

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (RAT) | BDBM50406692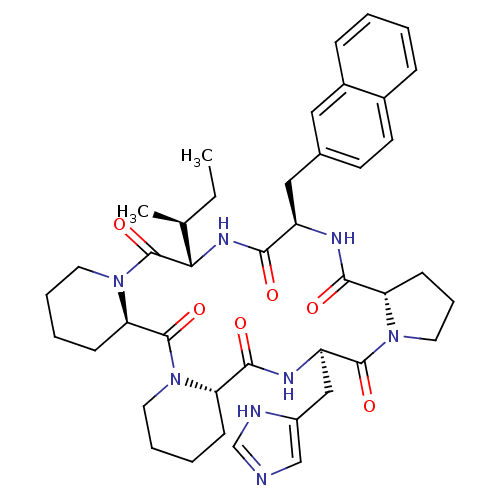 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894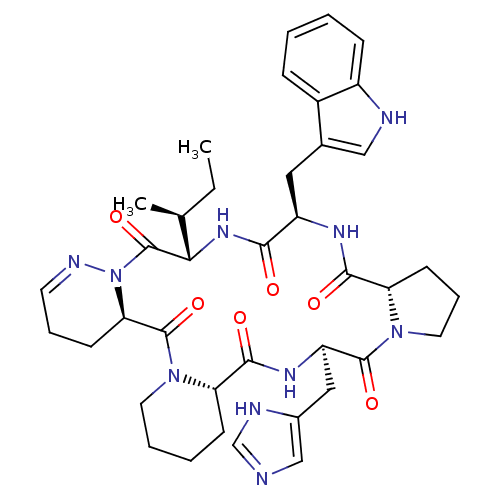 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit AVP stimulation of adenylate cyclase activity in the rat kidney medulla (AVP-V2) receptor | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM81894 (Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001311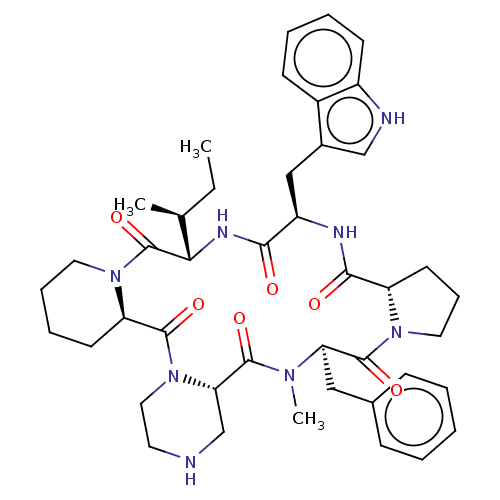 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001311 (24-benzyl-16-(1H-3-indolylmethyl)-25-methyl-13-[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50406692 (CHEMBL2112249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368435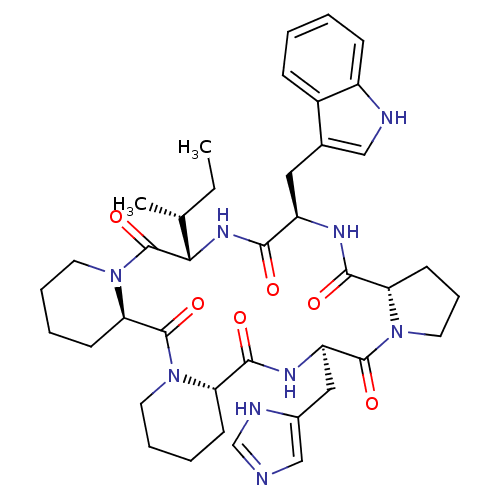 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001307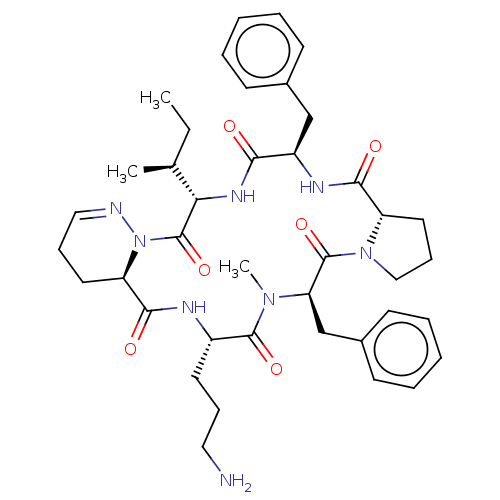 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]arginine vasopressin binding to AVP-V2 site in rat kidney medulla. | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50001309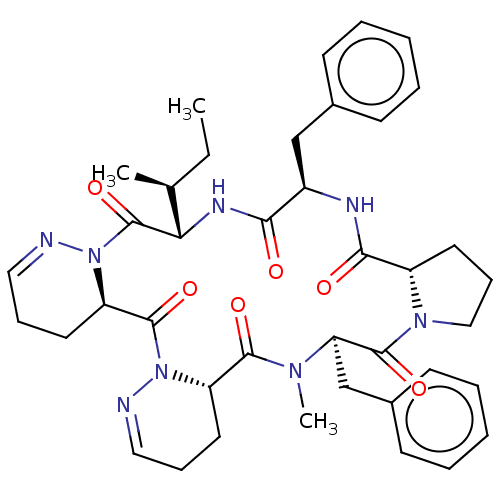 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat uterine receptor was determined using [3H]oxytocin as radioligand | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50368435 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50368435 (CHEMBL1790937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001307 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50001307 (18-(3-Amino-propyl)-6,15-dibenzyl-3-sec-butyl-16-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50109377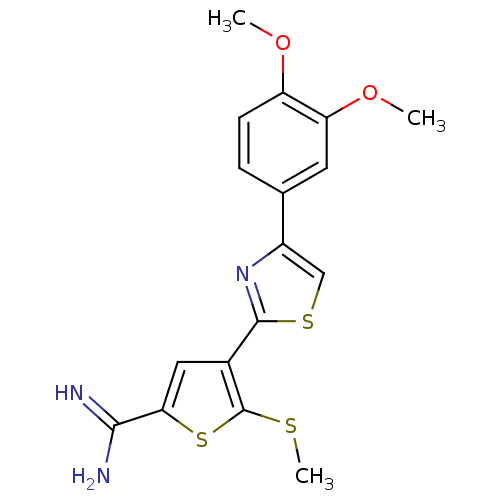 (4-[4-(3,4-Dimethoxy-phenyl)-thiazol-2-yl]-5-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098163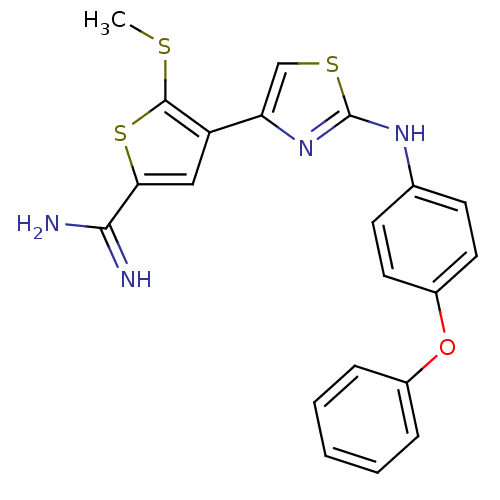 (5-Methylsulfanyl-4-[2-(4-phenoxy-phenylamino)-thia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098169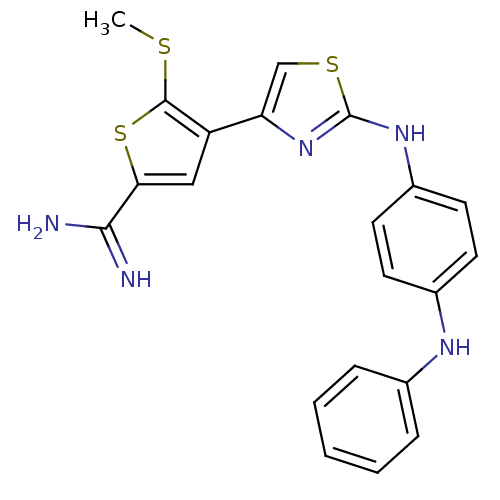 (5-Methylsulfanyl-4-[2-(4-phenylamino-phenylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098144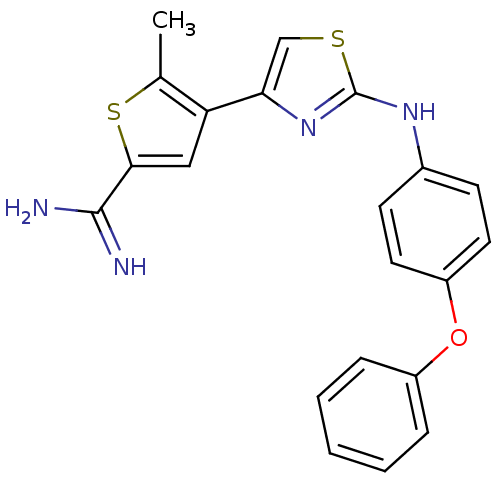 (5-Methyl-4-[2-(4-phenoxy-phenylamino)-thiazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099921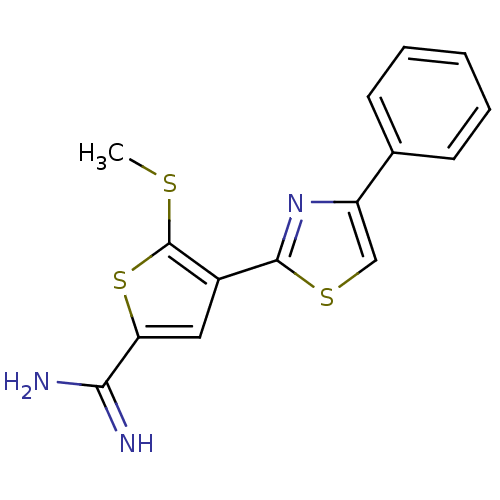 (5-Methylsulfanyl-4-(4-phenyl-thiazol-2-yl)-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50099900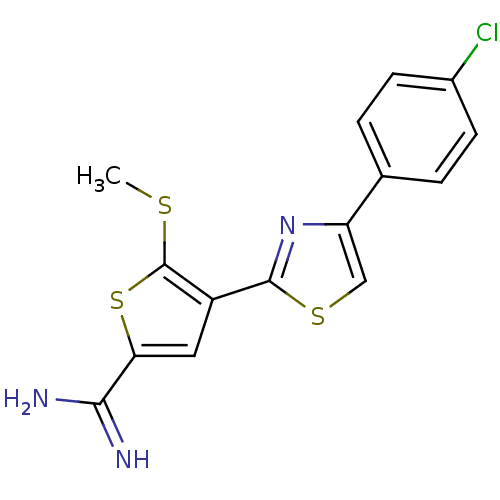 (4-[4-(4-Chloro-phenyl)-thiazol-2-yl]-5-methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50109381 (5-Methyl-4-(4-phenyl-thiazol-2-yl)-thiophene-2-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098127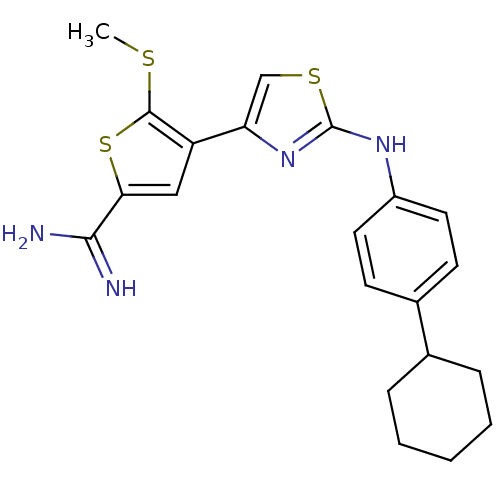 (4-[2-(4-Cyclohexyl-phenylamino)-thiazol-4-yl]-5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098141 (4-{2-[4-(4-Chloro-phenoxy)-phenylamino]-thiazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50109378 (5-Ethyl-4-(4-phenyl-thiazol-2-yl)-thiophene-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098139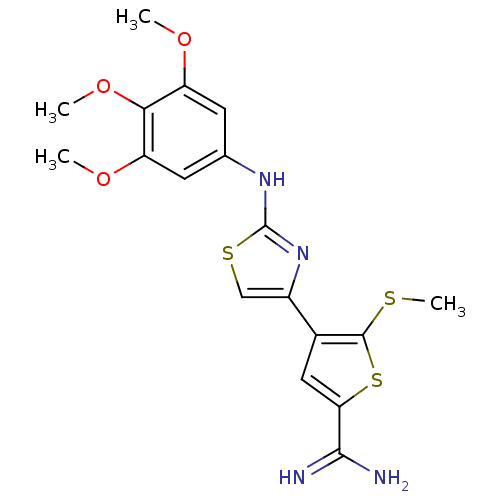 (5-Methylsulfanyl-4-[2-(3,4,5-trimethoxy-phenylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50109376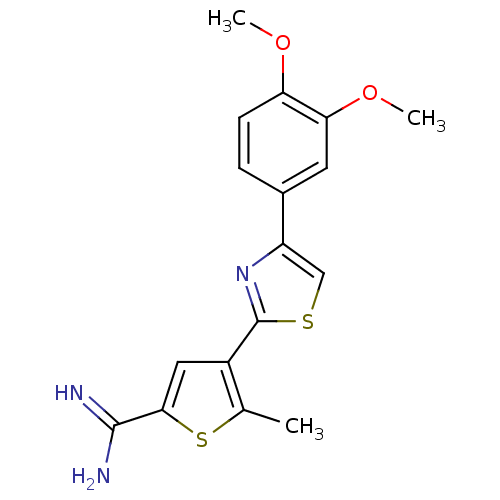 (4-[4-(3,4-Dimethoxy-phenyl)-thiazol-2-yl]-5-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098132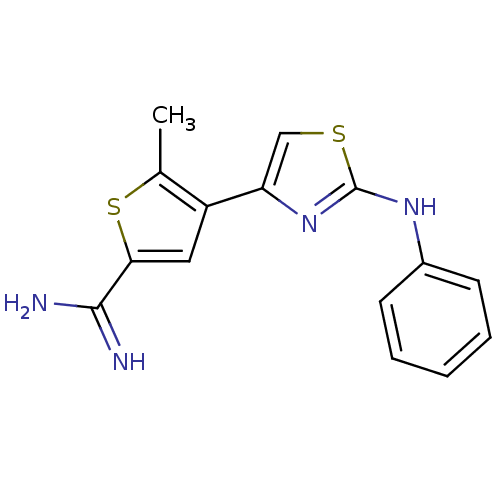 (5-Methyl-4-(2-phenylamino-thiazol-4-yl)-thiophene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098133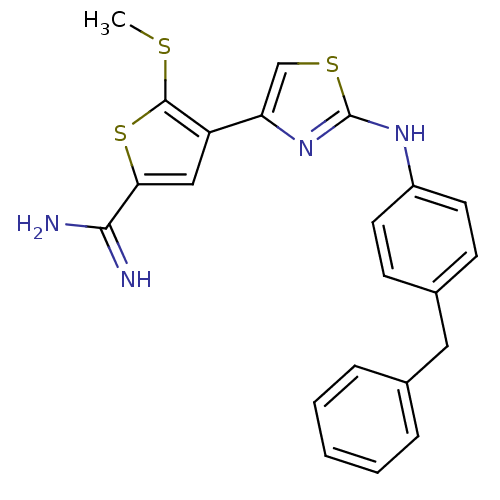 (4-[2-(4-Benzyl-phenylamino)-thiazol-4-yl]-5-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098131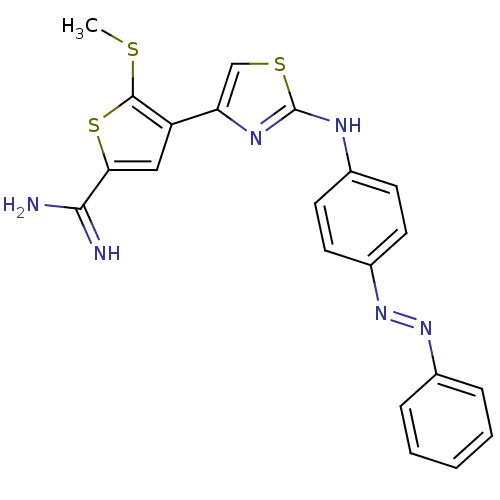 (5-Methylsulfanyl-4-[2-(4-phenylazo-phenylamino)-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098147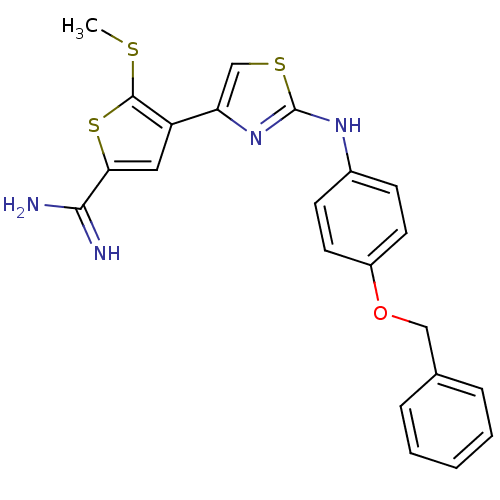 (4-[2-(4-Benzyloxy-phenylamino)-thiazol-4-yl]-5-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098166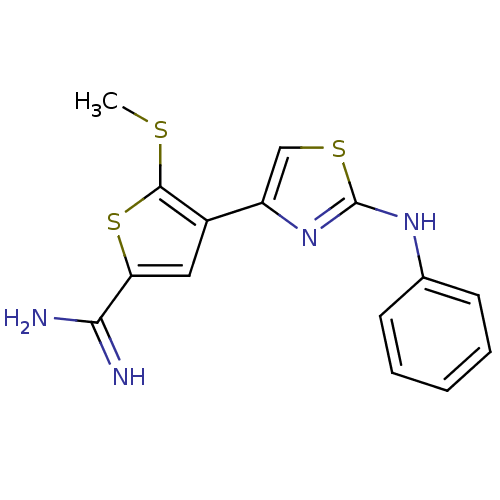 (5-Methylsulfanyl-4-(2-phenylamino-thiazol-4-yl)-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098149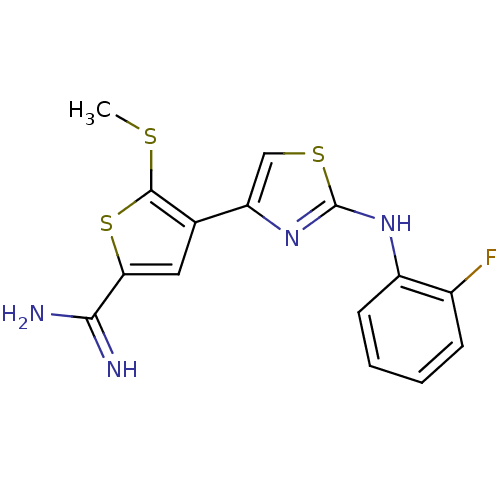 (4-[2-(2-Fluoro-phenylamino)-thiazol-4-yl]-5-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098164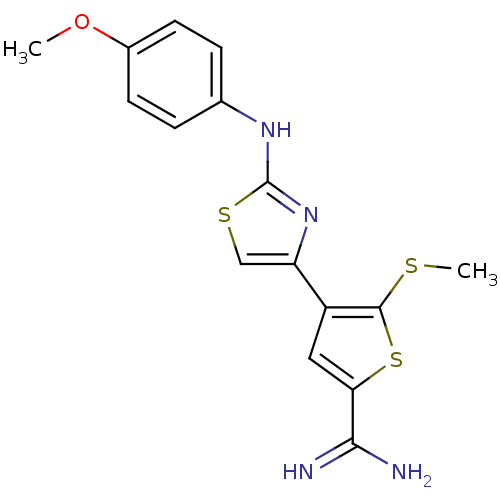 (4-[2-(4-Methoxy-phenylamino)-thiazol-4-yl]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098158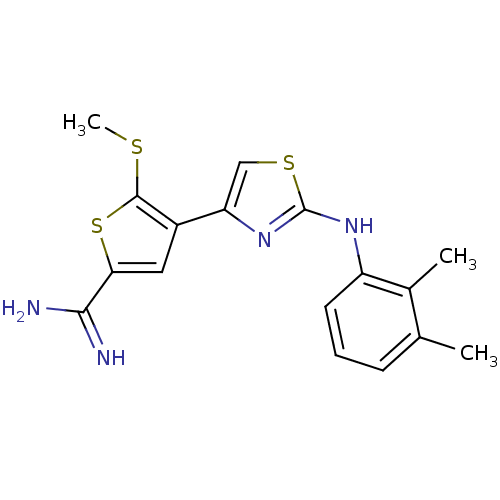 (4-[2-(2,3-Dimethyl-phenylamino)-thiazol-4-yl]-5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098142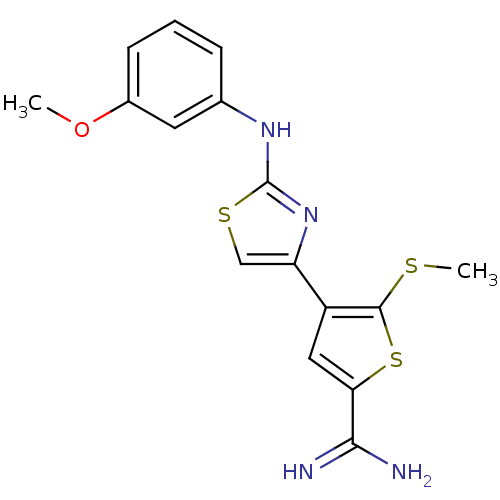 (4-[2-(3-Methoxy-phenylamino)-thiazol-4-yl]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098151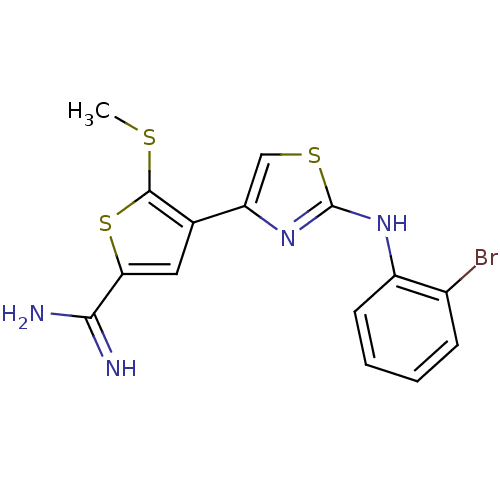 (4-[2-(2-Bromo-phenylamino)-thiazol-4-yl]-5-methyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098150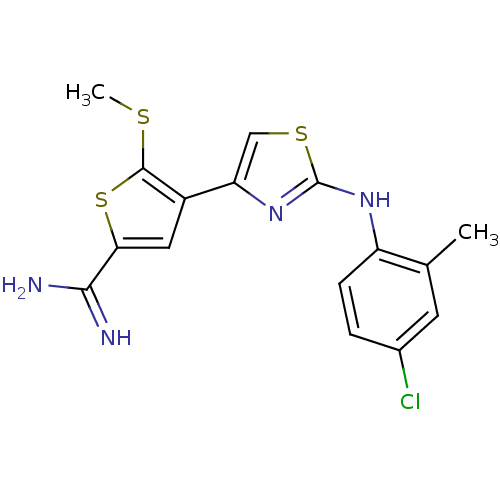 (4-[2-(4-Chloro-2-methyl-phenylamino)-thiazol-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098140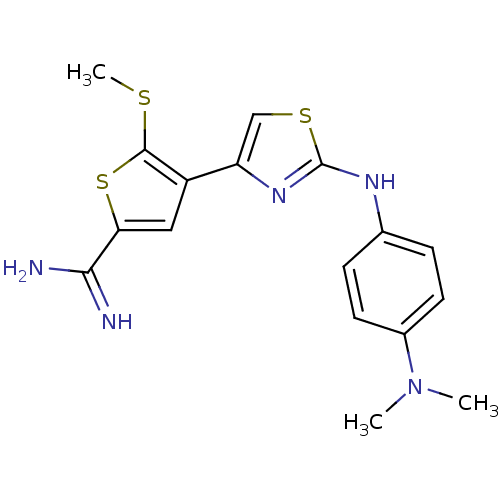 (4-[2-(4-Dimethylamino-phenylamino)-thiazol-4-yl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098137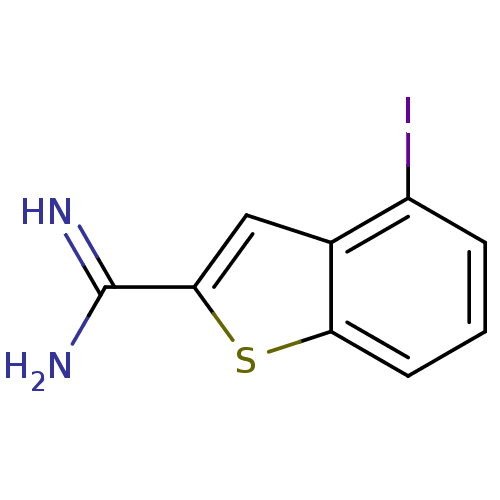 (2-amidino-4-iodobenzothiophene | 4-Iodo-benzo[b]th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human kidney cell urokinase | Bioorg Med Chem Lett 12: 491-5 (2002) BindingDB Entry DOI: 10.7270/Q2H994H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098137 (2-amidino-4-iodobenzothiophene | 4-Iodo-benzo[b]th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098143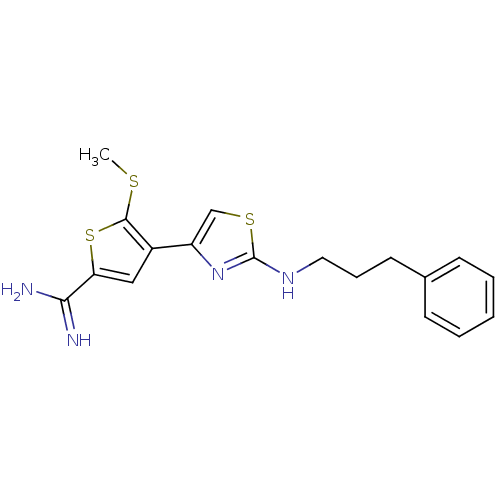 (5-Methylsulfanyl-4-[2-(3-phenyl-propylamino)-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50001309 ((cyclo-[L-propyl-D-phenylalanyl-L-isoleucyl-D-dehy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women | J Med Chem 35: 3905-18 (1992) BindingDB Entry DOI: 10.7270/Q2K64JP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098152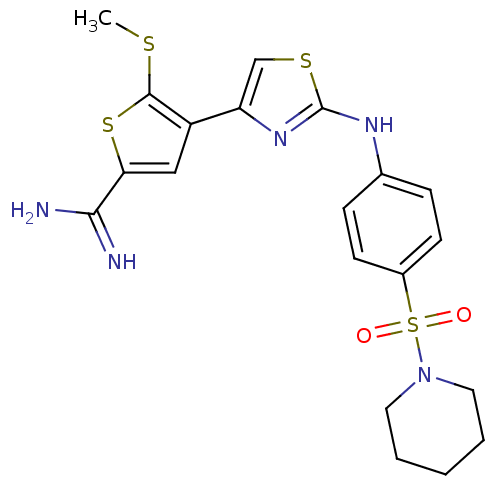 (5-Methylsulfanyl-4-{2-[4-(piperidine-1-sulfonyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098138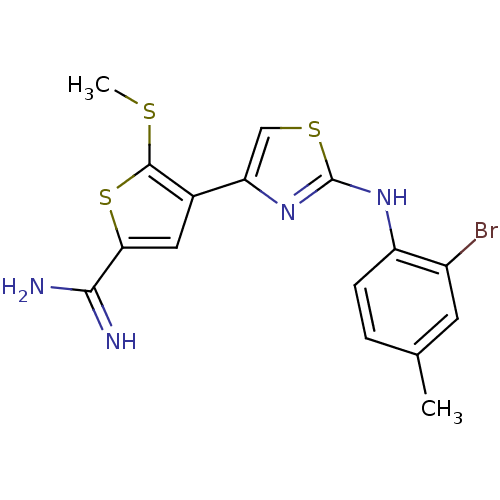 (4-[2-(2-Bromo-4-methyl-phenylamino)-thiazol-4-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098162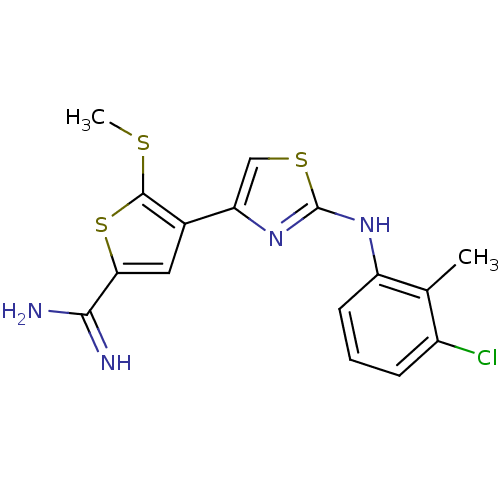 (4-[2-(3-Chloro-2-methyl-phenylamino)-thiazol-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098153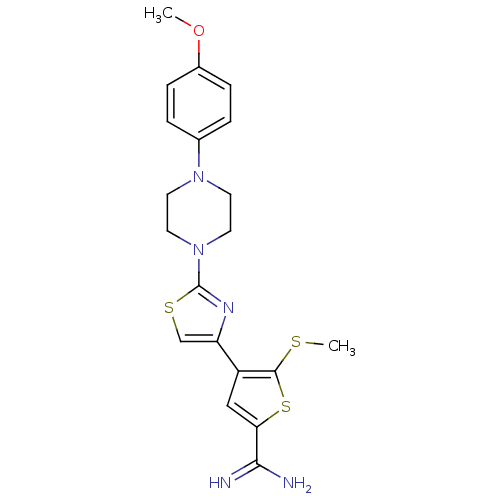 (4-{2-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098156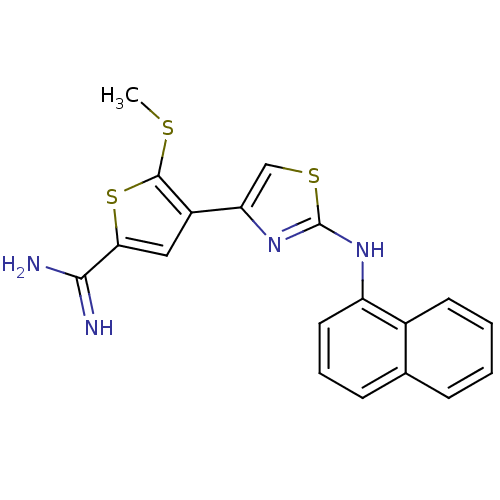 (5-Methylsulfanyl-4-[2-(naphthalen-1-ylamino)-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50098145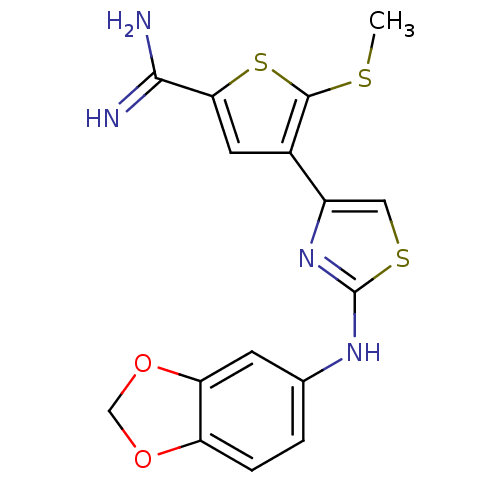 (4-[2-(Benzo[1,3]dioxol-5-ylamino)-thiazol-4-yl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against Urokinase-type plasminogen activator (microPa) | Bioorg Med Chem Lett 11: 915-8 (2001) BindingDB Entry DOI: 10.7270/Q2J67G5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |