N5S/c1-2-11-30-22-27-20(26-17-10-6-9-16(23)12-17)18-13-25-29(21(18)28-22)14-19(24)15-7-4-3-5-8-15/h3-10,12-13,19H,2,11,14H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224381

(1-(2-chloro-2-phenylethyl)-N-(3-fluorophenyl)-6-(m...)Show SMILES CSc1nc(Nc2cccc(F)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C20H17ClFN5S/c1-28-20-25-18(24-15-9-5-8-14(22)10-15)16-11-23-27(19(16)26-20)12-17(21)13-6-3-2-4-7-13/h2-11,17H,12H2,1H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224388
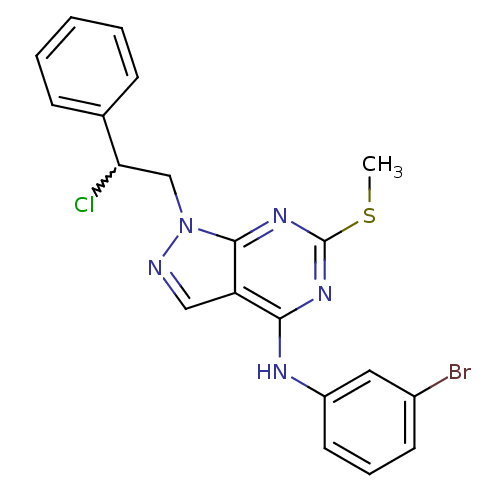
(CHEMBL238561 | N-(3-bromophenyl)-1-(2-chloro-2-phe...)Show SMILES CSc1nc(Nc2cccc(Br)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:18.19| Show InChI InChI=1S/C20H17BrClN5S/c1-28-20-25-18(24-15-9-5-8-14(21)10-15)16-11-23-27(19(16)26-20)12-17(22)13-6-3-2-4-7-13/h2-11,17H,12H2,1H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50170723

(1,1'-Hexamethylene bis(5-(p-chlorophenyl)biguanide...)Show SMILES NC(NC(N)=Nc1ccc(Cl)cc1)=NCCCCCCN=C(N)N=C(N)Nc1ccc(Cl)cc1 |w:13.14,20.20,23.23,5.5| Show InChI InChI=1S/C22H30Cl2N10/c23-15-5-9-17(10-6-15)31-21(27)33-19(25)29-13-3-1-2-4-14-30-20(26)34-22(28)32-18-11-7-16(24)8-12-18/h5-12H,1-4,13-14H2,(H5,25,27,29,31,33)(H5,26,28,30,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibitory constant against recombinant Trypanosoma cruzi trypanothione reductase was determined photometrically at 25 degree C in TR assay buffer (4... |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50170726
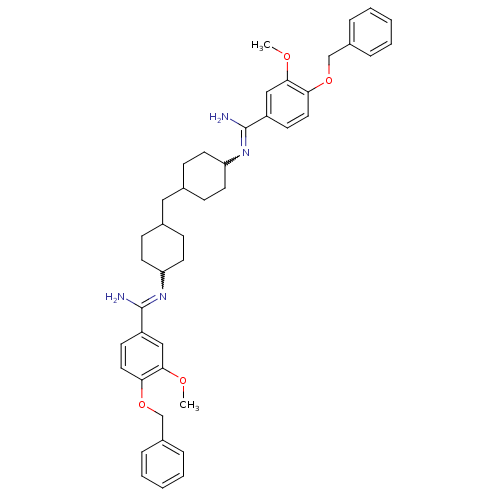
(4,4'-Bis(4-benzyloxy-3-methoxybenzimidoylamino)dic...)Show SMILES COc1cc(ccc1OCc1ccccc1)C(N)=NC1CCC(CC2CCC(CC2)N=C(N)c2ccc(OCc3ccccc3)c(OC)c2)CC1 |w:18.20,30.32,(-1.23,-7.92,;.33,-7.9,;1.12,-9.21,;2.66,-9.19,;3.45,-10.52,;2.7,-11.87,;1.15,-11.89,;.36,-10.56,;-1.16,-10.59,;-1.93,-11.94,;-3.47,-11.96,;-4.22,-13.3,;-5.75,-13.33,;-6.55,-12,;-5.79,-10.66,;-4.28,-10.63,;4.9,-11,;5.23,-12.5,;6.06,-9.97,;7.51,-10.45,;8.65,-9.42,;10.1,-9.89,;10.42,-11.4,;11.89,-11.87,;13.03,-10.84,;14.5,-11.33,;15.64,-10.3,;15.32,-8.79,;13.85,-8.33,;12.71,-9.35,;16.46,-7.77,;17.92,-8.23,;18.25,-9.74,;18.67,-6.88,;20.21,-6.83,;20.95,-5.48,;20.14,-4.16,;20.86,-2.8,;22.4,-2.73,;23.12,-1.38,;22.3,-.08,;23.02,1.3,;24.56,1.37,;25.38,.04,;24.66,-1.33,;18.59,-4.22,;17.78,-2.92,;18.5,-1.57,;17.87,-5.55,;9.28,-12.43,;7.83,-11.95,)| Show InChI InChI=1S/C43H52N4O4/c1-48-40-26-34(17-23-38(40)50-28-32-9-5-3-6-10-32)42(44)46-36-19-13-30(14-20-36)25-31-15-21-37(22-16-31)47-43(45)35-18-24-39(41(27-35)49-2)51-29-33-11-7-4-8-12-33/h3-12,17-18,23-24,26-27,30-31,36-37H,13-16,19-22,25,28-29H2,1-2H3,(H2,44,46)(H2,45,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibitory constant against recombinant Trypanosoma cruzi trypanothione reductase was determined photometrically at 25 degree C in TR assay buffer (4... |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50170727

(4,4'-Bis(4-benzyloxy-3-methoxybenzimidoylamino)-di...)Show SMILES COc1cc(ccc1OCc1ccccc1)C(N)=Nc1ccc(Oc2ccc(cc2)N=C(N)c2ccc(OCc3ccccc3)c(OC)c2)cc1 |w:30.32,18.20| Show InChI InChI=1S/C42H38N4O5/c1-47-39-25-31(13-23-37(39)49-27-29-9-5-3-6-10-29)41(43)45-33-15-19-35(20-16-33)51-36-21-17-34(18-22-36)46-42(44)32-14-24-38(40(26-32)48-2)50-28-30-11-7-4-8-12-30/h3-26H,27-28H2,1-2H3,(H2,43,45)(H2,44,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibitory constant against recombinant Trypanosoma cruzi trypanothione reductase was determined photometrically at 25 degree C in TR assay buffer (4... |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224383

(1-(2-chloro-2-(4-chlorophenyl)ethyl)-6-(methylthio...)Show SMILES CSc1nc(NCCc2ccccc2)c2cnn(CC(Cl)c3ccc(Cl)cc3)c2n1 |w:19.20| Show InChI InChI=1S/C22H21Cl2N5S/c1-30-22-27-20(25-12-11-15-5-3-2-4-6-15)18-13-26-29(21(18)28-22)14-19(24)16-7-9-17(23)10-8-16/h2-10,13,19H,11-12,14H2,1H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224374
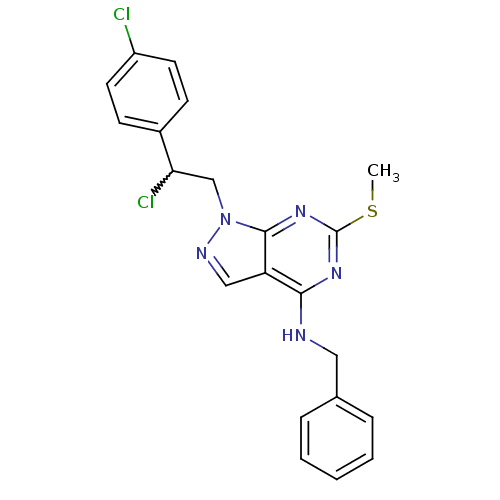
(CHEMBL241749 | N-benzyl-1-(2-chloro-2-(4-chlorophe...)Show SMILES CSc1nc(NCc2ccccc2)c2cnn(CC(Cl)c3ccc(Cl)cc3)c2n1 |w:18.19| Show InChI InChI=1S/C21H19Cl2N5S/c1-29-21-26-19(24-11-14-5-3-2-4-6-14)17-12-25-28(20(17)27-21)13-18(23)15-7-9-16(22)10-8-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224387
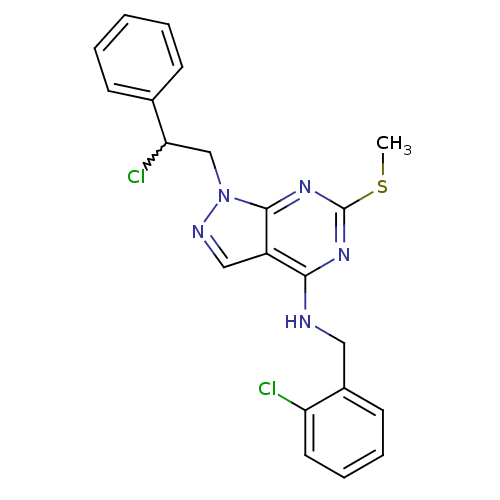
(1-(2-chloro-2-phenylethyl)-N-(2-chlorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2ccccc2Cl)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19Cl2N5S/c1-29-21-26-19(24-11-15-9-5-6-10-17(15)22)16-12-25-28(20(16)27-21)13-18(23)14-7-3-2-4-8-14/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142885
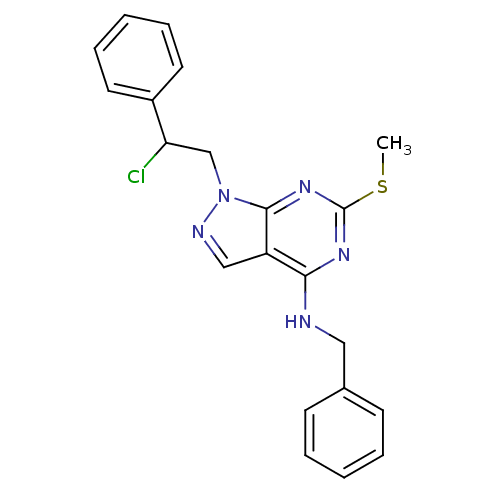
(Benzyl-[1-(2-chloro-2-phenyl-ethyl)-6-methylsulfan...)Show SMILES CSc1nc(NCc2ccccc2)c2cnn(CC(Cl)c3ccccc3)c2n1 Show InChI InChI=1S/C21H20ClN5S/c1-28-21-25-19(23-12-15-8-4-2-5-9-15)17-13-24-27(20(17)26-21)14-18(22)16-10-6-3-7-11-16/h2-11,13,18H,12,14H2,1H3,(H,23,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224366
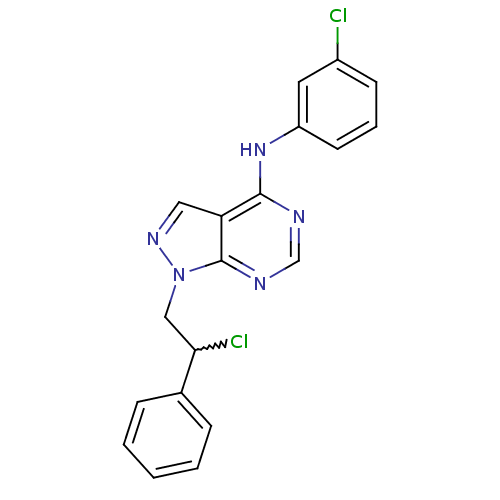
(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenyl)-1H-p...)Show SMILES ClC(Cn1ncc2c(Nc3cccc(Cl)c3)ncnc12)c1ccccc1 |w:1.0| Show InChI InChI=1S/C19H15Cl2N5/c20-14-7-4-8-15(9-14)25-18-16-10-24-26(19(16)23-12-22-18)11-17(21)13-5-2-1-3-6-13/h1-10,12,17H,11H2,(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224367

(1-(2-chloro-2-phenylethyl)-N-(2-chlorophenethyl)-6...)Show SMILES CSc1nc(NCCc2ccccc2Cl)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21Cl2N5S/c1-30-22-27-20(25-12-11-16-9-5-6-10-18(16)23)17-13-26-29(21(17)28-22)14-19(24)15-7-3-2-4-8-15/h2-10,13,19H,11-12,14H2,1H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224386
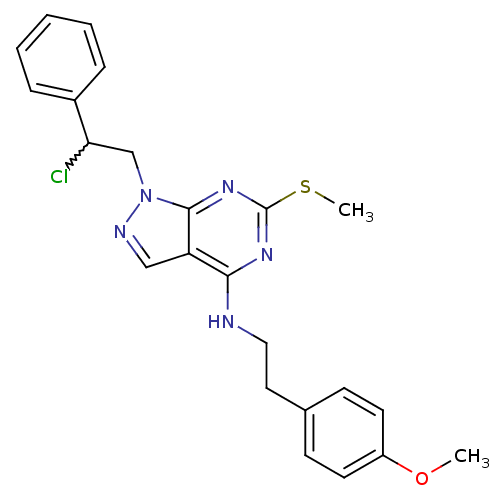
(CHEMBL399813 | N-(4-methoxyphenethyl)-1-(2-chloro-...)Show SMILES COc1ccc(CCNc2nc(SC)nc3n(CC(Cl)c4ccccc4)ncc23)cc1 |w:18.18| Show InChI InChI=1S/C23H24ClN5OS/c1-30-18-10-8-16(9-11-18)12-13-25-21-19-14-26-29(22(19)28-23(27-21)31-2)15-20(24)17-6-4-3-5-7-17/h3-11,14,20H,12-13,15H2,1-2H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224382
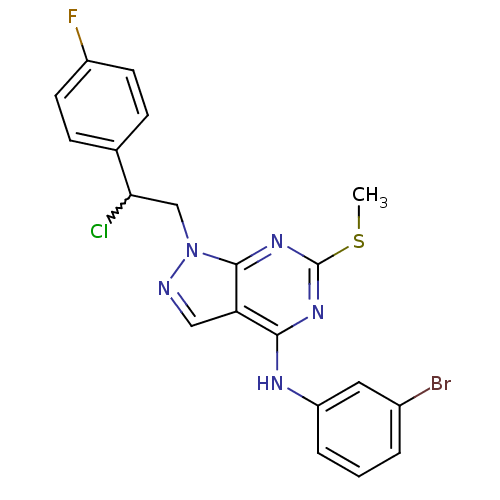
(CHEMBL399627 | N-(3-bromophenyl)-1-(2-chloro-2-(4-...)Show SMILES CSc1nc(Nc2cccc(Br)c2)c2cnn(CC(Cl)c3ccc(F)cc3)c2n1 |w:18.19| Show InChI InChI=1S/C20H16BrClFN5S/c1-29-20-26-18(25-15-4-2-3-13(21)9-15)16-10-24-28(19(16)27-20)11-17(22)12-5-7-14(23)8-6-12/h2-10,17H,11H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224372
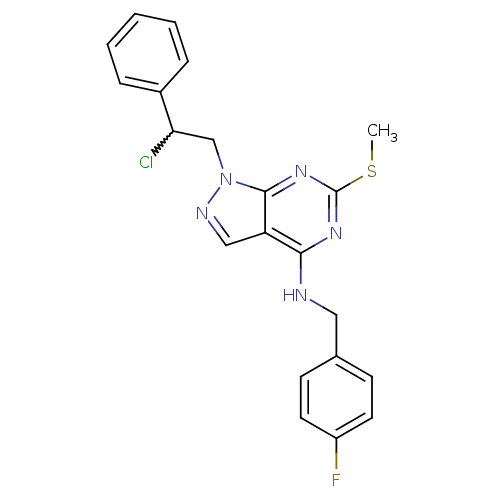
(1-(2-chloro-2-phenylethyl)-N-(4-fluorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2ccc(F)cc2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19ClFN5S/c1-29-21-26-19(24-11-14-7-9-16(23)10-8-14)17-12-25-28(20(17)27-21)13-18(22)15-5-3-2-4-6-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50170724

(1,8-Bis(4-benzyloxy-3-methoxybenzimidoylamino)octa...)Show SMILES COc1cc(ccc1OCc1ccccc1)C(=N)NCCCCCCCCNC(=N)c1ccc(OCc2ccccc2)c(OC)c1 Show InChI InChI=1S/C38H46N4O4/c1-43-35-25-31(19-21-33(35)45-27-29-15-9-7-10-16-29)37(39)41-23-13-5-3-4-6-14-24-42-38(40)32-20-22-34(36(26-32)44-2)46-28-30-17-11-8-12-18-30/h7-12,15-22,25-26H,3-6,13-14,23-24,27-28H2,1-2H3,(H2,39,41)(H2,40,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibitory constant against recombinant Trypanosoma cruzi trypanothione reductase was determined photometrically at 25 degree C in TR assay buffer (4... |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224384

(1-(2-chloro-2-phenylethyl)-N-(3-fluorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2cccc(F)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19ClFN5S/c1-29-21-26-19(24-11-14-6-5-9-16(23)10-14)17-12-25-28(20(17)27-21)13-18(22)15-7-3-2-4-8-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224380
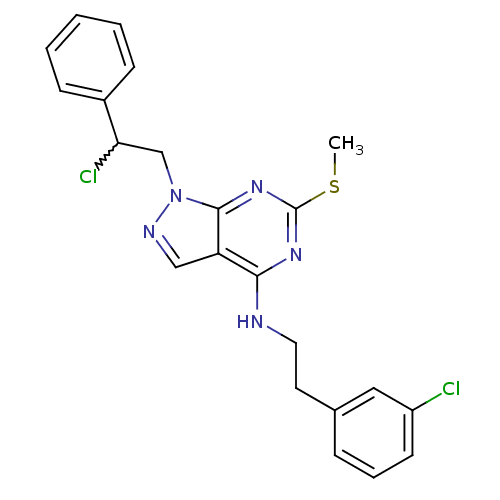
(1-(2-chloro-2-phenylethyl)-N-(3-chlorophenethyl)-6...)Show SMILES CSc1nc(NCCc2cccc(Cl)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21Cl2N5S/c1-30-22-27-20(25-11-10-15-6-5-9-17(23)12-15)18-13-26-29(21(18)28-22)14-19(24)16-7-3-2-4-8-16/h2-9,12-13,19H,10-11,14H2,1H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50370594

(CHEMBL1794917)Show SMILES N=C(CCCCCCCC(=N)NCC#Cc1ccccc1)NCC#Cc1ccccc1 Show InChI InChI=1S/C27H32N4/c28-26(30-22-12-18-24-14-6-4-7-15-24)20-10-2-1-3-11-21-27(29)31-23-13-19-25-16-8-5-9-17-25/h4-9,14-17H,1-3,10-11,20-23H2,(H2,28,30)(H2,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Competitive inhibitory constant against recombinant Trypanosoma cruzi trypanothione reductase was determined photometrically at 25 degree C in TR ass... |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50170722

(4-[2-(4-Nitro-phenyl)-2-oxo-acetylamino]-piperidin...)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)C(=O)NC1(CCNCC1)C(=O)NCCNC(=O)Cc1cccc2ccccc12 Show InChI InChI=1S/C28H29N5O6/c34-24(18-21-6-3-5-19-4-1-2-7-23(19)21)30-16-17-31-27(37)28(12-14-29-15-13-28)32-26(36)25(35)20-8-10-22(11-9-20)33(38)39/h1-11,29H,12-18H2,(H,30,34)(H,31,37)(H,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibitory constant against recombinant Trypanosoma cruzi trypanothione reductase was determined photometrically at 25 degree C in TR assay buffer (4... |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224369
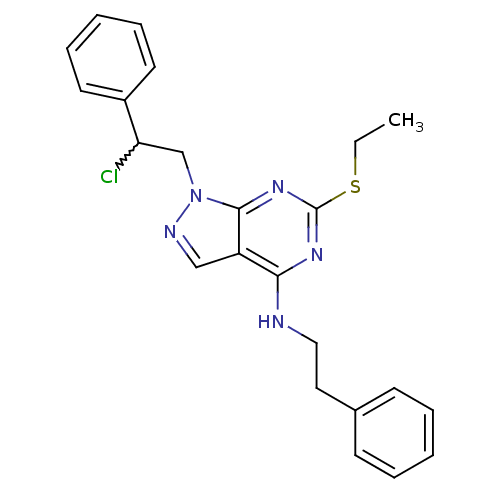
(1-(2-chloro-2-phenylethyl)-6-(ethylthio)-N-pheneth...)Show SMILES CCSc1nc(NCCc2ccccc2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C23H24ClN5S/c1-2-30-23-27-21(25-14-13-17-9-5-3-6-10-17)19-15-26-29(22(19)28-23)16-20(24)18-11-7-4-8-12-18/h3-12,15,20H,2,13-14,16H2,1H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224373

(1-(2-chloro-2-phenylethyl)-N-(3-fluorophenethyl)-6...)Show SMILES CSc1nc(NCCc2cccc(F)c2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C22H21ClFN5S/c1-30-22-27-20(25-11-10-15-6-5-9-17(24)12-15)18-13-26-29(21(18)28-22)14-19(23)16-7-3-2-4-8-16/h2-9,12-13,19H,10-11,14H2,1H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224379

(1-(2-chloro-2-phenylethyl)-N-(4-methylphenethyl)-6...)Show SMILES CSc1nc(NCCc2ccc(C)cc2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C23H24ClN5S/c1-16-8-10-17(11-9-16)12-13-25-21-19-14-26-29(22(19)28-23(27-21)30-2)15-20(24)18-6-4-3-5-7-18/h3-11,14,20H,12-13,15H2,1-2H3,(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224377
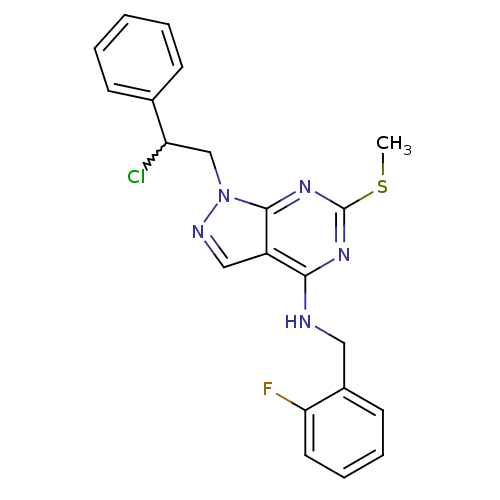
(1-(2-chloro-2-phenylethyl)-N-(2-fluorobenzyl)-6-(m...)Show SMILES CSc1nc(NCc2ccccc2F)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19ClFN5S/c1-29-21-26-19(24-11-15-9-5-6-10-18(15)23)16-12-25-28(20(16)27-21)13-17(22)14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224378
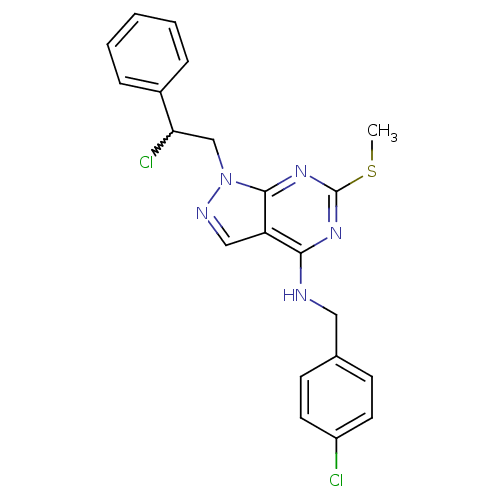
(CHEMBL239192 | N-(4-chlorobenzyl)-1-(2-chloro-2-ph...)Show SMILES CSc1nc(NCc2ccc(Cl)cc2)c2cnn(CC(Cl)c3ccccc3)c2n1 |w:19.20| Show InChI InChI=1S/C21H19Cl2N5S/c1-29-21-26-19(24-11-14-7-9-16(22)10-8-14)17-12-25-28(20(17)27-21)13-18(23)15-5-3-2-4-6-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224375
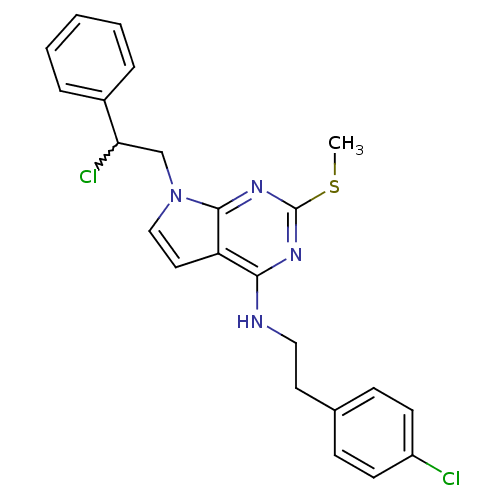
(CHEMBL401038 | N-(4-chlorophenethyl)-7-(2-chloro-2...)Show SMILES CSc1nc(NCCc2ccc(Cl)cc2)c2ccn(CC(Cl)c3ccccc3)c2n1 |w:20.21| Show InChI InChI=1S/C23H22Cl2N4S/c1-30-23-27-21(26-13-11-16-7-9-18(24)10-8-16)19-12-14-29(22(19)28-23)15-20(25)17-5-3-2-4-6-17/h2-10,12,14,20H,11,13,15H2,1H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50224370

(CHEMBL241750 | N-benzyl-1-(2-chloro-2-(4-fluorophe...)Show SMILES CSc1nc(NCc2ccccc2)c2cnn(CC(Cl)c3ccc(F)cc3)c2n1 |w:18.19| Show InChI InChI=1S/C21H19ClFN5S/c1-29-21-26-19(24-11-14-5-3-2-4-6-14)17-12-25-28(20(17)27-21)13-18(22)15-7-9-16(23)10-8-15/h2-10,12,18H,11,13H2,1H3,(H,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Glutathione reductase, mitochondrial
(Homo sapiens (Human)) | BDBM50170723

(1,1'-Hexamethylene bis(5-(p-chlorophenyl)biguanide...)Show SMILES NC(NC(N)=Nc1ccc(Cl)cc1)=NCCCCCCN=C(N)N=C(N)Nc1ccc(Cl)cc1 |w:13.14,20.20,23.23,5.5| Show InChI InChI=1S/C22H30Cl2N10/c23-15-5-9-17(10-6-15)31-21(27)33-19(25)29-13-3-1-2-4-14-30-20(26)34-22(28)32-18-11-7-16(24)8-12-18/h5-12H,1-4,13-14H2,(H5,25,27,29,31,33)(H5,26,28,30,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Heidelberg
Curated by ChEMBL
| Assay Description
Inhibitory constant against human glutathione reductase |
J Med Chem 48: 4793-802 (2005)
Article DOI: 10.1021/jm050027z
BindingDB Entry DOI: 10.7270/Q2XK8GB5 |
More data for this
Ligand-Target Pair | |
Type 1 InsP3 receptor isoform S2
(Sus scrofa) | BDBM50075183
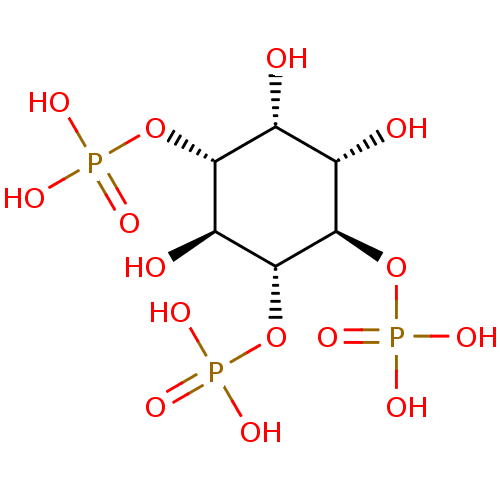
(1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...)Show SMILES O[C@@H]1[C@H](O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@@H](O)[C@@H]1OP(O)(O)=O Show InChI InChI=1S/C6H15O15P3/c7-1-2(8)5(20-23(13,14)15)6(21-24(16,17)18)3(9)4(1)19-22(10,11)12/h1-9H,(H2,10,11,12)(H2,13,14,15)(H2,16,17,18)/t1-,2+,3+,4-,5-,6-/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of compound was determined by its ability to displace specifically bound Inositol 1,4,5-trisphosphate receptor from purified porcine... |
Bioorg Med Chem Lett 5: 831-834 (1995)
Article DOI: 10.1016/0960-894X(95)00120-I
BindingDB Entry DOI: 10.7270/Q2ST7PTQ |
More data for this
Ligand-Target Pair | |
Type 1 InsP3 receptor isoform S2
(Sus scrofa) | BDBM50082885

(CHEMBL144954 | Phosphoric acid mono-((1R,2R,3S,4R,...)Show SMILES O[C@@H]1[C@H](O)[C@@H](OP(O)(O)=O)[C@@H](C[C@@H]1OP(O)(O)=O)OP(O)(O)=O Show InChI InChI=1S/C6H15O14P3/c7-4-2(18-21(9,10)11)1-3(19-22(12,13)14)6(5(4)8)20-23(15,16)17/h2-8H,1H2,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17)/t2-,3+,4-,5-,6-/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of compound was determined by its ability to displace specifically bound Inositol 1,4,5-trisphosphate receptor from purified porcine... |
Bioorg Med Chem Lett 5: 831-834 (1995)
Article DOI: 10.1016/0960-894X(95)00120-I
BindingDB Entry DOI: 10.7270/Q2ST7PTQ |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510218

(CHEMBL4439603)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSCCCc4cn(Cc5ccccc5)nn4)C3)c[nH]c12 |r| Show InChI InChI=1S/C24H30N8OS/c25-24-23-22(27-16-28-24)18(9-26-23)11-31-12-19(21(33)14-31)15-34-8-4-7-20-13-32(30-29-20)10-17-5-2-1-3-6-17/h1-3,5-6,9,13,16,19,21,26,33H,4,7-8,10-12,14-15H2,(H2,25,27,28)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510219
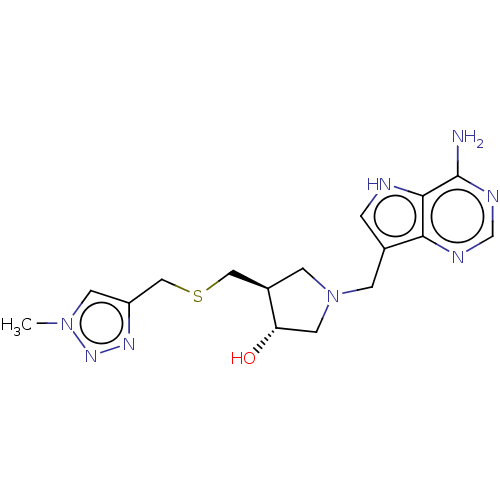
(CHEMBL4439224)Show SMILES Cn1cc(CSC[C@H]2CN(Cc3c[nH]c4c(N)ncnc34)C[C@@H]2O)nn1 |r| Show InChI InChI=1S/C16H22N8OS/c1-23-5-12(21-22-23)8-26-7-11-4-24(6-13(11)25)3-10-2-18-15-14(10)19-9-20-16(15)17/h2,5,9,11,13,18,25H,3-4,6-8H2,1H3,(H2,17,19,20)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510220

(CHEMBL4555970)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSCc4cn(CC=C)nn4)C3)c[nH]c12 |r| Show InChI InChI=1S/C18H24N8OS/c1-2-3-26-7-14(23-24-26)10-28-9-13-6-25(8-15(13)27)5-12-4-20-17-16(12)21-11-22-18(17)19/h2,4,7,11,13,15,20,27H,1,3,5-6,8-10H2,(H2,19,21,22)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510221

(CHEMBL4555691)Show SMILES CCCCn1cc(CSC[C@H]2CN(Cc3c[nH]c4c(N)ncnc34)C[C@@H]2O)nn1 |r| Show InChI InChI=1S/C19H28N8OS/c1-2-3-4-27-8-15(24-25-27)11-29-10-14-7-26(9-16(14)28)6-13-5-21-18-17(13)22-12-23-19(18)20/h5,8,12,14,16,21,28H,2-4,6-7,9-11H2,1H3,(H2,20,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510222

(CHEMBL4586326)Show SMILES CSC[C@H]1CN(Cc2c[nH]c3c(N)nccc23)C[C@@H]1O |r| Show InChI InChI=1S/C14H20N4OS/c1-20-8-10-6-18(7-12(10)19)5-9-4-17-13-11(9)2-3-16-14(13)15/h2-4,10,12,17,19H,5-8H2,1H3,(H2,15,16)/t10-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510223

(CHEMBL4444249)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSCc4cn(Cc5ccccc5)nn4)C3)c[nH]c12 |r| Show InChI InChI=1S/C22H26N8OS/c23-22-21-20(25-14-26-22)16(6-24-21)8-29-9-17(19(31)11-29)12-32-13-18-10-30(28-27-18)7-15-4-2-1-3-5-15/h1-6,10,14,17,19,24,31H,7-9,11-13H2,(H2,23,25,26)/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510230
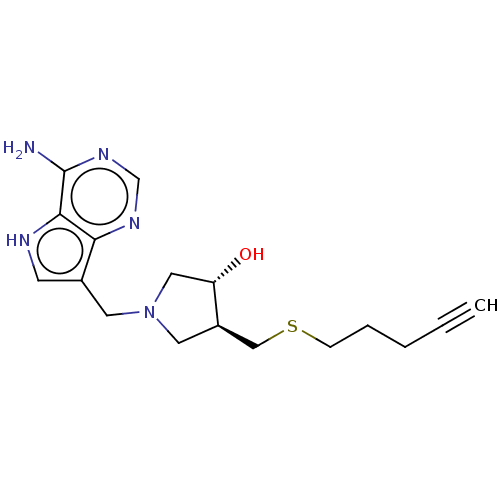
(CHEMBL4465346)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSCCCC#C)C3)c[nH]c12 |r| Show InChI InChI=1S/C17H23N5OS/c1-2-3-4-5-24-10-13-8-22(9-14(13)23)7-12-6-19-16-15(12)20-11-21-17(16)18/h1,6,11,13-14,19,23H,3-5,7-10H2,(H2,18,20,21)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510225

(CHEMBL4450105)Show SMILES CCCCn1cc(CCCSC[C@H]2CN(Cc3c[nH]c4c(N)ncnc34)C[C@@H]2O)nn1 |r| Show InChI InChI=1S/C21H32N8OS/c1-2-3-6-29-11-17(26-27-29)5-4-7-31-13-16-10-28(12-18(16)30)9-15-8-23-20-19(15)24-14-25-21(20)22/h8,11,14,16,18,23,30H,2-7,9-10,12-13H2,1H3,(H2,22,24,25)/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510226

(CHEMBL4469129)Show SMILES Cn1cc(CCCSC[C@H]2CN(Cc3c[nH]c4c(N)ncnc34)C[C@@H]2O)nn1 |r| Show InChI InChI=1S/C18H26N8OS/c1-25-8-14(23-24-25)3-2-4-28-10-13-7-26(9-15(13)27)6-12-5-20-17-16(12)21-11-22-18(17)19/h5,8,11,13,15,20,27H,2-4,6-7,9-10H2,1H3,(H2,19,21,22)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510227
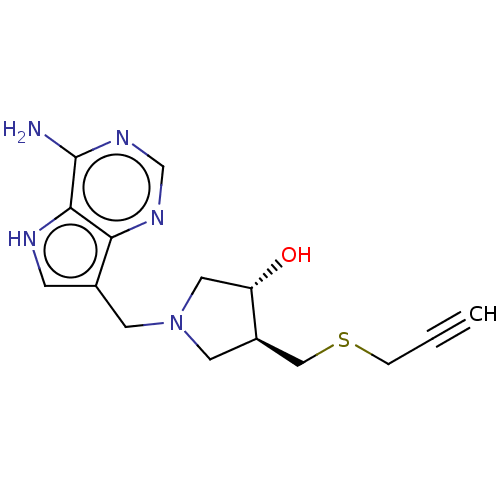
(CHEMBL4445386)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSCC#C)C3)c[nH]c12 |r| Show InChI InChI=1S/C15H19N5OS/c1-2-3-22-8-11-6-20(7-12(11)21)5-10-4-17-14-13(10)18-9-19-15(14)16/h1,4,9,11-12,17,21H,3,5-8H2,(H2,16,18,19)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510228
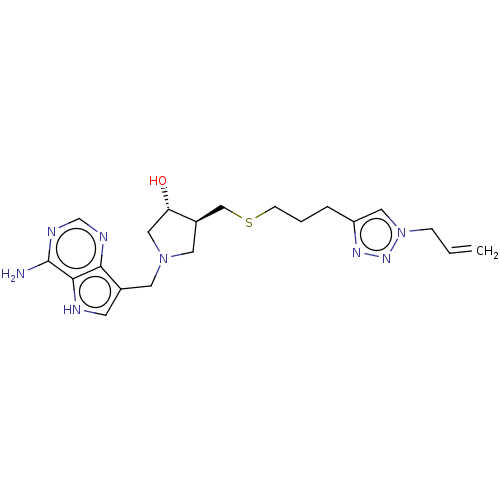
(CHEMBL4469234)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSCCCc4cn(CC=C)nn4)C3)c[nH]c12 |r| Show InChI InChI=1S/C20H28N8OS/c1-2-5-28-10-16(25-26-28)4-3-6-30-12-15-9-27(11-17(15)29)8-14-7-22-19-18(14)23-13-24-20(19)21/h2,7,10,13,15,17,22,29H,1,3-6,8-9,11-12H2,(H2,21,23,24)/t15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510229
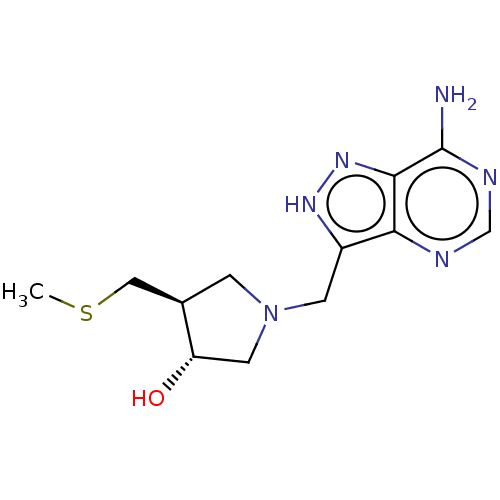
(CHEMBL4472065)Show SMILES CSC[C@H]1CN(Cc2[nH]nc3c(N)ncnc23)C[C@@H]1O |r| Show InChI InChI=1S/C12H18N6OS/c1-20-5-7-2-18(4-9(7)19)3-8-10-11(17-16-8)12(13)15-6-14-10/h6-7,9,19H,2-5H2,1H3,(H,16,17)(H2,13,14,15)/t7-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |
S-methyl-5'-thioadenosine phosphorylase
(Homo sapiens (Human)) | BDBM50510224

(CHEMBL4451807)Show SMILES Nc1ncnc2c(CN3C[C@H](O)[C@@H](CSc4ncccn4)C3)c[nH]c12 |r| Show InChI InChI=1S/C16H19N7OS/c17-15-14-13(21-9-22-15)10(4-20-14)5-23-6-11(12(24)7-23)8-25-16-18-2-1-3-19-16/h1-4,9,11-12,20,24H,5-8H2,(H2,17,21,22)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human N-terminal TEV cleavage site-fused-His6-tagged MTAP expressed in Escherichia coli BL21-CodonPlus(DE3)-RIPL comp... |
J Med Chem 62: 3286-3296 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01642
BindingDB Entry DOI: 10.7270/Q2JM2DXM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data