 Found 30 hits with Last Name = 'honda' and Initial = 'k'
Found 30 hits with Last Name = 'honda' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232582
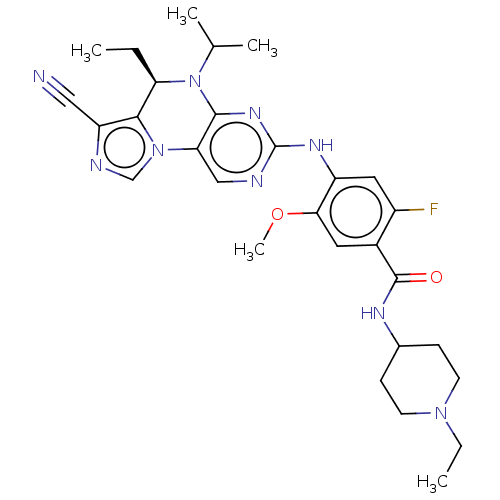
(CHEMBL4067710)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(CC)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C29H36FN9O2/c1-6-23-26-22(14-31)33-16-38(26)24-15-32-29(36-27(24)39(23)17(3)4)35-21-13-20(30)19(12-25(21)41-5)28(40)34-18-8-10-37(7-2)11-9-18/h12-13,15-18,23H,6-11H2,1-5H3,(H,34,40)(H,32,35,36)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232580

(CHEMBL4078098)Show SMILES CC[C@H]1N(C2CCCCC2)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C31H38FN9O2/c1-4-25-28-24(16-33)35-18-40(28)26-17-34-31(38-29(26)41(25)20-8-6-5-7-9-20)37-23-15-22(32)21(14-27(23)43-3)30(42)36-19-10-12-39(2)13-11-19/h14-15,17-20,25H,4-13H2,1-3H3,(H,36,42)(H,34,37,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232588

(CHEMBL4094634)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C(O)=O)c12 |r| Show InChI InChI=1S/C30H38N8O4/c1-4-22-26-25(29(40)41)32-17-37(26)23-16-31-30(35-27(23)38(22)20-7-5-6-8-20)34-21-10-9-18(15-24(21)42-3)28(39)33-19-11-13-36(2)14-12-19/h9-10,15-17,19-20,22H,4-8,11-14H2,1-3H3,(H,33,39)(H,40,41)(H,31,34,35)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232583

(CHEMBL4074778)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C30H37N9O2/c1-4-24-27-23(16-31)33-18-38(27)25-17-32-30(36-28(25)39(24)21-7-5-6-8-21)35-22-10-9-19(15-26(22)41-3)29(40)34-20-11-13-37(2)14-12-20/h9-10,15,17-18,20-21,24H,4-8,11-14H2,1-3H3,(H,34,40)(H,32,35,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232592

(CHEMBL4088932)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CN(C)C3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C26H30FN9O2/c1-6-20-23-19(9-28)30-13-35(23)21-10-29-26(33-24(21)36(20)14(2)3)32-18-8-17(27)16(7-22(18)38-5)25(37)31-15-11-34(4)12-15/h7-8,10,13-15,20H,6,11-12H2,1-5H3,(H,31,37)(H,29,32,33)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232579
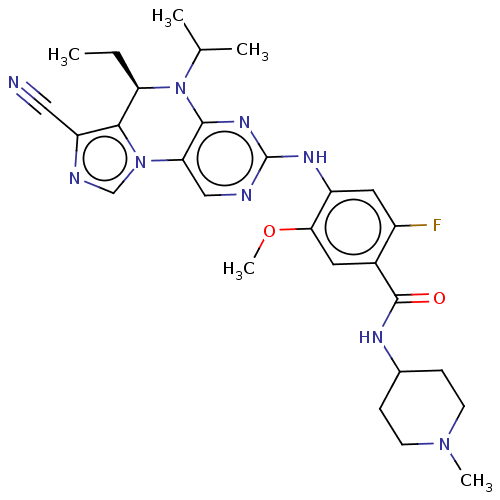
(CHEMBL4103908)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C28H34FN9O2/c1-6-22-25-21(13-30)32-15-37(25)23-14-31-28(35-26(23)38(22)16(2)3)34-20-12-19(29)18(11-24(20)40-5)27(39)33-17-7-9-36(4)10-8-17/h11-12,14-17,22H,6-10H2,1-5H3,(H,33,39)(H,31,34,35)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232585

(CHEMBL4063398)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C(N)=O)c12 |r| Show InChI InChI=1S/C30H39N9O3/c1-4-22-26-25(27(31)40)33-17-38(26)23-16-32-30(36-28(23)39(22)20-7-5-6-8-20)35-21-10-9-18(15-24(21)42-3)29(41)34-19-11-13-37(2)14-12-19/h9-10,15-17,19-20,22H,4-8,11-14H2,1-3H3,(H2,31,40)(H,34,41)(H,32,35,36)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232584
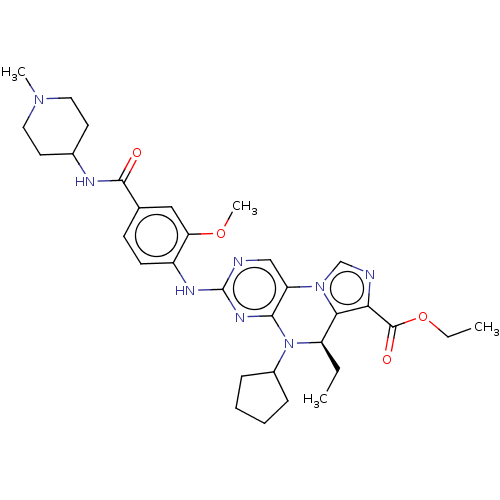
(CHEMBL4074112)Show SMILES CCOC(=O)c1ncn-2c1[C@@H](CC)N(C1CCCC1)c1nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc-21 |r| Show InChI InChI=1S/C32H42N8O4/c1-5-24-28-27(31(42)44-6-2)34-19-39(28)25-18-33-32(37-29(25)40(24)22-9-7-8-10-22)36-23-12-11-20(17-26(23)43-4)30(41)35-21-13-15-38(3)16-14-21/h11-12,17-19,21-22,24H,5-10,13-16H2,1-4H3,(H,35,41)(H,33,36,37)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232590
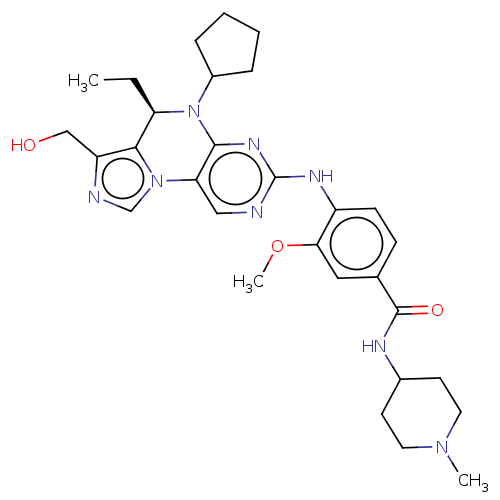
(CHEMBL4101459)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(CO)c12 |r| Show InChI InChI=1S/C30H40N8O3/c1-4-24-27-23(17-39)32-18-37(27)25-16-31-30(35-28(25)38(24)21-7-5-6-8-21)34-22-10-9-19(15-26(22)41-3)29(40)33-20-11-13-36(2)14-12-20/h9-10,15-16,18,20-21,24,39H,4-8,11-14,17H2,1-3H3,(H,33,40)(H,31,34,35)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232578

(CHEMBL4066163)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C30H36FN9O2/c1-4-24-27-23(15-32)34-17-39(27)25-16-33-30(37-28(25)40(24)19-7-5-6-8-19)36-22-14-21(31)20(13-26(22)42-3)29(41)35-18-9-11-38(2)12-10-18/h13-14,16-19,24H,4-12H2,1-3H3,(H,35,41)(H,33,36,37)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232581

(CHEMBL4096151)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3cc(F)c(cc3OC)C(=O)N[C@H]3CC[C@@H](CC3)N3CCN(CC4CC4)CC3)ncc2-n2cnc(C#N)c12 |r,wU:2.1,26.30,wD:23.23,(65.63,.49,;65.63,2.03,;64.3,2.79,;62.97,2.04,;62.96,.5,;61.62,-.27,;64.29,-.28,;61.65,2.82,;60.32,2.04,;58.99,2.81,;57.66,2.02,;57.66,.48,;58.99,-.28,;59,-1.82,;60.34,-2.58,;57.67,-2.6,;56.33,-1.83,;56.33,-.29,;54.99,.48,;54.99,2.02,;57.67,-4.14,;59.01,-4.9,;56.34,-4.91,;56.34,-6.45,;55.01,-7.22,;55.01,-8.75,;56.34,-9.53,;57.68,-8.75,;57.68,-7.21,;56.34,-11.06,;55.01,-11.82,;55,-13.36,;56.33,-14.13,;56.32,-15.67,;54.99,-16.44,;53.45,-16.43,;54.21,-17.77,;57.67,-13.37,;57.68,-11.82,;58.98,4.34,;60.31,5.12,;61.65,4.34,;62.98,5.12,;63.31,6.61,;64.83,6.75,;65.44,5.36,;66.95,5.02,;68.45,4.69,;64.3,4.34,)| Show InChI InChI=1S/C36H47FN10O2/c1-5-30-33-29(18-38)40-21-46(33)31-19-39-36(43-34(31)47(30)22(2)3)42-28-17-27(37)26(16-32(28)49-4)35(48)41-24-8-10-25(11-9-24)45-14-12-44(13-15-45)20-23-6-7-23/h16-17,19,21-25,30H,5-15,20H2,1-4H3,(H,41,48)(H,39,42,43)/t24-,25-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232587

(CHEMBL4093737)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C(=O)N(C)O)c12 |r| Show InChI InChI=1S/C31H41N9O4/c1-5-23-27-26(30(42)38(3)43)33-18-39(27)24-17-32-31(36-28(24)40(23)21-8-6-7-9-21)35-22-11-10-19(16-25(22)44-4)29(41)34-20-12-14-37(2)15-13-20/h10-11,16-18,20-21,23,43H,5-9,12-15H2,1-4H3,(H,34,41)(H,32,35,36)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232591
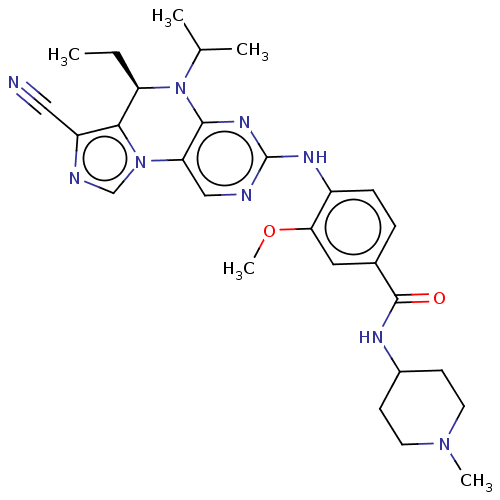
(CHEMBL4085987)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C28H35N9O2/c1-6-22-25-21(14-29)31-16-36(25)23-15-30-28(34-26(23)37(22)17(2)3)33-20-8-7-18(13-24(20)39-5)27(38)32-19-9-11-35(4)12-10-19/h7-8,13,15-17,19,22H,6,9-12H2,1-5H3,(H,32,38)(H,30,33,34)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50232586
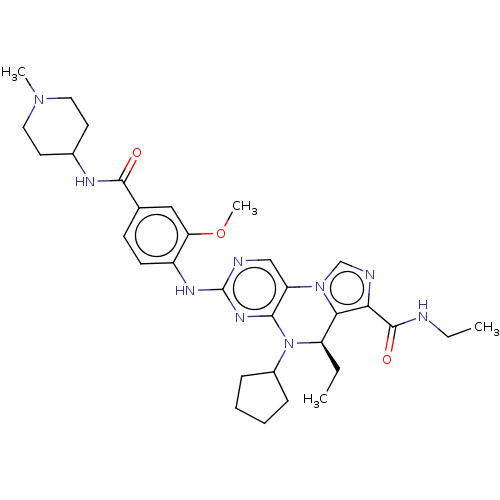
(CHEMBL4085036)Show SMILES CCNC(=O)c1ncn-2c1[C@@H](CC)N(C1CCCC1)c1nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc-21 |r| Show InChI InChI=1S/C32H43N9O3/c1-5-24-28-27(31(43)33-6-2)35-19-40(28)25-18-34-32(38-29(25)41(24)22-9-7-8-10-22)37-23-12-11-20(17-26(23)44-4)30(42)36-21-13-15-39(3)16-14-21/h11-12,17-19,21-22,24H,5-10,13-16H2,1-4H3,(H,33,43)(H,36,42)(H,34,37,38)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PLK1 expressed in baculovirus infected sf21 cells using Biotin-AGAGTVPESIHSFIGDGLV as substrate by TR-FRET assay |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50240296

(CHEMBL4095506)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C71H99N17O19/c1-35(2)25-46(72)62(98)87-58(38(6)90)70(106)85-52(27-40-13-9-8-10-14-40)66(102)80-49(22-24-57(94)95)64(100)84-54(30-43-32-75-34-77-43)68(104)82-51(28-41-17-19-44(92)20-18-41)67(103)83-53(29-42-31-76-47-16-12-11-15-45(42)47)65(101)78-37(5)61(97)79-48(21-23-56(73)93)63(99)81-50(26-36(3)4)69(105)88-59(39(7)91)71(107)86-55(33-89)60(74)96/h8-20,31-32,34-39,46,48-55,58-59,76,89-92H,21-30,33,72H2,1-7H3,(H2,73,93)(H2,74,96)(H,75,77)(H,78,101)(H,79,97)(H,80,102)(H,81,99)(H,82,104)(H,83,103)(H,84,100)(H,85,106)(H,86,107)(H,87,98)(H,88,105)(H,94,95)/t37-,38+,39+,46-,48-,49-,50-,51-,52-,53-,54-,55-,58-,59-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled P4 peptide binding to MDM2 (25 to 109 residues) (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM29643
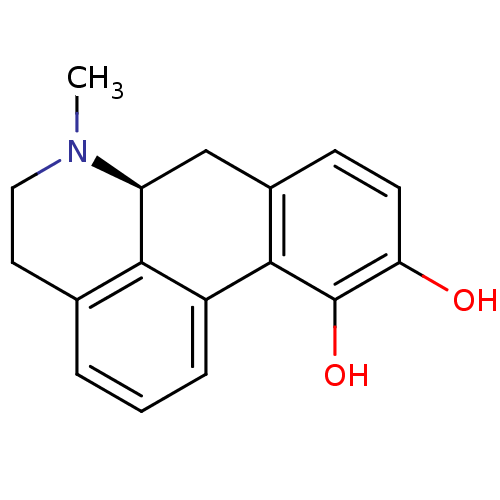
((6aS)-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled L-p53 binding to L-MDM2 (25 to 109 residues) (unknown origin) by FAM labeled P4 peptide based fluorescence polarization... |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled D-p53 binding to D-MDM2 (25 to 109 residues) (unknown origin) by FAM labeled P4 peptide based fluorescence polarization... |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled L-p53 binding to L-MDM2 (25 to 109 residues) (unknown origin) by FAM labeled P4 peptide based fluorescence polarization... |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50240295
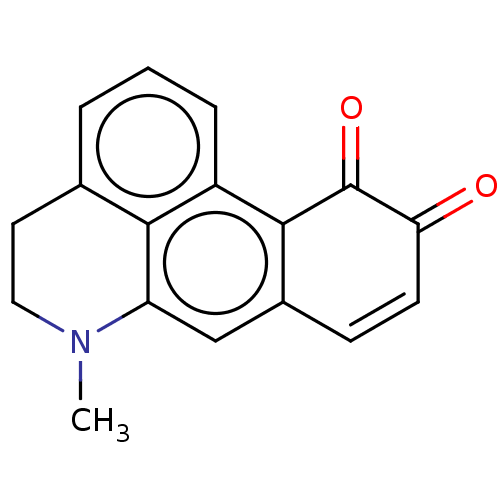
(CHEMBL4068121)Show InChI InChI=1S/C17H13NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,9H,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled P4 peptide binding to MDM2 (25 to 109 residues) (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436446

(CHEMBL2397155)Show SMILES COc1ccc(COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(C)=O)C(=O)N[C@@H](Cc2cn(c3ccccc23)S(=O)(=O)c2c(C)cc(C)cc2C)C(=O)N[C@@H](CC(C)C)C(=O)NN)cc1 |r| Show InChI InChI=1S/C46H61N7O10S/c1-26(2)20-37(44(57)52-47)49-43(56)38(23-33-24-53(39-13-11-10-12-35(33)39)64(60,61)41-29(6)21-28(5)22-30(41)7)50-42(55)36(19-14-31(8)54)48-45(58)40(27(3)4)51-46(59)63-25-32-15-17-34(62-9)18-16-32/h10-13,15-18,21-22,24,26-27,36-38,40H,14,19-20,23,25,47H2,1-9H3,(H,48,58)(H,49,56)(H,50,55)(H,51,59)(H,52,57)/t36-,37-,38-,40-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) binding to FAM-LTFEHYWAQLTS-NH2 after 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 3802-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.094
BindingDB Entry DOI: 10.7270/Q2VD70WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50232580

(CHEMBL4078098)Show SMILES CC[C@H]1N(C2CCCCC2)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C31H38FN9O2/c1-4-25-28-24(16-33)35-18-40(28)26-17-34-31(38-29(26)41(25)20-8-6-5-7-9-20)37-23-15-22(32)21(14-27(23)43-3)30(42)36-19-10-12-39(2)13-11-19/h14-15,17-20,25H,4-13H2,1-3H3,(H,36,42)(H,34,37,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50232578

(CHEMBL4066163)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C30H36FN9O2/c1-4-24-27-23(15-32)34-17-39(27)25-16-33-30(37-28(25)40(24)19-7-5-6-8-19)36-22-14-21(31)20(13-26(22)42-3)29(41)35-18-9-11-38(2)12-10-18/h13-14,16-19,24H,4-12H2,1-3H3,(H,35,41)(H,33,36,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50232580

(CHEMBL4078098)Show SMILES CC[C@H]1N(C2CCCCC2)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C31H38FN9O2/c1-4-25-28-24(16-33)35-18-40(28)26-17-34-31(38-29(26)41(25)20-8-6-5-7-9-20)37-23-15-22(32)21(14-27(23)43-3)30(42)36-19-10-12-39(2)13-11-19/h14-15,17-20,25H,4-13H2,1-3H3,(H,36,42)(H,34,37,38)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50232578

(CHEMBL4066163)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3cc(F)c(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C30H36FN9O2/c1-4-24-27-23(15-32)34-17-39(27)25-16-33-30(37-28(25)40(24)19-7-5-6-8-19)36-22-14-21(31)20(13-26(22)42-3)29(41)35-18-9-11-38(2)12-10-18/h13-14,16-19,24H,4-12H2,1-3H3,(H,35,41)(H,33,36,37)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50232583

(CHEMBL4074778)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2-n2cnc(C#N)c12 |r| Show InChI InChI=1S/C30H37N9O2/c1-4-24-27-23(16-31)33-18-38(27)25-17-32-30(36-28(25)39(24)21-7-5-6-8-21)35-22-10-9-19(15-26(22)41-3)29(40)34-20-11-13-37(2)14-12-20/h9-10,15,17-18,20-21,24H,4-8,11-14H2,1-3H3,(H,34,40)(H,32,35,36)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 1311-1315 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.009
BindingDB Entry DOI: 10.7270/Q2K939RQ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM22774
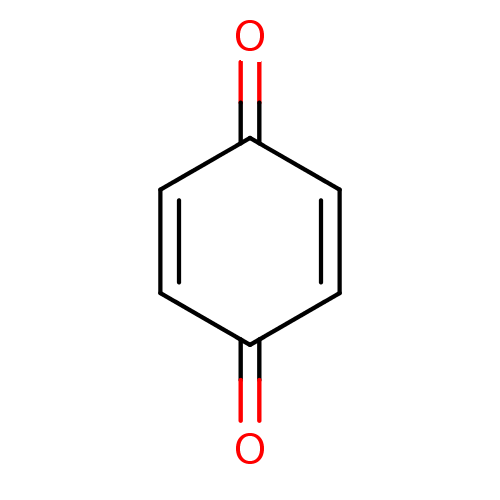
(1,4-Benzoquinone | Benzil-related compound, 53 | C...)Show InChI InChI=1S/C6H4O2/c7-5-1-2-6(8)4-3-5/h1-4H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled p53 binding to MDM2 (25 to 109 residues) (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436444

(CHEMBL2397153)Show SMILES COc1ccc(COC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(=O)OCc2ccccc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)OC2CCCCCC2)C(=O)N[C@@H](C)C(=O)NN)cc1 |r| Show InChI InChI=1S/C49H72N8O13/c1-29(2)25-38(47(64)56-42(30(3)4)48(65)55-39(46(63)51-32(6)44(61)57-50)26-41(59)70-36-17-13-8-9-14-18-36)54-45(62)37(23-24-40(58)68-27-33-15-11-10-12-16-33)53-43(60)31(5)52-49(66)69-28-34-19-21-35(67-7)22-20-34/h10-12,15-16,19-22,29-32,36-39,42H,8-9,13-14,17-18,23-28,50H2,1-7H3,(H,51,63)(H,52,66)(H,53,60)(H,54,62)(H,55,65)(H,56,64)(H,57,61)/t31-,32-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) binding to FAM-LTFEHYWAQLTS-NH2 after 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 3802-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.094
BindingDB Entry DOI: 10.7270/Q2VD70WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50436445

(CHEMBL2397154)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CSSc1ncccc1[N+]([O-])=O)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CCC[NH+]=C([NH-])NS(=O)(=O)c1c(C)cc(C)cc1C)C(=O)OC |r,w:47.48| Show InChI InChI=1S/C44H61N9O11S3/c1-10-27(3)35(39(56)48-31(41(57)63-9)18-14-21-47-42(45)52-67(61,62)36-28(4)22-26(2)23-29(36)5)51-37(54)32(24-30-16-12-11-13-17-30)49-38(55)33(50-43(58)64-44(6,7)8)25-65-66-40-34(53(59)60)19-15-20-46-40/h11-13,15-17,19-20,22-23,27,31-33,35H,10,14,18,21,24-25H2,1-9H3,(H7,45,47,48,49,50,51,52,54,55,56,58)/t27-,31-,32-,33-,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) binding to FAM-LTFEHYWAQLTS-NH2 after 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 23: 3802-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.094
BindingDB Entry DOI: 10.7270/Q2VD70WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled P4 peptide binding to MDM2 (25 to 109 residues) (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM26188
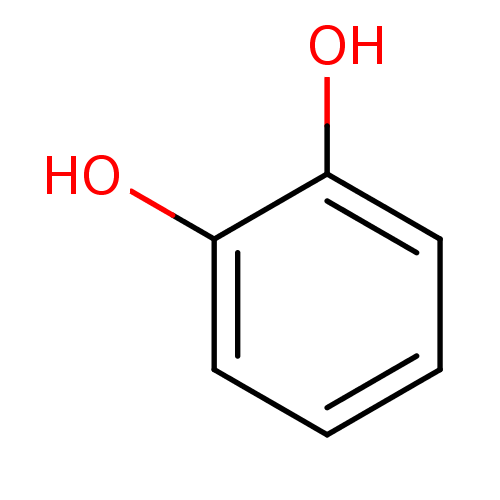
(α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of biotin-labelled p53 binding to MDM2 (25 to 109 residues) (unknown origin) by surface plasmon resonance method |
Bioorg Med Chem Lett 27: 2571-2574 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.082
BindingDB Entry DOI: 10.7270/Q2571F44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data